1. Oxalic Acid, a Versatile Toxin and Broad-Spectrum Pathogenicity Factor
Of the various virulence factors, extensive research has been conducted on the relationship between white mold disease and the production of OA by
S. sclerotiorum [
37,
38]. This fungus is well-known for its ability to acidify the surroundings via OA secretion. The OA-deficient mutants of the fungus have been shown to be poorly pathogenic [
39], suggesting that the pathogenesis of
S. sclerotiorum requires accumulating high quantities of OA. While OA-minus mutants can still infect and colonize hosts to variable degrees, it is appropriate to view OA more as a colonization factor rather than a crucial compatibility element [
31]. OA is not directly toxic to plant cells but plays a more sophisticated role as a signaling molecule that seizes control of host cells and creates an environment favorable for
S. sclerotiorum growth. OA alters a number of physiological processes in host plants. OA promotes cell wall degradation by increasing the activity of polygalacturonases, inhibiting plant-protection enzymes, subduing oxidative burst, deregulating stomatal guard cell closure, mediating pH signaling, inducing apoptosis-like cell death, and altering cellular redox status in plants (
Figure 4) [
14,
35,
39,
40,
41,
42,
43]. The production of OA seems to suit the overall strategy of
S. sclerotiorum to acidify its ambient environment to facilitate infection in several ways. Many of the fungal enzymes (such as polygalacturonase) secreted during the invasion of plant tissues have maximal activities at a low pH [
40]. It is postulated that OA could increase
Sclerotinia virulence by adjusting the apoplastic pH to a level conducive to the enzymatic breakdown of plant cell walls. OA can also weaken plant cell walls via acidity, thereby facilitating invasion [
40]. Interestingly, no ROS production is detected at acidic pH [
43]. It is evident that
S. sclerotinia uses OA to elicit apoptosis-like programmed cell death, and the induction of this process is commenced by a reducing environment generated by the fungus in the host cell [
35]. As a direct consequence of this redox manipulation, the fungus subverts host defense responses, inhibits oxidative burst, and prepares the infection court for disease establishment [
35]. These findings imply that the effect of OA on the reduction of pH in the ambient environment is significantly greater than that of OA alone as an essential component of the infection process caused by
S. sclerotiorum. Intriguingly, the regulation of host defense by OA appears to be biphasic. In the early phases of infection, OA suppresses host defenses by culminating ROS buildup and callose deposition in the host [
35]. OA sequesters Ca
2+ liberated during cell wall collapse, thereby protecting the development of
S. sclerotiorum hyphae from hazardous Ca
2+ concentrations [
44]. In the later stages of infection, OA quashes antioxidant enzyme activities and stimulates ROS generation, concurrently damaging the membranes of host cells [
45]. These findings suggest that OA is a cross-functional toxin crucial to the widespread pathogenic success of
S. sclerotiorum.
The dicarboxylic acid OA can be produced through the oxidation of glyoxylate or glycolaldehyde or the hydrolysis of oxaloacetate [
46]. However, current research indicates that OA biosynthesis entirely depends on the oxaloacetate acetylhydrolase (OAH) activity [
26]. OAH mediates the C-C cleavage of oxaloacetate during the OA biosynthesis process [
31]. Depending on the growth substrate, either the tricarboxylic acid (TCA) cycle or the glyoxylate cycle may produce oxaloacetate precursor [
47]. Experimental evidence from
S. sclerotiorum and
B. cinerea gene-deletion mutants supports the hypothesis that the expressed enzyme activity is responsible for catalyzing the last step in the OA biosynthesis process [
48]. Three types of OA-degrading enzymes are found throughout evolutionary lineages: OxDC (EC 4.1.1.2), OxOx (EC 1.2.3.4), and oxalylCoA decarboxylase (OXC, EC 4.1.1.8).
S. sclerotiorum exhibits oxalate decarboxylase (OxDCs) activity but not OxOx [
49], while only bacterial species have been identified to possess OXC activity. OxDC catalyzes the production of formate and CO
2 from OA, and its activity is widespread among fungi and bacteria [
50].
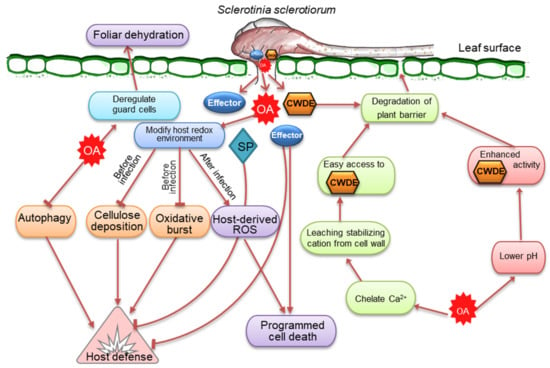
Figure 4. The frontline weapons or virulence factors of Sclerotinia sclerotiorum important for developing and regulating the infection process in plants. S. sclerotiorum releases oxalic acid (OA), which modulates reactive oxygen species (ROS) and the activity of cell wall-degrading enzymes (CWDEs) while dampening host defense responses and suppressing autophagy. At the early stage, OA suppresses plant defensive responses and modifies the host redox state by inhibiting callose deposition and host oxidative burst and establishing a reducing environment. At a later stage, OA-induced ROS causes apoptotic-like programmed cell death and disease. Because of its affinity for Ca2+, OA may degrade plant barriers by draining the stabilization of cations from the plant cell wall. OA accumulation also lowers the pH and activates CWDEs, which are necessary to destroy infection surfaces. Effectors and other secreted proteins (SP) have a similar role in cell death and host defensive responses.
