Echinococcosis is considered a neglected disease in most European countries. However, migratory flows of populations, long-term stays in endemic areas, uninterrupted tourism (travel to Echinococcus-endemic countries), traveling dogs and dog translocations from endemic areas, and inappropriate hygiene practices are potential factors that alarm public health officials. Identifying a cyst-like mass in the liver or lung of an individual with a travel history of likely exposure to sheepdogs in an area where the parasite Echinococcus (E.) granulosus (sive cysticus) is endemic advocates for a prompt preliminary diagnosis of cystic echinococcosis (CE), no matter the age of the affected individuals.
- Echinococcosis
- liver
- cysts
1. Introduction
2. Echinococcosis
3. Epidemiology
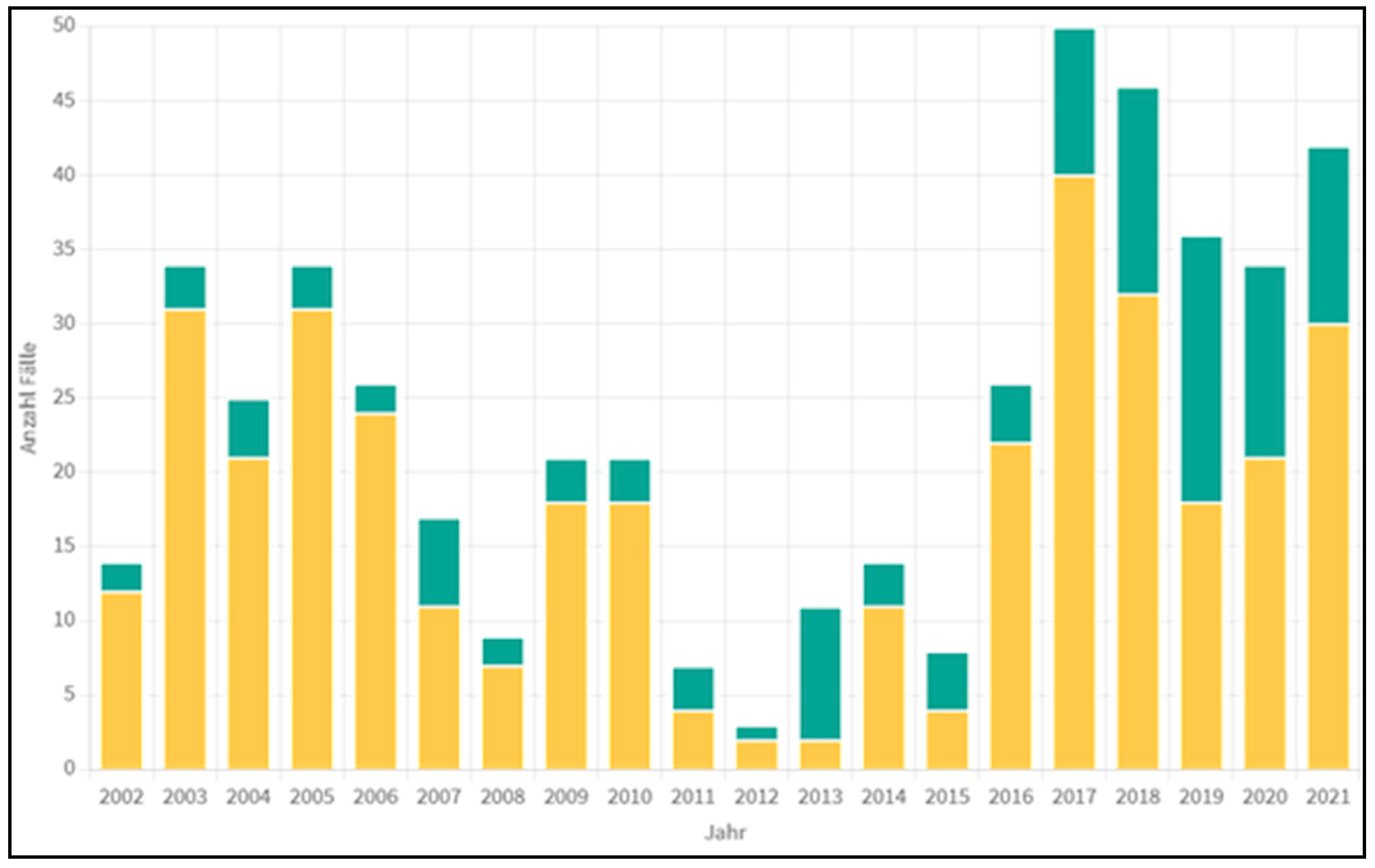
4. Statistical Data from Austria
5. The Parasite Biology
6. Clinical Symptomatology
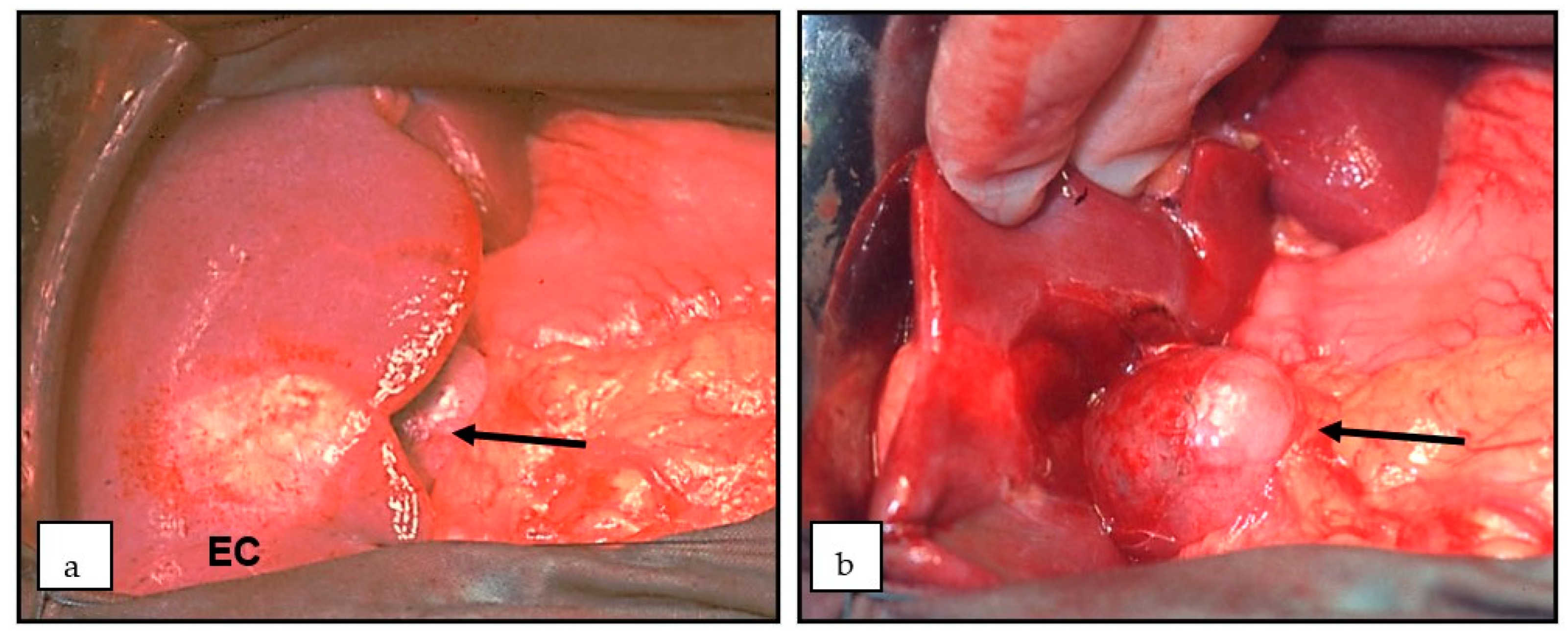
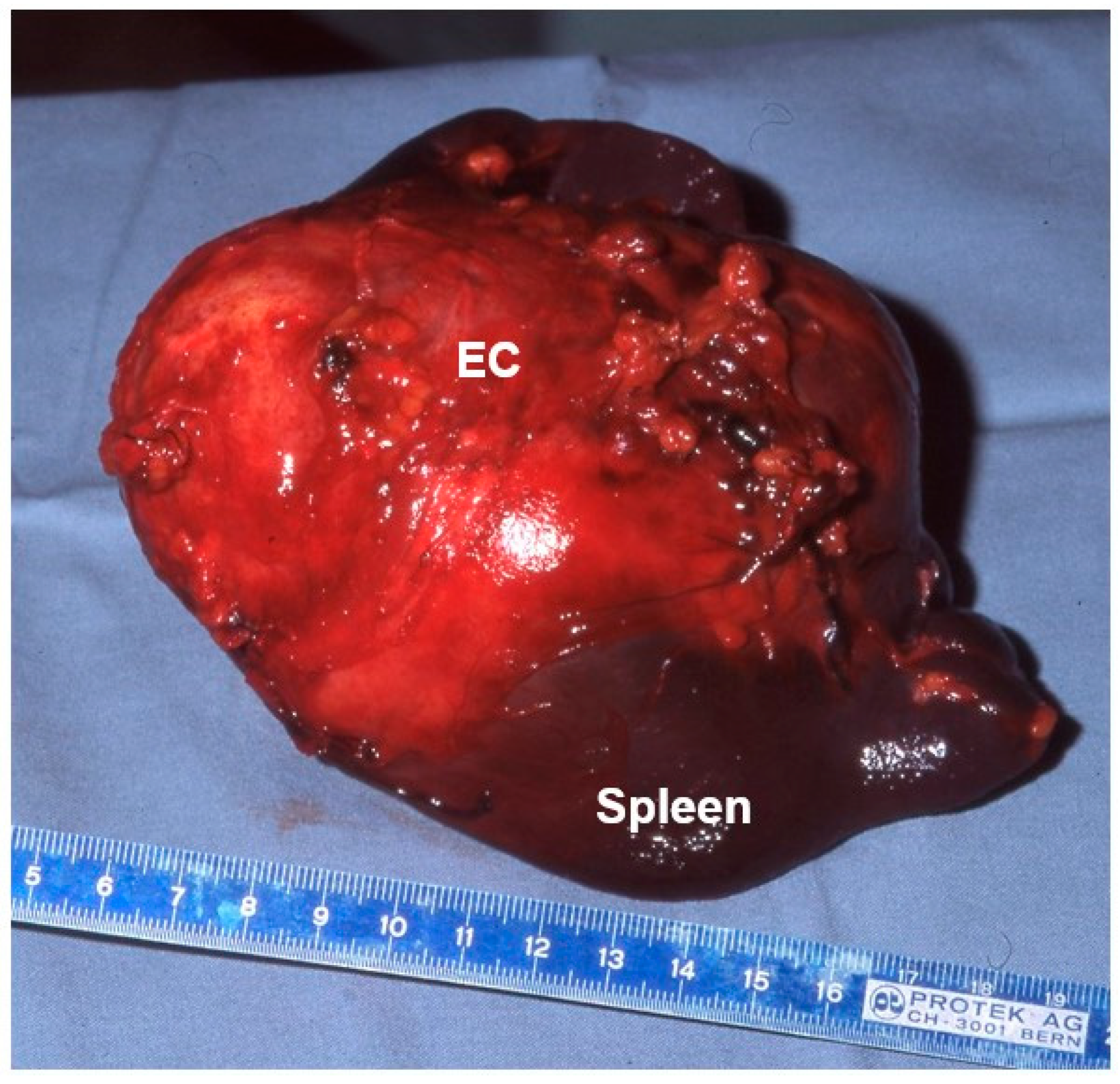
7. Diagnostics
The diagnostic procedures include sonography (better contrast-enhanced sonography (CEUS)), a CT scan, and, if possible, an MRI of the abdomen [14][17][18]. In addition, a lung X-ray is indicated because lung involvement—usually asymptomatic—is possible [19].
8. Therapy
8.1. Alveolar Echinococcosis
8.2. Cystic Echinococcosis
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13071343
References
- Albendazole: New indication. Useful adjunct in hydatid disease. Prescrire Int. 2000, 9, 139–142.
- da Silva, A.M. Human echinococcosis: A neglected disease. Gastroenterol. Res. Pract. 2010, 2010, 583297.
