Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Fungal infections, named mycosis, can cause severe invasive and systemic diseases that can even lead to death. In recent years, epidemiological data have recorded an increase in cases of severe fungal infections, caused mainly by a growing number of immunocompromised patients and the emergence of fungal pathogenic forms that are increasingly resistant to antimycotic drug treatments.
- antifungal
- drugs
- fungal infections
- mycosis
- antimicrobial resistance
1. Fungal Infections
Serious fungal infections mainly occur in immunosuppressed individuals, such as patients infected by the Human Immunodeficiency Virus (HIV) or Severe Combined Immunodeficiency (SCID), individuals with endocrine-metabolic disorders, or those undergoing antineoplastic chemotherapy or immunosuppressive therapy after organ transplantation [1]. Fungal infections pose a risk for critically ill patients in healthcare facilities. Patients hospitalized with severe COVID-19 are at risk of healthcare-associated infections, including candidemia, and various fungal infections in COVID-19 patients have been reported worldwide [2][3]. In particular, aspergillosis, cryptococcosis, and zygomycosis (mucormycosis) are overall the most common systemic mycoses. The main risk factors associated with systemic mycosis infections include critical illness, neutropenia, solid tumor, glucocorticoid therapy, diabetes, and age. The increased risk of severe invasive systemic mycoses in immunocompromised patients highlights the importance of immune defenses against commensal microorganisms [4][5]. Among the fungal pathogens most responsible for severe systemic mycoses are strains of Candida albicans, Candida glabrata, and Aspergillus spp. In particular, the fungus of the Aspergillus species is very persistent in the hospital environment causing a wide range of infections including life-threatening systemic ones especially in patients with severe immune system impairment. Clinical data indicate that other fungal families are responsible for serious systemic mycoses, as in the case of Trichosporon, reported mainly in patients with hematological diseases [6], but also Zygomycetes, Fusarium, and Scedosporium spp. [6][7]. In clinical practice, an accurate, early and timely diagnosis combined with effective antifungal drug treatment are of paramount importance for the proper management of systemic fungal infections to avoid serious consequences in patients. Early diagnosis of invasive fungal infections is the central challenge in routine clinical practice and forms the fundamental basis for targeted treatment [8][9][10]. The diagnosis of an invasive fungal infection is based on three elements: clinical examination, imaging and microbiological confirmation/proof of the causative agent. In the case of clinical suspicion of systemic fungal infection, confirmation is almost exclusively by blood culture. Recently, the World Health Organization (WHO) developed the first fungal priority pathogens list (FPPL) that includes the 19 fungi representing the greatest threat to public health divided into three categories: critical, high and medium priority [11]. The critical priority group includes four pathogens: Cryptococcus neoformans, C. auris, Candida albicans and Aspergillus fumigatus. The high priority group includes seven pathogens: Nakaseomyces glabrata (Candida glabrata), Histoplasma spp., eumycetoma causative agents, Mucorales, Fusarium spp., Candida tropicalis and Candida parapsilosis. The medium priority group includes eight pathogens: Scedosporium spp., Lomentospora prolificans, Coccidioides spp., Pichia kudriavzeveii (Candida krusei), Cryptococcus gattii, Talaromyces marneffei, Pneumocystis jirovecii and Paracoccidioides spp. Some of these fungal pathogens (e.g., Paracoccidioides spp.) are confined to certain geographical areas and therefore are not considered a priority globally. However, in the areas where these pathogens are endemic, they are associated with a significant burden of disease. In addition, some pathogens cause infection and represent a health threat in specific populations only; e.g., Pneumocystis jirovecii is one of the main pathogens causing opportunistic infections in people living with HIV/AIDS, but it ranked low in the FPPL [11]. All the 19 pathogens lack comprehensive information on the burden of disease, in terms of data from formal surveillance and linkage to clinical outcomes, and susceptibility, mostly from Low–Middle Income Countries (LMIC), likely due to limited access to medical mycology laboratories in resource-limited settings.
