Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Immunology
|
Infectious Diseases
Host defense peptides (HDPs), also known as antimicrobial peptides, are an important component of the innate immune system. HDPs possess both antimicrobial and immunomodulatory properties. HDPs and their derivatives are being actively explored for antimicrobial therapies. A host-directed approach to stimulate the synthesis of endogenous HDPs is also being developed to treat infections with a minimum risk for developing antimicrobial resistance.
- host defense peptides
- antimicrobial resistance
- antimicrobial peptides
- antibiotic alternatives
1. Introduction
Antimicrobial resistance has emerged as a major world health concern over past decades [1]. Excessive usage of antibiotics in human medicine and agriculture production has driven the emergence of resistant bacterial strains [1]. This issue has led to a significant increase in deaths associated with infections that were once treatable with antibiotics [1]. Development of novel antibiotics and antimicrobial approaches has become an intensive area of research [2]. Host defense peptides (HDPs), also known as antimicrobial peptides, elicit strong antimicrobial activity and regulate the immune response to protect against infections while minimizing inflammation [3,4]. As an important chemical barrier of the innate immune system, HDPs are small peptides (approximately 15–45 amino acids) found in virtually all organisms including mammals, birds, reptiles, amphibians, plants, and fungi [3,4,11].
1.1. Classifications of HDPs
Two major HDP families in vertebrate animals are cathelicidins and defensins [3,4]. Cathelicidins are produced abundantly in myeloid cells such as neutrophils and monocytes as well as skin keratinocytes and epithelial cells lining the respiratory, gastrointestinal, and urogenital tracts [12]. Synthesized initially as a precursor, cathelicidins are proteolytically cleaved from the cathelin pro-segment to become biologically active and released upon activation [12]. Defensins are characterized by the presence of six cysteine residues in defined positions [13]. Based on the location and spacing pattern of the cysteines, defensins are further divided into three subfamilies, namely α-, β-, and θ-defensins [13]. The α-defensins are produced abundantly by neutrophils and intestinal Paneth cells, while β-defensins are produced largely by the skin keratinocytes and epithelial cells of the gastrointestinal and urogenital tracts [13]. On the other hand, θ-defensins have a unique cyclic structure and have only been found in primates [14]. Like cathelicidins, α- and θ-defensins are translated as inactive precursors that are proteolytically cleaved at highly conserved sites to become mature active peptides to be stored in the granules of neutrophils or Paneth cells [14].
1.2. Mechanism of Antimicrobial Action of HDPs
HDPs are active against both Gram-positive and Gram-negative bacteria, fungi, parasites, and enveloped viruses, as well as cancer cells [3,4]. Several models, such as barrel stave, carpet, and toroidal pore, have been proposed to explain the antibacterial activity of HDPs [3,4] (Figure 1). The most accepted mechanism is based on the cationic and amphipathic natures of HDPs, which allow these peptides to interact with the outer membrane of bacteria composed of negatively charged phospholipid head groups. The cationicity of HDPs drives their attraction to the bacterial membrane causing disruption of the membrane structure leading to the leakage of cellular contents and bacterial lysis [3,4]. Additionally, other antibacterial mechanisms have been revealed with several HDPs (Figure 1). For example, human α-defensin 6 (HD6) forms a nanonet structure to entrap bacteria to prevent translocation into intestinal epithelium [15]. Proline-rich HDPs, such as indolicidin, kill bacteria by binding to intracellular targets such as DNA without membrane lysis [16]. Proline-arginine rich PR-39 directly inhibits or degrades proteins involved in DNA replication in the bacterial nucleus [17]. Furthermore, human β-defensin 3 (HBD3), has been shown to inhibit bacterial cell wall synthesis by interacting with the cell wall building block, lipid II [18].