- Djuricic, S.M.; Grebeldinger, S.; Kafka, D.I.; Djan, I.; Vukadin, M.; Vasiljevic, Z.V. Cystic echinococcosis in children—The seventeen-year experience of two large medical centers in Serbia. Parasitol. Int. 2010, 59, 257–261.
- Petropoulos, A.S.; Chatzoulis, G.A. Echinococcus Granulosus in Childhood: A Retrospective Study of 187 Cases and Newer Data. Clin. Pediatr. 2019, 58, 864–888.
- Jonaityte, E.; Judickas, M.; Tamuleviciene, E.; Seskute, M. Alveolar Echinococcosis in Children. Case Rep. Pediatr. 2020, 2020, 5101234.
- Tappe, D.; Stich, A.; Frosch, M. Emergence of polycystic neotropical echinococcosis. Emerg. Infect. Dis. 2008, 14, 292–297.
- Xiao, N.; Qiu, J.; Nakao, M.; Li, T.; Yang, W.; Chen, X.; Schantz, P.M.; Craig, P.S.; Ito, A. Echinococcus shiquicus n. sp., a taeniid cestode from Tibetan fox and plateau pika in China. Int. J. Parasitol. 2005, 35, 693–701.
- Gottstein, B. Epidemiology and systematics of cystic and alveolar hydatid disease. Chirurg 2000, 71, 1–8.
- Austrian Agency for Health and Food Safety GmbH: Pathogens from A to Z; Actualised at 20 December 2022. Available online: https://www.ages.at/en/ (accessed on 17 January 2023).
- Pakala, T.; Molina, M.; Wu, G.Y. Hepatic Echinococcal Cysts: A Review. J. Clin. Transl. Hepatol. 2016, 4, 39–46.
- Behera, P.K.; Satpathy, S. Hydatidosis of female genital tract: A case report. Indian J. Pathol. Microbiol. 2003, 46, 78–79.
- Nunnari, G.; Pinzone, M.R.; Gruttadauria, S.; Celesia, B.M.; Madeddu, G.; Malaguarnera, G.; Pavone, P.; Cappellani, A.; Cacopardo, B. Hepatic echinococcosis: Clinical and therapeutic aspects. World J. Gastroenterol. 2012, 18, 1448–1458.
- Yoshida, T.; Kamiyama, T.; Okada, T.; Nakanishi, K.; Yokoo, H.; Kamachi, H.; Matsushita, M.; Sato, N.; Sasaki, F.; Todo, S. Alveolar echinococcosis of the liver in children. J. Hepatobiliary Pancreat. Sci. 2010, 17, 152–157.
- Eckert, J.; Deplazes, P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004, 17, 107–135.
- Senyuz, O.F.; Celayir, A.C.; Kilic, N.; Celayir, S.; Sarimurat, N.; Erdogan, E.; Yeker, D. Hydatid disease of the liver in childhood. Pediatr. Surg. Int. 1999, 15, 217–220.
- Foroughi, M.; Bahador, A.; Beizavi, Z. Rapid Growth of Hydatid Cyst: A Pediatric Case Report. Iran J. Parasitol. 2021, 16, 164–167.
- Kratzer, W.; Gruener, B.; Kaltenbach, T.E.; Ansari-Bitzenberger, S.; Kern, P.; Fuchs, M.; Mason, R.A.; Barth, T.F.; Haenle, M.M.; Hillenbrand, A.; et al. Proposal of an ultrasonographic classification for hepatic alveolar echinococcosis: Echinococcosis multilocularis Ulm classification-ultrasound. World J. Gastroenterol. 2015, 21, 12392–12402.
- Stojkovic, M.; Rosenberger, K.; Kauczor, H.U.; Junghanss, T.; Hosch, W. Diagnosing and Staging of Cystic Echinococcosis: How Do CT and MRI Perform in Comparison to Ultrasound? PLoS Negl Trop Dis. 2012, 6, e1880.
- Cevik, M.; Boleken, M.E.; Kurkcuoglu, I.C.; Eser, I.; Dorterler, M.E. Pulmonary hydatid disease is difficult recognized in children. Pediatr. Surg. Int. 2014, 30, 737–741.
- Turgut, A.T.; Akhan, O.; Bhatt, S.; Dogra, V.S. Sonographic spectrum of hydatid disease. Ultrasound Q. 2008, 24, 17–29.
