Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Orthopedic oncology has begun to use three-dimensional-printing technology, which is expected to improve the accuracy of osteotomies, ensure a safe margin, and facilitate precise surgery. However, several difficulties should be considered.
- three-dimensional printed guide
- patients-specific guide
- bone tumor
- limb-sparing surgery
- orthopedic oncology
- pelvic tumor
- tumor of the sacrum
- patient-specific implant
1. Advantages of the 3D-Printing Technique
1.1. Resection with Safe Margins
One of the most important advantages of PSGs is the achievement of an accurate resection of bone tumors with safe margins [1][2][3]. From previous cadaver studies, manual resection can result in inaccuracies of up to 5–15 mm, which might lead to unexpected intralesional resection [4][5][6][7]. A PSG can be applied for the preparation of massive bone allografts (MBAs) for the reconstruction of bone defects following bone-tumor resection (Figure 1) [8]. A PSG can help surgeons achieve a more precise cutting of the MBA using the same predefined section planes for the resection of bone tumors [8]. Bellanove et al. reported a case series of four patients and the outcomes of the resection of a malignant bone tumor in the proximal tibia using a PSG and MBA [9]. Safe resection margins were achieved, and satisfactory postoperative radiographs were obtained [9]. In addition, radiological union at the graft–-host junction was observed at 4–12 months [9].
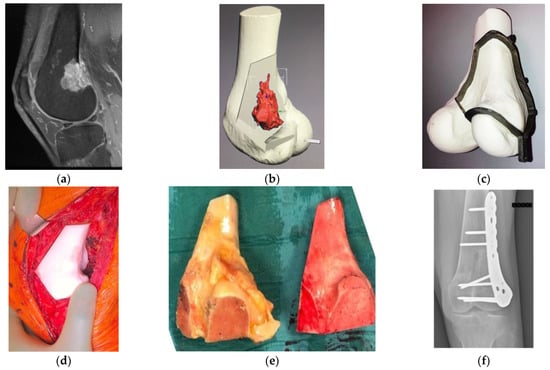
Figure 1. Osteosarcoma in the distal femur of a 56-year-old man. (a) High-intensity signal was seen in the sagittal MRI image (T1-fat suppression with contrast) at the posterior to the lateral side of the distal femur. (b) Resection planning in the computer-aided design phase. The location of the tumor was highlighted in red via an integrated image of CT and MRI and resection planes were determined with a discussion between surgeons and engineers. (c) Surgical planning with a PSG and an artificial bone. To preserve the surface of the knee joint and bone stock, hemi-cortical resection with the PSG, followed by reconstruction with a massive bone allograft was planned. The fitting of the PSG to the bone was confirmed. (d) Intraoperatively, after the resection with the PSG, the compatibility of the bone defect with the allograft, which was subsequently resected using the PSG, was confirmed by the artificial bone spacer. (e) Resected specimen (left) and resected massive bone allograft (right) via the PSG. Researchers confirmed the identical fitting of a massive bone allograft to the large defect after the tumor resection. (f) Postoperative radiograph after the resection of the tumor and reconstruction with massive bone allograft. CT, computed tomography; PSG, patient-specific cutting guide; MRI, magnetic resonance imaging.
1.2. Reconstruction of Bone Defects
The design of conventional prosthetic implants has improved, although, it is important to consider the potential failure of initial fixation due to inadequate matching of implants and host bone tissue, which affects the bone–implant interaction, leading to bone atrophy or implant loosening [10][11]. A patient-specific implant (PSI) is used to ensure a good fit for bone defects for accurate placement of prostheses; however, its clinical utility should be validated over the long term [3] (Figure 2). Kieser et al. reported mid-term outcomes (a median follow-up period of 38 months) of a PSI for large bone defects in the acetabular region [12]. Of the 36 patients evaluated, one patient experienced early implant migration with subsequent stabilization; two patients experienced failure of osteointegration; and no patient exhibited aseptic loosening [12]. Liu et al. reported the results of a retrospective study of a P2–P3 resection of pelvic tumors, with a median follow-up period of 36 months [13]. Among the 19 patients treated with PSG + PSI, aseptic loosening occurred in four patients [13] (Figure 2).