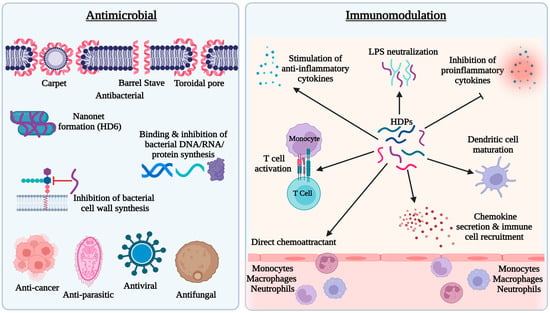
Figure 1. Antimicrobial and immunomodulatory activities of host defense peptides (HDPs). HDPs possess direct antimicrobial activity against bacteria, fungi, viruses, and parasites mainly through membrane disruption. A few HDPs also inhibit bacteria by forming nanonets or targeting bacterial DNA, RNA, proteins, or lipid II of the cell wall. Immunomodulatory effects of HDPs mainly include recruitment and activation of immune cells and regulation of inflammatory response.
1.3. Role of HDPs in Innate and Adaptive Immunity
In addition to direct antimicrobial activity, HDPs possess immunomodulatory activities [3,12] (Figure 1). HDPs are capable of directly recruiting different types of immune cells to the site of infection or indirectly through stimulation of chemokine production. For example, human cathelicidin LL-37 is chemotactic for neutrophils, monocytes, and T lymphocytes through engaging formyl peptide receptor 2 [19], while human β-defensins such as HBD2 and HBD3 chemoattract neutrophils and monocytes through interacting with chemokine receptor CCR2 [20] and they recruit dendritic cells and T cells through CCR6 [21]. Additionally, HDPs such as LL-37 and HBD3 can induce the expression of a variety of chemokines, such as CCL2/monocyte chemoattractant protein-1, CCL3/macrophage inflammatory protein-1α, CCL4/macrophage inflammatory protein-1β, CXCL1/Gro-α, and CCL22/macrophage-derived chemokine, which attract monocytes, macrophages, neutrophils, dendritic cells, and T cells subsequently to the sites of inflammation [22]. In addition to chemoattraction, many HDPs further induce maturation and activation of the cells involved in innate and adaptive immunity. For example, β-defensins induce the expression of co-stimulatory molecules, CD80, CD86, and CD40, on monocytes and dendritic cells in a Toll-like receptor-dependent manner [23,24].
Although they are capable of eliciting proinflammatory and immune responses to facilitate the clearance of pathogens, HDPs mediate largely anti-inflammatory responses during inflammation and infection by inhibiting the expression of proinflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α), while stimulating the production of anti-inflammatory cytokines such as IL-10 [25,26] (Figure 1). Furthermore, cationic HDPs also bind to anionic lipopolysaccharides (LPS) to neutralize its effect on inflammation [3,12].
1.4. Transcriptional Regulation of HDPs
As an important component of innate defense, certain HDPs such as human α-defensins and HBD1 are constitutively produced, while other HDPs like HBD2, HBD3, and HBD4 are induced upon infection and injury [27,28]. Pathogen-associated molecular patterns (PAMPs), such as LPS, bacterial DNA, and flagellin, can induce the expression of HDPs [27]. For example, Psuedomonas aeruginosa rhamnolipids act as a PAMP to activate transcription factors, such as nuclear factor κB (NF-κB) and activator protein-1 (AP-1), causing induction of HBD2 in keratinocytes [29,30]. Proinflammatory cytokines such as TNF-α and IL-1α are known to induce the expression of HDPs such as HBD2 [31].
Additionally, a diverse group of small-molecule compounds have been shown to induce expression of HDPs [7,8,9,10]. Enhancing HDP synthesis by short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate has been well documented [10]. Medium-chain fatty acids are also capable of regulating HDP synthesis, albeit with reduced efficacy [32,33]. Amino acids such as L-arginine, L-isoleucine, leucine, and valine can induce β-defensin synthesis in the intestinal epithelium [34]. Histone deacetylase (HDAC) inhibitors, such as entinostat, valproic acid, and trichostatin, are very effective at upregulating HDP synthesis [9]. Other compounds such as vitamin D3, zinc, β-glucan, fructan, and lactose are also capable of inducing HDP synthesis [8]. Furthermore, probiotic bacteria such as Lactobacillus strains have the ability to upregulate β-defensin synthesis as well [35]. Recent development of several high-throughput screening assays has led to identification of a number of HDP-inducing compounds and their efficacy in disease control and prevention is being characterized [36,37,38,39,40,41].
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics12040660
This entry is offline, you can click here to edit this entry!
