Traumatic brain injury (TBI) affects 69 million people every year. One of the main limitations in managing TBI patients is the lack of univocal diagnostic criteria, including the absence of standardized assessment methods and guidelines. Immediacy and accuracy in diagnosis and management of TBIs are critically unmet medical needs. Especially in the context of sports-associated TBI, there is a strong need for prognostic indicators to help diagnose and identify at-risk subjects to avoid their returning to play while the brain is still highly vulnerable. Fluid biomarkers may emerge as new prognostic indicators to develop more accurate prediction models, improving risk stratification and clinical decision-making.
- traumatic brain injury (TBI)
- sports-related TBI
- brain injury markers/biomarkers
- TBI biomarker
- outcome assessment
1. Introduction
2. Current Guidelines
| Risk Factor | New Orleans Criteria | Canadian CT Head Rule | NICE 2014 | NCWFS | Scandinavian |
|---|---|---|---|---|---|
| Headache | High risk | High risk | Medium risk | ||
| Vomiting | High risk | High risk | High risk | High risk | Medium risk |
| Post-traumatic seizure | High risk | High risk | High risk | ||
| Intoxication (drug or alcohol) | High risk | High risk | |||
| Persistent anterograde amnesia | High risk | High risk | |||
| Age | High risk > 60 years | High risk > 65 years | High risk > 65 years | ||
| Clinical signs of skull fracture | High risk | High risk | High risk | High risk | High risk |
| Contusion of the skull | High risk | High risk | High risk | High risk | High risk |
| Signs official fracture | High risk | ||||
| Contusion of the face | High risk | ||||
| GCS score deterioration | High risk | High risk | High risk | ||
| Pedestrian versus vehicle | Medium risk | High risk | |||
| Ejected from vehicle | Medium risk | High risk | |||
| Fall from height | Medium risk | High risk | |||
| Prolonged post-traumatic amnesia | Medium risk | High risk | High risk | Medium risk | |
| GCS < 15 at presentation | High risk | High risk | High risk | ||
| Loss of consciousness | High risk | Medium risk | |||
| Neurologic deficit | High risk | High risk | Medium risk | ||
| Anticoagulation therapy | High risk | High risk | High risk | ||
| High-energy trauma | |||||
| Multiple injuries | |||||
| Pre-traumatic seizure | High risk | ||||
| Unclear trauma mechanism | |||||
| Previous neurosurgery | High risk | ||||
| S100B ≥ 0.1 μg/L | Medium risk |
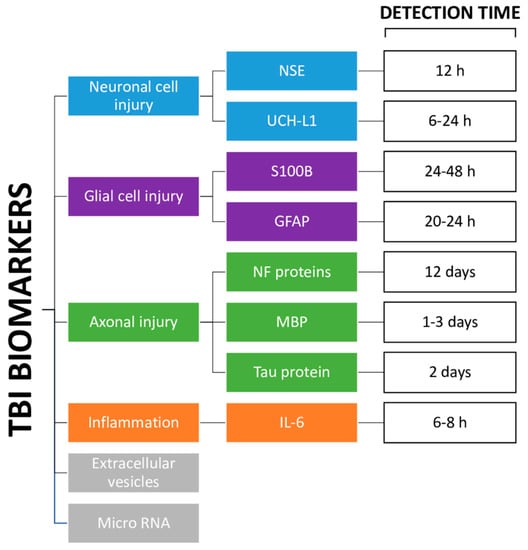
3. Markers
3.1. Markers of Neuronal Cell Body Injury
3.2. Markers of Glial Cell Injury
3.3. Markers of Axonal Injury
3.4. Markers of Inflammation
3.5. Other Markers
This entry is adapted from the peer-reviewed paper 10.3390/jcm12072563
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2019, 130, 1080–1097.
- Holm, L.; Cassidy, J.D.; Carroll, L.; Borg, J. Summary of the WHO collaborating centre for neurotrauma task force on mild traumatic brain injury. J. Rehabil. Med. 2005, 37, 137–141.
- Stein, S.C.; Spettell, C.; Young, G.; Ross, S.E. Limitations of neurological assessment in mild head injury. Brain Inj. 1993, 7, 425–430.