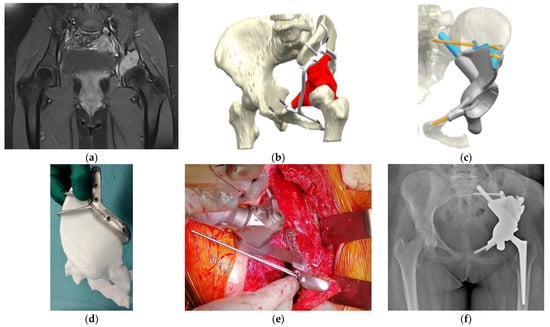
Figure 2. Osteosarcoma in the pelvis in a 14-year-old girl. (a) MRI image (T1-fat suppression with contrast) shows an osteolytic lesion in the left acetabulum with a high-intensity signal. (b) Preoperative planning for the P2–P3 resection of the pelvis using a PSG and reconstruction using a PSI. The tumor’s location was highlighted in red. The PSG was designed to have a sufficient margin with hooks for better attachment of the pelvic bone. The PSG was segmented for detachment into small parts, enabling a step-by-step surgical procedure. (c) In the computer-aided-design phase, the insertion of the PSI after the resection of the tumor and the points of fixation of the PSI were simulated. The actual cutting points were confirmed with a 3D-printed bone model. The points of fixation of cancellous screws and stems were also determined, and the appropriate length of drilling or the sizes of planned screws or stems were calculated. (d) The actual cutting points were preoperatively simulated with an artificial, 3D-printed bone model. (e) An intraoperative image of the resection along with the PSG. This procedure was supported by Waldemar Link GmbH & Co. KG (Norderstedt, Germany). (f) Postoperative radiograph after the resection of the tumor and reconstruction with a PSI. PSI, patient-specific implant; PSG, patient-specific cutting guide; MRI, magnetic resonance imaging.
1.3. Understanding of Anatomy and Surgical Planning
Surgical planning is important for successful outcomes at challenging sites such as the pelvis and sacrum. This planning is improved with a better understanding of the spatial relationship between important adjacent structures and tumors [14][15]. By using a sterile 3D-printed model during the operation, proper orientation of the anatomy can be provided for surgeons and assistants [16][17]. Goyal et al. described the utility of education in orthopedic residents using a 3D-printed model [18]. They compared education with lectures only (Group 1) or lectures with 3D-printed-model guidance (Group 2). The post-test scores for fracture classification and surgical approach were significantly higher in the 3D model group (Group 1 vs. 2: 2.5–6 vs. 4.4–10%; p < 0.05) [18]. With a better and more accurate understanding of anatomy, surgeons are confident in maintaining a safe surgical margin without compromising important structures [19]. The educational approach, when combined with the 3D model and the PSG, enables precise control of safe margins by demonstrating osteotomies with oscillating blades in the predefined resection planes to young surgeons [8].
1.4. Reduction of Surgical Invasiveness and Operation Time
Several studies have shown that PSGs can reduce blood loss and operation time during bone tumor surgery [20][21]. In a retrospective comparative study, Liu et al. conducted a retrospective comparative study of patients with malignant bone tumors in the pelvis (P2–P3) treated with or without PSGs (n = 19/19), and they found that operation time (PSG group vs. control group: 209 vs. 272 min; p = 0.003) and blood loss (PSG group vs. control group: 1390 vs. 2248 mL; p = 0.002) were better in the PSG group [13]. In contrast, a randomized control study by Wang et al. described the efficacy of the PSG for the resection of malignant bone tumors around the knee joint (n = 33, control group; n = 33, PSG group) [21]. They proved the superiority of the PSG in terms of blood loss (control group vs. PSG group: 689 vs. 647 mL; p = 0.003) [21], but no difference was observed in operation time (control group vs. PSG group: 136 vs. 145 min; p = 0.685) [21].
The PSG is expected to enable surgeons to reproduce a virtual surgical plan with similar accuracy but with less bone resection time when compared with navigation assistance during surgery [22]. Bosma et al. compared PSGs and navigation-assisted osteotomy for knee-joint resection in a cadaver study that involved 16 simulated tumors around the knee in four cadavers [6]. The PSG group had a significantly lower total bone resection time (navigation group vs. PSG group: 17 vs. 5 min; p < 0.001), and the maximum distance between the planned osteotomy and the achieved osteotomy was superior in the PSG group (PSG group vs. navigation-assisted group: 1.9 vs. 3.6 mm, p = 0.042) [6].