2. Systemic Mycosis and Pharmacological Therapy
The drugs used in the treatment of fungal infections are traditionally divided into two distinct groups: drugs for systemic use and drugs for topical use. However, this classification is not always applicable since some drugs (imidazole, triazoles, and polyenes) can be used both topically and systemically. Indeed, many superficial mycoses can be treated with both posology. The antifungal chemotherapy of systemic fungal infections has been enriched in recent years by a number of innovative and active, broad-spectrum compounds with a discrete therapeutic index, although greater efforts must be made by the scientific world to increase the therapeutic armamentarium available to clinicians [12]. To date, three main classes of antimycotics are used for treating invasive fungal infections: polyenes, azoles, and echinocandins. Among the polyene antimycotics, amphotericin B is the most potent drug currently available for the treatment of systemic fungal infections, and the reference compound. Amphotericin B has fungicidal and fungistatic activities against numerous fungal pathogens responsible for systemic fungal infections, such as Candida albicans, Cryptococcus neoformans, Histoplasma capsulatum, and Aspergillus. It is frequently used to treat recurrent fungal infections in patients with compromised defense mechanisms (e.g., patients on immunosuppressive therapy, chemotherapy, and AIDS patients). The drug binds to sterols, particularly to the ergosterol contained in the membrane of susceptible fungi, creating pores from which vital cellular substances escape [13]. Resistance to amphotericin B can occur when structural modifications lead to the reduction in ergosterol in the membrane. Due to the significant toxic effects, patients undergoing antifungal therapy with amphotericin B should be hospitalized at least in the early period of therapy. A preliminary dose administration should provide the degree of reaction of the patient by evaluating the severity of undesirable effects in order to define a therapeutic regimen suitable for the individual and possibly the use of preventive therapy. The effects are generally classified into two groups: infusion-related toxicity and delayed toxicity. To date, amphotericin B remains the drug of first choice in the treatment of systemic fungal infections. The absorption of amphotericin B in the gastrointestinal tract is negligible. Because of the strong bond of the drug to the human tissues, its half-life is approximately 2 weeks. In recent years, two lipid-based formulations developed for oral administration, named amB lipid complex (ABLC) and liposomal amphotericin (L-AmB), have been authorized, characterized by greater selective toxicity towards sensitive fungi, and increased tolerability especially in terms of nephrotoxicity. Indeed, these lipidic formulations interact with ergosterol, leading to increased permeability to univalent and divalent cations and fungal cell death [14]. The most widely used triazoles in clinical practice are itraconazole, posaconazole, and voriconazole; other agents in the class are fluconazole and isavuconazole [15]. Fluconazole inhibits the synthesis of sterol present in the fungal membrane, as it hampers the conversion of lanosterol to ergosterol by inhibition of the enzyme 14-alpha-demethylase, causing increased cell permeability and subsequent loss of cellular constituents. Fluconazole finds clinical indication mainly against Candida spp. and Cryptococcus spp., both in prophylaxis and therapy. Fluconazole is the drug of choice for treatment of cryptococcal meningoencephalitis pathologies that occur in immunocompromised patients; however, the drug performed poorly compared to other azoles for the treatment of progressive disseminated histoplasmosis (PDH), a serious fungal infection that affects people living with HIV [16]. Fluconazole is excreted renally and presents toxic effects of lower intensity than ketoconazole. The most common side effect pertains to gastrointestinal disorders [17]. Isavuconazole, on the other hand, is indicated mainly in the treatment of invasive aspergillosis and in the treatment of mucormycosis. Isavuconazole is available as intravenous or oral formulations, and it shows less drug–drug interactions and decreased toxicity [18]. Like isavuconazole, posaconazole activity against Mucorales is species dependent, and it can be used as a salvage therapy option for patients who are nonresponsive to other treatments [19][20]. Posaconazole is generally well tolerated, except for minor gastrointestinal side effects due to oral administration. Itraconazole is the most potent triazole compound available. It has a broader spectrum of action than fluconazole, including all Candida and Aspergillus species, and other rare fungal infections such as Blastomyces, Cryptococcus, and Sporothrix. Specifically, itraconazole is indicated in the prophylaxis of systemic invasive mycoses caused by aspergillosis in patients with leukemia or bone marrow transplantation. It is also effective against oropharyngeal candidiasis, esophageal and vaginal as well as onychomycosis, griseofulvin-resistant roundworms (worms). Itraconazole is well absorbed after oral administration, is distributed in many tissues including bone and adipose. Itraconazole is metabolized in the liver, and its plasma levels of vary considerably between subjects. Hepatotoxicity can occasionally occur which can be controlled by reducing drug doses [21]. Voriconazole is an antifungal drug used in the treatment or prophylaxis of invasive aspergillosis and candidiasis [22]. Serum concentrations of voriconazole can have considerable interpatient variability, with demonstrated higher hepatotoxicity rate in Asian vs. non-Asian populations [23]. A systematic review and meta-analysis selected the optimal trough concentration of voriconazole for adult patients with invasive fungal infections between 1.0 and 4.0 μg/mL [24]. Finally, echinocandins are a recently marketed class of antifungals, including drugs such as anidulafungin, caspofungin, and micafungin. These antifungals have a unique mechanism of action among antifungal drugs, making them attractive. Echinocandins inhibit the fungal wall synthesis selectively by blocking the activity of β-1-3-D glucan synthetase enzymes, leading to susceptibility of fungal cell to osmotic lysis. Echinocandins are indicated for the treatment of various forms of Candida and Aspergillus spp. [25]. The WHO has endorsed their importance by adding them to the 2021 Essential Medicines List for both adults and children.