- Calame, P.; Weck, M.; Busse-Cote, A.; Brumpt, E.; Richou, C.; Turco, C.; Doussot, A.; Bresson-Hadni, S.; Delabrousse, E. Role of the radiologist in the diagnosis and management of the two forms of hepatic echinococcosis. Insights Imaging 2022, 13, 68.
- Gharbi, H.A.; Hassine, W.; Brauner, M.W.; Dupuch, K. Ultrasound examination of the hydatic liver. Radiology 1981, 139, 459–463.
- Caoduro, C.; Porot, C.; Vuitton, D.A.; Bresson-Hadni, S.; Grenouillet, F.; Richou, C.; Boulahdour, H.; Blagosklonov, O. The role of delayed 18F-FDG-PET imaging in the follow-up of patients with alveolar echinococcosis. J. Nucl. Med. 2013, 54, 358–363.
- Bulakci, M.; Ilhan, M.; Bademler, S.; Yilmaz, E.; Gulluoglu, M.; Bayraktar, A.; Asik, M.; Guloglu, R. Efficacy of ultrasound-guided core-needle biopsy in the diagnosis of hepatic alveolar echinococcosis: A retrospective analysis. Parasite 2016, 23, 19.
- Sulima, M.; Nahorski, W.; Gorycki, T.; Wolyniec, W.; Waz, P.; Felczak-Korzybska, I.; Szostakowska, B.; Sikorska, K. Ultrasound images in hepatic alveolar echinococcosis and clinical stage of the disease. Adv. Med. Sci. 2019, 64, 324–330.
- Kratzer, W.; Weimer, H.; Schmidberger, J. Echinococcosis: A Challenge for Liver Sonography. Ultraschall Med. 2022, 43, 120–145.
- Graeter, T.; Kratzer, W.; Oeztuerk, S.; Haenle, M.M.; Mason, R.A.; Hillenbrand, A.; Kull, T.; Barth, T.F.; Kern, P.; Gruener, B. Proposal of a computed tomography classification for hepatic alveolar echinococcosis. World J. Gastroenterol. 2016, 22, 3621–3631.
- Brunetti, E.; Kern, P.; Vuitton, D.A. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010, 114, 1–16.
- Faraj, W.; Abi Faraj, C.; Kanso, M.; Nassar, H.; Hoteit, L.; Farsakoury, R.; Zaghal, A.; Yaghi, M.; Jaafar, R.F.; Khalife, M. Hydatid Disease of the Liver in the Middle East: A Single Center Experience. Surg. Infect. 2022, 23, 29–34.
- Calame, P.; Doussot, A.; Turco, C.; Colpart, P.; Heyd, B.; Delabrousse, E. Local invasion of hepatic alveolar echinococcosis should not be underestimated: Lessons learned from imaging-pathologic correlation. Diagn. Interv. Imaging 2021, 102, 189–192.
- Gupta, N.; Javed, A.; Puri, S.; Jain, S.; Singh, S.; Agarwal, A.K. Hepatic hydatid: PAIR, drain or resect? J. Gastrointest. Surg. 2011, 15, 1829–1836.
- Stojkovic, M.; Weber, T.F.; Junghanss, T. Clinical management of cystic echinococcosis: State of the art and perspectives. Curr. Opin. Infect. Dis. 2018, 31, 383–392.
- Demir, S.; Ilikan, G.B.; Erturk, A.; Oztorun, C.I.; Guney, D.; Azili, M.N.; Senel, E.; Tiryaki, H.T. A serious complication of liver hydatid cysts in children: Cystobiliary fistulas. Pediatr. Surg. Int. 2020, 36, 611–620.
- Tersigni, C.; Venturini, E.; Montagnani, C.; Bianchi, L.; Chiappini, E.; de Martino, M.; Galli, L. Should Pediatricians Be Aware of Cystic Echinococcosis? A Literature Review. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 161–168.
- Junghanss, T.; da Silva, A.M.; Horton, J.; Chiodini, P.L.; Brunetti, E. Clinical management of cystic echinococcosis: State of the art, problems, and perspectives. Am. J. Trop. Med. Hyg. 2008, 79, 301–311.