- Easter, J.S.; Haukoos, J.S.; Meehan, W.P.; Novack, V.; Edlow, J.A. Will Neuroimaging Reveal a Severe Intracranial Injury in This Adult with Minor Head Trauma? JAMA 2015, 314, 2672.
- Shackford, S.R.; Wald, S.L.; Ross, S.E.; Cogbill, T.H.; Hoyt, D.B.; Morris, J.A.; Mucha, P.A.; Pachter, H.L.; Sugerman, H.J. The clinical utility of computed tomographic scanning and neurologic examination in the management of patients with minor head injuries. J. Trauma Inj. Infect. Crit. Care 1992, 33, 385–394.
- Langfitt, T.W.; Obrist, W.D.; Alavi, A.; Grossman, R.I.; Zimmerman, R.A.; Jaggi, J.; Uzzell, B.P.; Reivich, M.; Patton, D.R. Computerized tomography, magnetic resonance imaging, and positron emission tomography in the study of brain trauma. J. Neurosurg. 1986, 64, 760–767.
- Lee, B.; Newberg, A. Neuroimaging in traumatic brain imaging. Neurorx 2005, 2, 372–383.
- Eisele, A.; Hill-Strathy, M.; Michels, L.; Rauen, K. Magnetic Resonance Spectroscopy following Mild Traumatic Brain Injury: A Systematic Review and Meta-Analysis on the Potential to Detect Posttraumatic Neurodegeneration. Neurodegener. Dis. 2020, 20, 2–11.
- Lefevre-Dognin, C.; Cogné, M.; Perdrieau, V.; Granger, A.; Heslot, C.; Azouvi, P. Definition and epidemiology of mild traumatic brain injury. Neurochirurgie 2020, 67, 218–221.
- Danna-Dos-Santos, A.; Mohapatra, S.; Santos, M.; Degani, A.M. Long-term effects of mild traumatic brain injuries to oculomotor tracking performances and reaction times to simple environmental stimuli. Sci. Rep. 2018, 8, 4583.
- Ghaith, H.S.; Nawar, A.A.; Gabra, M.D.; Abdelrahman, M.E.; Nafady, M.H.; Bahbah, E.I.; Ebada, M.A.; Ashraf, G.M.; Negida, A.; Barreto, G.E. A Literature Review of Traumatic Brain Injury Biomarkers. Mol. Neurobiol. 2022, 59, 4141–4158.
- McDonald, S.J.; Shultz, S.R.; Agoston, D.V. The Known Unknowns: An Overview of the State of Blood-Based Protein Biomarkers of Mild Traumatic Brain Injury. J. Neurotrauma 2021, 38, 2652–2666.
- Helmrich, M.I.R.A.R.; Lingsma, H.F.; Turgeon, A.F.; Yamal, J.-M.; Steyerberg, E.W. Prognostic Research in Traumatic Brain Injury: Markers, Modeling, and Methodological Principles. J. Neurotrauma 2021, 38, 2502–2513.
- Foks, K.A.; Cnossen, M.C.; Dippel, D.W.; Maas, A.I.; Menon, D.; van der Naalt, J.; Steyerberg, E.W.; Lingsma, H.F.; Polinder, S.; on behalf of CENTER-TBI investigators and participants. Management of Mild Traumatic Brain Injury at the Emergency Department and Hospital Admission in Europe: A Survey of 71 Neurotrauma Centers Participating in the CENTER-TBI Study. J. Neurotrauma 2017, 34, 2529–2535.
- Stiell, I.G.; Wells, G.A.; Vandemheen, K.; Clement, C.; Lesiuk, H.; Laupacis, A.; McKnight, R.D.; Verbeek, R.; Brison, R.; Cass, D.; et al. The Canadian CT Head Rule for patients with minor head injury. Lancet 2001, 357, 1391–1396.
- Ingebrigtsen, T.; Romner, B.; Kock-Jensen, C. Scandinavian Guidelines for Initial Management of Minimal, Mild, and Moderate Head Injuries. J. Trauma Inj. Infect. Crit. Care 2000, 48, 760–766.