The optimal duration of antifungal therapy is still an unresolved issue depending on several factors, mainly the following: immunological status of the host, type of pathogen and its drug sensitivity, adequateness, and promptness of initial antifungal therapy [26].
3. Antifungal Resistance Mechanisms
Resistance to antifungal drug treatments can be distinguished in intrinsic (primary) forms, which are genetically encoded and associated with fungal species independently of drug exposure, and acquired (secondary) forms, which develop as a consequence of exposure to a certain factor, often an antifungal drug or its structural analogue [27]. Fungal resistance to a specific antifungal compound extends to its entire class. Therefore, the resistance to any class of antifungal drugs can significantly limit the patient’s treatment options. One of the causes of the increase in invasive fungal infections include the emergence of pathogenic forms resistant to common antifungal treatments and the limited access to new pharmacological agents. There are no fungal drug-resistance transposons or plasmids that can pass easily between isolates. However, the growing number of antifungal agents that has been in use for at least twenty years increases the risk of the development of resistant microbes. Indeed, over the last decade, the consumption of systemic antifungal agents has increased globally, with a compound annual growth rate of over 6%, and the High-Income Countries (HIC) have become major consumers of antifungal agents [28]. For example, long-term treatment regimens interfere with and complicate the treatment of underlying diseases, reducing compliance and increasing the risk of drug toxicity and resistance. In addition, the ability of fungi to rapidly mutate by adapting to environmental conditions facilitates the emergence of forms resistant to antifungal treatments, with an inevitable increase in minimum inhibitory concentrations (MIC) to be used during therapy. To date, Candida spp. are considered among the pathogenic fungi most implicated in posing an urgent threat to global health [29][30]. Although the molecular characterization of the mechanism of resistance of Candida forms to antifungal treatment is not fully understood, some evidence associated mutation in ERG11 and TAC1B as being responsible for fluconazole resistance, and it mainly associated mutations in the FKS gene as being primarily responsible for resistance to echinocandin treatment [31], resulting in upregulation of multidrug efflux transporters and reduced sensitivity to glucan synthase. Some evidence shows that a high percentage of C. auris isolates are resistant to fluconazole, approximately 30%–50% are resistant to amphotericin B, and a small percentage are resistant to echinocandins [31][32]. In recent years, more and more forms of Aspergillus resistant to antifungal treatments with azoles have been identified, causing high morbidity and mortality as well as an increase in resistance of some Aspergillus forms to amphotericin B [33]. Furthermore, the ERG6 gene coding for the sterol-methyltransferase enzyme responsible for altering the molecular target of amphotericin B has been identified. Evidence from several studies demonstrated resistant forms of Aspergillus spp. in vitro [34], although clear data and studies fully describing the correlation between amphotericin B MIC values and clinical outcomes in certain patient populations are lacking. The molecular mechanisms of resistance to triazoles occur mainly through increased expression of lanosterol 14-alpha demethylase, alterations in the binding site and increased synthesis of transmembrane transport proteins that lead to drug excretion and decreased intracellular accumulation [34]. Figure 1 shows the main mechanisms of resistance to antifungal treatments.
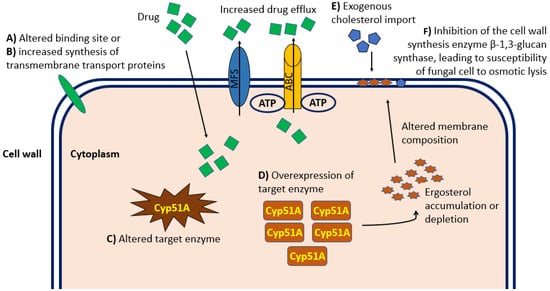
Figure 1. Graphical representation at the cellular level of the principal mechanisms of antifungal drug resistance relevant in the clinical practice. Molecular mechanisms of resistance to antifungal therapy occur mainly through alterations in the cellular binding site (A) or increased synthesis of transmembrane transport proteins (B), which both lead to reduced intracellular accumulation. In addition, alteration (C) or overexpression (D) of the enzyme targeted by the drug can cause Ergosterol intracellular accumulation and consequent altered composition and permeability of the cell wall. Import of exogenous cholesterol (E) can alter the cell wall composition and permeability resulting in inadequate intracellular drug concentration. Finally, inhibition of the cell wall synthesis enzyme β-1,3-glucan synthase (F) can lead to fungal cell susceptibility to osmotic lysis. ABC, ATP-binding cassette transporter family; MFS, major facilitator superfamily of transporters.