2. Limitations of the 3D-Printing Technique
2.1. Delay and Cost of Surgery
Lead time is required for the design and manufacturing of PSGs. The intervals between diagnosis and surgery are related to the diagnosis of the bone tumor—longer intervals in chemo-sensitive tumors, such as osteosarcoma or Ewing sarcoma; shorter intervals in chemo-resistant tumors, such as chondrosarcoma [23]. If there was adequate time before surgery, it would be possible to prepare the PSG [23]. With the advances in technology, any delay during preparation has been shortened, and Rustemeyer et al. mentioned a 2–4-week delay in PSG- or PSI-assisted surgeries for maxillofacial applications [24]. However, Martelli et al. reported that production delays were a limitation in 19.6% of studies that used 3D-printed devices [25]. Outsourcing the production of a PSG or PSI could take 4–6 weeks for the process of design and manufacturing [26]; whereas, with the development of 3D printing devices, low-cost in-house 3D-printing technology has been utilized, and the introduction of PSGs is becoming more simplified [27]. Calvo-Haro et al. reported that the average working hours for processing were 12 h and the operating time for 3D printing was 10 h based on their experience with the manufacturing process of the anatomical model, or PSG, for a total of 623 orthopedic surgeries in a single institution [28]. They also reported that the proficiency or complexity of the model might influence the time taken for the process [28]. Frizziero et al. reported that PSGs designed for pediatric orthopedic femoral osteotomies can be provided for approximately EUR 300 in 1.5 h (printing time) with a low-end fused deposition modeling 3D printer [27]. Although the cost of 3D printing is influenced by the size of the model, the material used, and the quality of the resolution, Fidanza et al. found that the production cost for anatomical full-touch real-size bio-models is less than EUR 5 per model for material (polylactic acid) and less than EUR 1000 for hardware installation [29].
2.2. Learning Curves
Unlike the navigation system, PSGs cannot provide accurate feedback on the patient’s position during surgery [7][23][30]. This inaccurate process may result in an unexpected deviation of the PSG setting from the planned correct position without consciousness. Thus, the subjective feeling of fitting the bony contour is important. Methods to verify the intraoperative position of the PSG should be developed [31]. Wong et al. first reported the results of a combined technique with a PSG and a navigation technique in three patients with primary bone tumors around the knee joints [32]. The combination technique revealed that the mean maximum deviation errors in osteotomy were 1.64 ± 0.35 mm [32]. Considering the accuracy of the navigation system of 2.43–3.60 mm in previous studies [6][31][33][34], this combination technique in joint-preserving tumor surgery suggested acceptable results [31][32].
Most software programs that facilitate the virtual process of PSG installation do not consider the soft tissues surrounding the surgical site [35]. In the real world, an installation should be planned considering the ligaments, cartilage, tendons, and muscles [35]. Mustahsan et al. reported a cadaver comparison study, which simulated wide resections of a bone sarcoma on 24 cadaver femurs, with and without soft tissue coverage, and concluded that soft tissue coverage caused random positioning errors of PSGs without a spike shape (smooth vs. soft tissue covered: 5.0 vs. 6.5 mm, mean deviation of the cutting planes from the planned plane) [36]. Furthermore, Dong et al. reported a retrospective study of PSG-guided malignant bone-tumor resection (pelvic = 10, femoral = 4, and tibia = 3; 64 osteotomies of tumor-affected bone) [16]. Although 63 of 64 (98%) osteotomies achieved wide resection and negative margins, one osteotomy had a contaminated margin due to the unexpected swing of the saw and flexibility of the guide [16]. The basic skills of orthopedic surgeons remain important for appropriate exposure of the surrounding tissue and accurate osteotomies [37].
Currently, preoperative planning can be achieved in a more realistic manner using a haptic component [17][35]. This technology allows surgeons to simulate the actual procedure with the tactile sensation of nearby soft tissue and the placement of the PSG [17][35]. Additionally, several optical feedback systems, including augmented reality or intraoperative reference of preoperative images, are feasible tools for correcting PSG positioning and improving the accuracy of bone cuts [36][38]. These new technologies may aid orthopedic surgeons in their learning curve.
2.3. Properties of Surgical Materials
Metallic or non-metallic materials can be used in 3D-printing technology [39]. The safety of these materials should be ensured in accordance with national and industrial standards [39]. Metallic guides are sufficiently strong to prevent unexpected chips or guide deformities during surgery using a surgical saw or electronic heating tools [39][40]. However, the running cost for the preparation of metallic materials is higher because the modeling technology should utilize selective laser melting or electron-beam melting [41].