2. Arsenal of Cell Wall-Degrading Enzymes Reflects Broad Host Preference
An essential component of the lifecycle of
S. sclerotiorum is its capacity to break down a wide range of complex plant polysaccharides by CWDEs (
Figure 4). Cellulose, hemicellulose, and pectin form a complex network of polysaccharides that constitute plant cell walls [
51]. This network is the target of CAZymes, a group of proteins and enzymes that break down plant cell wall polysaccharides into simple monomers [
52]. These monomers serve as carbon sources and allow access to internal plant tissues. At least 118 CAZymes are explicitly associated with plant cell wall degradation by
S. sclerotiorum; however, many other CAZymes presumably contribute to this activity [
53]. Several endo- and exo-polygalacturonases (PGs) are known to break down unesterified pectate polymers, middle lamella structural polysaccharides, and primary plant cell wall components. Endo-PGs catalyze the hydrolysis of homogalacturonan, whereas exo-PGs fragment the substrate and release potential nutrients by removing monomeric or dimeric glycosyl groups from pectic cell wall polysaccharides [
54]. PGs are also recognized as potential virulence factors in several pathosystems [
55,
56,
57]; however, convincing proof for their universal involvement in virulence is missing. The principal hemicellulose constituent of plant cell walls, xylan, is comprised of xylose and arabinose connected by β-(1–4)-linked xylopyranose. Endo β-1, 4-xylanase is the major enzyme that degrades xylan [
58]. Studies have highlighted the critical functions of endo-β-1, 4-xylanase in the development and pathogenicity of
S. sclerotiorum [
59]. Other important groups of mannan- and cellulose-degrading enzymes are also highly upregulated [
53]. These indicate that
S. sclerotiorum utilizes a diverse array of enzymes that play significant roles in its infection strategy across many host species.
3. Secretory Effectors, Critical Components in the Necrotrophic Infection Strategy
Numerous small, secreted proteins of
S. sclerotiorum have been reported as having effector-like features or effector-like activities, which are released either extracellularly or within the cytoplasm of plant cells to disarm host defense mechanisms and enhance fungal infection (
Figure 4) [
33,
60]. Until now, a number of
S. sclerotiorum effectors associated with virulence have been identified. During the infection of soybean seedlings,
S. sclerotiorum produces a basic endopolygalacturonase (PG) isoform early in the process that elicits Ca
2+-mediated signaling and programmed cell death [
61]. A polygalacturonase (PG) enzyme, called
Sspg1d, reacts with proteins that have a C2 domain in
Brassica napus [
62]. Necrosis and ethylene-inducing peptides encoded by two genes,
SsNep1 and
SsNep2, induce necrosis in tobacco (
Nicotiana tabacum) leaves during heterologous expression [
63]. A protein with a signal peptide and 302 amino acid residues in the integrin alpha N-terminal domain superfamily, SSITL, inhibits defense reactions in
Arabidopsis thaliana [
64]. A putative-secreted protein disrupting pathogen virulence has also been reported [
65]. A cutinase encoded by
SsCut induces cell death in an array of plants, including rice (
Oryzae staiva)
, G. max,
maize (
Zea mays),
B. napus, wheat (
Triticum aestivum), and
A. thaliana [
66]. A small cysteine-rich protein containing a cyanoviron-N homology (CVNH) domain, SsSSVP1, is required for pathogen virulence [
67]. This putative effector is believed to alter plant energy metabolism in order to promote
S. sclerotiorum infection. A small virulence-related protein 1, without obvious functional domains, has been found explicitly in
S. sclerotiorum and
B. cinerea [
68].
In addition, several other effectors, such as chorismate mutase (
SsCM1) [
69], compound appressorium formation-related protein 1 (
SsCaf1) [
70], BAX inhibitor-1 (
SsBI1) [
71], superoxide dismutase (
SsSodI) [
72], catalase (
SsCat1) [
73], a protein analogous to a
Magnaporthe grisea protein elicitor (
SsPemG1) [
74], and ceratoplantanin effector (
SsCP1) [
75], have been documented in
S. sclerotiorum. The vast majority of the putative proteinaceous effectors characterized in
S. sclerotiorum cause cell death in host tissue.
S. sclerotiorum genome analysis has recently identified a novel class of necrosis-inducing effector 2 (
SsNE2) [
76]. On the contrary, a few biotrophy-related candidate effectors have been spotted, such as salicylate hydroxylase and a lysin motif (LysM) domain protein [
33]. These effector genes were found to be upregulated within the first 24 h after infection. In biotrophic and hemibiotrophic pathogens, recognizing the effectors often leads to a hypersensitive reaction (HR) to contain the pathogens (Jones and Dangl, 2006) [
77]. Contrariwise, for necrotrophic infections, the elicitation of HR responses by effectors delivers substrates to stimulate host colonization [
26,
78]. Additionally,
S. sclerotiorum exhibits a host-specific expression of candidate effectors, as Guyon et al. [
79] showed that the expression patterns of 16 effector candidates varied significantly between the hosts
S. lycospersicum,
Nicotiana benthamiana, and susceptible and resistant accessions of
A. thaliana. According to previous studies, the host-specific expression of putative effectors was more limited than anticipated. Only one of the effector candidate genes (sscle 08g064180) identified by Derbyshire et al. [
80] and Amselem et al. [
53] was expressed differently between
B. napus and
Lupinus angustifolius, where the expression was significantly higher in
B. napus compared to
L. angustifolius. This putative effector is anticipated to have a coiled-coil domain, although little is known about the role of such domain in mediating effectors’ functions.
This entry is adapted from the peer-reviewed paper 10.3390/cells12071063