Systemic fungal infections, such as bacterial infections, should be included in antimicrobial stewardship programs as an essential component, while health policies should ensure equitable and affordable access to quality antifungal agents, mostly in LMIC.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11041063
References
- Rogawansamy, S.; Gaskin, S.; Taylor, M.; Pisaniello, D. An Evaluation of Antifungal Agents for the Treatment of Fungal Contamination in Indoor Air Environments. Int. J. Environ. Res. Public Health 2015, 12, 6319–6332.
- Chandley, P.; Subba, P.; Rohatgi, S. COVID-19-Associated Mucormycosis: A Matter of Concern Amid the SARS-CoV-2 Pandemic. Vaccines 2022, 10, 1266.
- Corrêa-Junior, D.; Andrade, I.B.; Alves, V.; de Araújo, S.G.R.; Frasest, S. Clinical Challenges of Emerging and Re-Emerging Yeast Infections in the Context of the COVID-19 Pandemic. Microorganisms 2022, 10, 2223.
- Kapitan, M.; Niemiec, M.J.; Steimle, A.; Frick, J.S.; Jacobsen, I.D. Fungi as Part of the Microbiota and Interactions with Intestinal Bacteria. Curr. Top Microbiol. Immunol. 2019, 422, 265–301.
- Vitiello, A.; Ferrara, F.; Zovi, A. The direct correlation between microbiota and SARS-CoV-2 infectious disease. Inflammopharmacology 2023, 1, 1–8.
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The Changing Epidemiology of Invasive Fungal Infections. Methods Mol. Biol. 2017, 1508, 17–65.
- Liao, Y.; Lu, X.; Yang, S.; Luo, Y.; Chen, Q.; Yang, R. Epidemiology and outcome of trichosporon fungemia: A review of 185 reported cases from 1975 to 2014. Open Forum Infect. Dis. 2015, 2, ofv141.
- Martin-Loeches, I.; Antonelli, M.; Cuenca-Estrella, M.; Dimopoulos, G.; Einav, S.; De Waele, J.J.; Garnacho-Montero, J.; Kanj, S.S.; Machado, F.R.; Montravers, P.; et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019, 45, 789–805.
- Tortorano, A.M.; Richardson, M.; Roilides, E.; van Diepeningen, A.; Caira, M.; Munoz, P.; Johnson, E.; Meletiadis, J.; Pana, Z.D.; Lackner, M.; et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp, Scedosporium spp. and others. Clin. Microbiol. Infect. 2014, 20 (Suppl. S3), 27–46.
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. S1), e1–e38.
- World Health Organization. WHO Fungal Priority Pathogens List to guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022; Licence: CC BY-NC-SA 3.0 IGO. .
- Robbins, N.; Wright, G.D.; Cowen, L.E. Antifungal Drugs: The Current Armamentarium and Development of New Agents. Microbiol. Spectr. 2016, 4, 5.
- Hamill, R.J. Amphotericin B formulations: A comparative review of efficacy and toxicity. Drugs 2013, 73, 919–934.
- Zhong, X.; Yang, J.; Liu, H.; Liu, H.; Yang, Z.; Luo, P. Potential lipid-based strategies of amphotericin B designed for oral ad-ministration in clinical application. Drug Deliv. 2023, 30, 2161671.
- Peyton, L.R.; Gallagher, S.; Hashemzadeh, M. Triazole antifungals: A review. Drugs Today 2015, 51, 705–718.
- Murray, M.; Hine, P. Treating progressive disseminated histoplasmosis in people living with HIV. Cochrane Database Syst. Rev. 2020, 4, CD013594.
- Govindarajan, A.; Bistas, K.G.; Ingold, C.J.; Aboeed, A. Fluconazole; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Ellsworth, M.; Ostrosky-Zeichner, L. Isavuconazole: Mechanism of Action, Clinical Efficacy, and Resistance. J. Fungi 2020, 6, 324.
- Meena, D.S.; Kumar, D.; Bohra, G.K. Combination therapy in Mucormycosis: Current evidence from the world literature, a mini review. J. Mycol. Med. 2023, 33, 101332.
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421.