In the case of non-metallic materials, material characteristics should be considered before their use in the surgical field. A PSG must be sterilized without compromising its mechanical resistance and design geometry [27]. As the PSG is administered directly into the bone, the risk of infection should be closely monitored [42]. Autoclaving is generally used as a sterilization method [27]. Thus, tolerance to aggressive steam heat cycles should be noted. High-molecular polymers, such as acrylonitrile butadiene styrene, polyethylene terephthalate glycol, and simple polylactic acid, cannot withstand temperatures above 50 °C without significant loss of mechanical properties [43]. As an alternative sterilization method, ethylene oxide at 37 °C for 16 h may be used [43]. Conversely, polyether-ketone has thermomechanical resistance and biocompatibility but at a higher cost [44]. For non-metallic materials, high-temperature polylactic acid may be used because it is printable with fused deposition modeling and has the capacity for aggressive steam heat cycles while maintaining the same designed geometry [27][45].
This entry is adapted from the peer-reviewed paper 10.3390/curroncol30040292
References
- Biscaccianti, V.; Fragnaud, H.; Hascoët, J.Y.; Crenn, V.; Vidal, L. Digital chain for pelvic tumor resection with 3D-printed surgical cutting guides. Front. Bioeng. Biotechnol. 2022, 10, 991676.
- García-Sevilla, M.; Mediavilla-Santos, L.; Ruiz-Alba, M.T.; Pérez-Mañanes, R.; Calvo-Haro, J.A.; Pascau, J. Patient-specific desktop 3D-printed guides for pelvic tumour resection surgery: A precision study on cadavers. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 397–406.
- Park, J.W.; Kang, H.G. Application of 3-dimensional printing implants for bone tumors. Clin. Exp. Pediatr. 2022, 65, 476–482.
- Khan, F.A.; Lipman, J.D.; Pearle, A.D.; Boland, P.J.; Healey, J.H. Surgical technique: Computer-generated custom jigs improve accuracy of wide resection of bone tumors. Clin. Orthop. Relat. Res. 2013, 471, 2007–2016.
- Helguero, C.G.; Kao, I.; Komatsu, D.E.; Shaikh, S.; Hansen, D.; Franco, J.; Khan, F. Improving the accuracy of wide resection of bone tumors and enhancing implant fit: A cadaveric study. J. Orthop. 2015, 12 (Suppl. S2), S188–S194.
- Bosma, S.E.; Wong, K.C.; Paul, L.; Gerbers, J.G.; Jutte, P.C. A cadaveric comparative study on the surgical accuracy of freehand, computer navigation, and patient-specific instruments in joint-preserving bone tumor resections. Sarcoma 2018, 2018, 4065846.
- Sallent, A.; Vicente, M.; Reverté, M.M.; Lopez, A.; Rodríguez-Baeza, A.; Pérez-Domínguez, M.; Velez, R. How 3D patient-specific instruments improve accuracy of pelvic bone tumour resection in a cadaveric study. Bone Joint Res. 2017, 6, 577–583.
- Evrard, R.; Schubert, T.; Paul, L.; Docquier, P.L. Quality of resection margin with patient specific instrument for bone tumor resection. J. Bone Oncol. 2022, 34, 100434.
- Bellanova, L.; Paul, L.; Docquier, P.L. Surgical guides (patient-specific instruments) for pediatric tibial bone sarcoma resection and allograft reconstruction. Sarcoma 2013, 2013, 787653.
- Hoang, D.; Perrault, D.; Stevanovic, M.; Ghiassi, A. Surgical applications of three-dimensional printing: A review of the current literature & how to get started. Ann. Transl. Med. 2016, 4, 456.
- Li, Z.; Chen, G.; Xiang, Y.; Muheremu, A.; Wu, X.; He, P.; Fan, H.; Liu, J.; Chen, C.; Yang, L.; et al. Treatment of massive iliac chondrosarcoma with personalized three-dimensional printed tantalum implant: A case report and literature review. J. Int. Med. Res. 2020, 48, 300060520959508.
- Kieser, D.C.; Ailabouni, R.; Kieser, S.C.J.; Wyatt, M.C.; Armour, P.C.; Coates, M.H.; Hooper, G.J. The use of an Ossis custom 3D-printed tri-flanged acetabular implant for major bone loss: Minimum 2-year follow-up. Hip. Int. 2018, 28, 668–674.