- Undén, J.; the Scandinavian Neurotrauma Committee (SNC); Ingebrigtsen, T.; Romner, B. Scandinavian guidelines for initial management of minimal, mild and moderate head injuries in adults: An evidence and consensus-based update. BMC Med. 2013, 11, 50.
- Stiell, I.; Clement, C.M.; Rowe, B.H.; Schull, M.; Brison, R.; Cass, D.; Eisenhauer, M.A.; McKnight, R.D.; Bandiera, G.; Holroyd, B.; et al. Comparison of the Canadian CT Head Rule and the New Orleans Criteria in Patients with Minor Head Injury. JAMA 2005, 294, 1511–1518.
- Goodacre, S. Hospital admissions with head injury following publication of NICE guidance. Emerg. Med. J. 2008, 25, 556–557.
- Yates, D.; Aktar, R.; Hill, J. Assessment, investigation, and early management of head injury: Summary of NICE guidance. BMJ 2007, 335, 719–720.
- National Institute for Health and Care Excellence (NICE). Head Injury: Assessment and Early Management. Guidance. Available online: https://www.nice.org.uk/guidance/cg176 (accessed on 17 December 2022).
- Fabbri, A.; Servadei, F.; Marchesini, G.; Dente, M.; Iervese, T.; Spada, M.; Vandelli, A. Clinical Performance of NICE Recommendations versus NCWFNS Proposal in Patients with Mild Head Injury. J. Neurotrauma 2005, 22, 1419–1427.
- Servadei, F.; Teasdale, G.; Merry, G. Defining Acute Mild Head Injury in Adults: A Proposal Based on Prognostic Factors, Diagnosis, and Management. J. Neurotrauma 2001, 18, 657–664.
- Huibregtse, M.E.; Bazarian, J.J.; Shultz, S.R.; Kawata, K. The biological significance and clinical utility of emerging blood biomarkers for traumatic brain injury. Neurosci. Biobehav. Rev. 2021, 130, 433–447.
- Shahim, P.; Zetterberg, H. Neurochemical Markers of Traumatic Brain Injury: Relevance to Acute Diagnostics, Disease Monitoring, and Neuropsychiatric Outcome Prediction. Biol. Psychiatry 2021, 91, 405–412.
- FDA Authorizes Marketing of First Blood Test to Aid in the Evaluation of Concussion in Adults. 2020. Available online: https://www.fda.gov/news-events/press-announcements/fda-authorizes-marketing-first-blood-test-aid-evaluation-concussion-adults (accessed on 16 December 2022).
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589.
- Nitta, M.E.; Savitz, J.; Nelson, L.D.; Teague, T.K.; Hoelzle, J.B.; McCrea, M.A.; Meier, T.B. Acute elevation of serum inflammatory markers predicts symptom recovery after concussion. Neurology 2019, 93, e497–e507.
- Meier, T.B.; Huber, D.L.; Bohorquez-Montoya, L.; Nitta, M.E.; Savitz, J.; Teague, T.K.; Bazarian, J.J.; Hayes, R.L.; Nelson, L.D.; McCrea, M.A. A Prospective Study of Acute Blood-Based Biomarkers for Sport-Related Concussion. Ann. Neurol. 2020, 87, 907–920.
- Edwards, K.A.; Gill, J.M.; Pattinson, C.L.; Lai, C.; Brière, M.; Rogers, N.J.; Milhorn, D.; Elliot, J.; Carr, W. Interleukin-6 is associated with acute concussion in military combat personnel. BMC Neurol. 2020, 20, 209.
- Shaw, A.C.; Goldstein, D.R.; Montgomery, R.R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013, 13, 875–887.
- Visser, K.; Koggel, M.; Blaauw, J.; van der Horn, H.J.; Jacobs, B.; van der Naalt, J. Blood-based biomarkers of inflammation in mild traumatic brain injury: A systematic review. Neurosci. Biobehav. Rev. 2021, 132, 154–168.
- Wang, W.; Kwon, E.J.; Tsai, L.-H. MicroRNAs in learning, memory, and neurological diseases: Figure 1. Learn. Mem. 2012, 19, 359–368.