- Ito, H.; Ogawa, R. Itraconazole and Voriconazole: The Complexity of Dose Adjustments. Clin. Infect. Dis. 2022, 75, 552.
- Douglas, A.P.; Smibert, O.C.; Bajel, A.; Halliday, C.L.; Lavee, O.; McMullan, B.; Yong, M.K.; van Hal, S.J.; Chen, S. Australasian Antifungal Guidelines Steering Committee. Consensus guidelines for the diagnosis and management of invasive aspergillosis. Intern. Med. J. 2021, 7, 143–176.
- Takesue, Y.; Hanai, Y.; Oda, K.; Hamada, Y.; Ueda, T.; Mayumi, T.; Matsumoto, K.; Fujii, S.; Takahashi, Y.; Miyazaki, Y.; et al. Clinical Practice Guideline for the Therapeutic Drug Monitoring of Voriconazole in Non-Asian and Asian Adult Patients: Consensus Review by the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. Clin. Ther. 2022, 44, 1604–1623.
- Hanai, Y.; Hamada, Y.; Kimura, T.; Matsumoto, K.; Takahashi, Y.; Fujii, S.; Nishizawa, K.; Miyazaki, Y.; Takesue, Y. Favorable Effects of Voriconazole Trough Concentrations Exceeding 1 μg/mL on Treatment Success and All-Cause Mortality: A Systematic Review and Meta-Analysis. J. Fungi 2021, 7, 306.
- Biagi, M.J.; Wiederhold, N.P.; Gibas, C.; Wickes, B.L.; Lozano, V.; Bleasdale, S.C.; Danziger, L. Development of high-level echinocandin resistance in a patient with recurrent Candida auris candidemia secondary to chronic candiduria. Open Forum Infect. Dis. 2019, 6, ofz262.
- Bassetti, M.; Giacobbe, D.; Berruti, M.; Del Puente, F.; Vena, V. Adequate duration of therapy in severe fungal infections. Curr. Opin. Crit. Care 2020, 26, 466–472.
- Ben-Ami, R.; Kontoyiannis, D.P. Resistance to Antifungal Drugs. Infect. Dis. Clin. North Am. 2021, 35, 279–311.
- Pathadka, S.; Yan, V.K.C.; Neoh, C.F.; Al-Badriyeh, D.; Kong, D.C.M.; Slavin, M.A.; Cowling, B.J.; Hung, I.F.N.; Wong, I.C.K.; Chanet, E.W. Global Consumption Trend of Antifungal Agents in Humans From 2008 to 2018: Data From 65 Middle- and High-Income Countries. Drugs 2022, 82, 1193–1205.
- Rabaan, A.A.; Eljaaly, K.; Alfouzan, W.A.; Al Mutair, A.; Alhumaid, S.; Alfaraj, A.H.; Aldawood, Y.; Alsaleh, A.A.; Albayat, H.; Al Azmi, R.; et al. Psychogenetic, genetic and epigenetic mechanisms in Candida auris: Role in drug resistance. J. Infect. Public Health 2023, 16, 257–263.
- Kaur, J.; Nobile, C.J. Antifungal drug-resistance mechanisms in Candida biofilms. Curr. Opin. Microbiol. 2023, 71, 102237.
- Rybak, J.M.; Munoz, J.F.; Barker, K.S.; Parker, J.E.; Esquivel, B.D.; Berkow, E.L.; Lockhart, S.H.; Gade, L.; Palmer, G.E.; White, T.C.; et al. Mutations in TAC1B: A novel genetic determinant of clinical fluconazole resistance in Candida auris. mBio 2020, 11, e00365-20.
- Izadi, A.; Gharehbolagh, A.S.; Sadeghi, F.; Talebi, M.; Darmiani, K.; Zarrinnia, A.; Zarei, F.; Peymaeei, F.; Khojasteh, S.; Borman, A.M.; et al. Drug repurposing against Candida auris: A systematic review. Mycoses 2022, 65, 784–793.
- Khojasteh, S.; Abastabar, M.; Haghani, I.; Valadan, R.; Ghazanfari, S.; Abbasi, K.; Ahangarkani, F.; Zarrinfar, H.; Khodavaisy, S.; Badali, H.; et al. Five-year surveillance study of clinical and environmental Triazole-Resistant Aspergillus fumigatus isolates in Iran. Mycoses 2023, 66, 98–105.
- Sen, P.; Vijay, M.; Singh, S.; Hameed, S.; Vijayaraghavan, P. Understanding the environmental drivers of clinical azole resistance in Aspergillus species. Drug Target Insights. 2022, 16, 25–35.
This entry is offline, you can click here to edit this entry!