- Liu, X.; Liu, Y.; Lu, W.; Liao, S.; Du, Q.; Deng, Z.; Lu, W. Combined application of modified three-dimensional printed anatomic templates and customized cutting blocks in pelvic reconstruction after pelvic tumor resection. J. Arthroplasty 2019, 34, 338–345.e1.
- Riggs, K.W.; Dsouza, G.; Broderick, J.T.; Moore, R.A.; Morales, D.L.S. 3D-printed models optimize preoperative planning for pediatric cardiac tumor debulking. Transl. Pediatr. 2018, 7, 196–202.
- Hu, H.; Liu, W.; Zeng, Q.; Wang, S.; Zhang, Z.; Liu, J.; Zhang, Y.; Shao, Z.; Wang, B. The personalized shoulder reconstruction assisted by 3d printing technology after resection of the proximal humerus tumours. Cancer. Manag. Res. 2019, 11, 10665–10673.
- Dong, C.; Beglinger, I.; Krieg, A.H. Personalized 3D-printed guide in malignant bone tumor resection and following reconstruction-17 cases in pelvic and extremities. J. Surg. Oncol. 2022, 42, 101733.
- Kovler, I.; Joskowicz, L.; Weil, Y.A.; Khoury, A.; Kronman, A.; Mosheiff, R.; Liebergall, M.; Salavarrieta, J. Haptic computer-assisted patient-specific preoperative planning for orthopedic fractures surgery. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 1535–1546.
- Goyal, S.; Chua, C.; Chen, Y.S.; Murphy, D.; O ’Neill, G.K. Utility of 3D printed models as adjunct in acetabular fracture teaching for Orthopaedic trainees. BMC Med. Educ. 2022, 22, 595.
- Ritacco, L.E.; Milano, F.E.; Farfalli, G.L.; Ayerza, M.A.; Muscolo, D.L.; Aponte-Tinao, L.A. Accuracy of 3-D planning and navigation in bone tumor resection. Orthopedics 2013, 36, e942–e950.
- Ma, L.; Zhou, Y.; Lin, Z.; Wang, Y.; Zhang, Y.; Xia, H.; Mao, C. 3D-printed guiding templates for improved osteosarcoma resection. Sci. Rep. 2016, 6, 23335.
- Wang, F.; Zhu, J.; Peng, X.; Su, J. The application of 3D printed surgical guides in resection and reconstruction of malignant bone tumor. Oncol. Lett. 2017, 14, 4581–4584.
- Wong, K.C.; Sze, K.Y.; Wong, I.O.; Wong, C.M.; Kumta, S.M. Patient-specific instrument can achieve same accuracy with less resection time than navigation assistance in periacetabular pelvic tumor surgery: A cadaveric study. Int. J. Comput. Assist. Radiol. Surg. 2016, 11, 307–316.
- Wong, K.C. 3D-printed patient-specific applications in orthopedics. Orthop. Res. Rev. 2016, 8, 57–66.
- Rustemeyer, J.; Melenberg, A.; Sari-Rieger, A. Costs incurred by applying computer-aided design/computer-aided manufacturing techniques for the reconstruction of maxillofacial defects. J. Craniomaxillofac. Surg. 2014, 42, 2049–2055.
- Martelli, N.; Serrano, C.; van den Brink, H.; Pineau, J.; Prognon, P.; Borget, I.; El Batti, S. Advantages and disadvantages of 3-dimensional printing in surgery: A systematic review. Surgery 2016, 159, 1485–1500.
- Kadakia, R.J.; Wixted, C.M.; Allen, N.B.; Hanselman, A.E.; Adams, S.B. Clinical applications of custom 3D printed implants in complex lower extremity reconstruction. 3D Print Med. 2020, 6, 29.
- Frizziero, L.; Santi, G.M.; Leon-Cardenas, C.; Donnici, G.; Liverani, A.; Papaleo, P.; Napolitano, F.; Pagliari, C.; Di Gennaro, G.L.; Stallone, S.; et al. In-house, fast FDM prototyping of a custom cutting guide for a lower-risk pediatric femoral osteotomy. Bioengineering 2021, 8, 71.
- Calvo-Haro, J.A.; Pascau, J.; Mediavilla-Santos, L.; Sanz-Ruiz, P.; Sánchez-Pérez, C.; Vaquero-Martín, J.; Perez-Mañanes, R. Conceptual evolution of 3D printing in orthopedic surgery and traumatology: From “do it yourself” to “point of care manufacturing”. BMC Musculoskelet Disord. 2021, 22, 360.
- Fidanza, A.; Perinetti, T.; Logroscino, G.; Saracco, M. 3D Printing Applications in Orthopaedic Surgery: Clinical Experience and Opportunities. Appl. Sci. 2022, 12, 3245.
- Leeuwen, J.A.; Grøgaard, B.; Nordsletten, L.; Röhrl, S.M. Comparison of planned and achieved implant position in total knee arthroplasty with patient-specific positioning guides. Acta Orthop. 2015, 86, 201–207.
- Wong, K.C.; Kumta, S.M.; Sze, K.Y.; Wong, C.M. Use of a patient-specific CAD/CAM surgical jig in extremity bone tumor resection and custom prosthetic reconstruction. Comput. Aided Surg. 2012, 17, 284–293.
- Wong, K.C.; Sze, L.K.Y.; Kumta, S.M. Complex joint-preserving bone tumor resection and reconstruction using computer navigation and 3D-printed patient-specific guides: A technical note of three cases. J. Orthop. Translat. 2021, 29, 152–162.
- Aponte-Tinao, L.A.; Ritacco, L.E.; Ayerza, M.A.; Muscolo, D.L.; Farfalli, G.L. Multiplanar osteotomies guided by navigation in chondrosarcoma of the knee. Orthopedics 2013, 36, e325–e330.
- Zhang, Y.; Zhang, Q.; Zhong, L.; Qiu, L.; Xu, L.; Sun, Y.; Niu, X.; Zhang, L. New perspectives on surgical accuracy analysis of image-guided bone tumour resection surgery. Int. Orthop. 2020, 44, 987–994.
- Mensel, C.; Gundtoft, P.H.; Brink, O. Preoperative templating in orthopaedic fracture surgery: The past, present and future. Injury 2022, 53 (Suppl. S3), S42–S46.
- Mustahsan, V.M.; He, G.; Helguero, C.G.; Blum, C.L.; Komatsu, D.E.; Pentyala, S.; Kao, I.; Khan, F. Novel positioning feedback system as a guidance in bone tumor resection. Surg. Innov. 2022, 30, 15533506221106070.
- Van den Broeck, J.; Wirix-Speetjens, R.; Vander Sloten, J. Preoperative analysis of the stability of fit of a patient-specific surgical guide. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 38–47.
- Cho, H.S.; Park, Y.K.; Gupta, S.; Yoon, C.; Han, I.; Kim, H.S.; Choi, H.; Hong, J. Augmented reality in bone tumour resection: An experimental study. Bone Joint Res. 2017, 6, 137–143.
- Meng, M.; Wang, J.; Sun, T.; Zhang, W.; Zhang, J.; Shu, L.; Li, Z. Clinical applications and prospects of 3D printing guide templates in orthopaedics. J. Orthop. Translat. 2022, 34, 22–41.
- Bruns, N.; Krettek, C. 3D-printing in trauma surgery: Planning, printing and processing. Unfallchirurg 2019, 122, 270–277.
- Gokuldoss, P.K.; Kolla, S.; Eckert, J. Additive manufacturing processes: Selective laser melting, electron beam melting and binder jetting-selection guidelines. Materials 2017, 10, 672.
- Peniston, S.J.; Choi, S.J. Effect of sterilization on the physicochemical properties of molded poly(L-lactic acid). J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 67–77.
- Pérez-Mañanes, R.; Burró, J.A.; Manaute, J.R.; Rodriguez, F.C.; Martín, J.V. 3D surgical printing cutting guides for open-wedge high tibial osteotomy: Do it yourself. J. Knee Surg. 2016, 29, 690–695.
- Zhao, Y.; Yuchan, K.Z.; Chen, L.F. Mechanical characterization of biocompatible PEEK by FDM. J. Manuf. Process. 2020, 56, 28–42.
- Chen, J.V.; Tanaka, K.S.; Dang, A.B.C.; Dang, A. Identifying a commercially-available 3D printing process that minimizes model distortion after annealing and autoclaving and the effect of steam sterilization on mechanical strength. 3D Print Med. 2020, 6, 9.
This entry is offline, you can click here to edit this entry!
