Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell & Tissue Engineering
This entry provides a summary of the main features of the periodontium and describes the currently available treatments for periodontal disease.
- periodontium
- periodontitis
- periodontal regeneration
1. Introduction
Periodontitis is a chronic inflammatory infection of the periodontium, the structure responsible for ensuring tooth attachment and stability [1]. Bacterial dental plaque accumulation causes this infection, which can lead to the inflammation and destruction of the periodontium and, ultimately, tooth loss [2]. Advanced stages of the disease require regenerative procedures to restore the lost tissues, which include membranes for guided tissue regeneration (GTR) and bone grafts. However, these current treatments lack effective strategies to induce tissue repair and coordinated regeneration of all periodontal tissues, increasing the demand for alternative solutions to improve clinical outcomes [3].
2. Periodontal Tissue Features and Periodontal Disease
The periodontium is a complex structure composed of hard and soft tissues that support the tooth. It has an important role of ensuring tooth attachment to the bone of the jaw and allowing the teeth to withstand the forces of mastication. The periodontium consists of alveolar bone, root cementum, and periodontal ligament (PDL) [7]. The anatomy of the periodontium is illustrated in Figure 1.
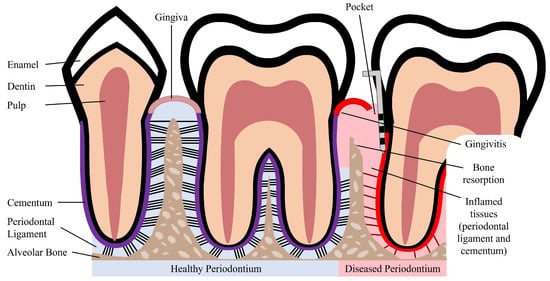
Figure 1. Schematic illustration of a healthy and diseased periodontium.
The alveolar bone is the part of the maxilla or mandible that contains the sockets that surround and anchor the teeth. The alveolar bone is a highly mineralized, hard tissue composed of 60% (w/w) inorganic material, 25% (w/w) organic material, and 15% water [8]. In the root of the teeth, the alveolar bone is connected to the root cementum through the PDL, as can be observed in Figure 1. The alveolar bone is perforated by channels, which allow the passage of blood vessels and nerve fibers that extend to within the pulp of the teeth [9]. Similar to what occurs in other types of bones, alveolar bone is maintained through constant bone remodeling. Because the teeth are continuously making minor movements and there is a functional demand due to the forces of mastication, the alveolar bone undergoes constant remodeling. Bone remodeling relies on a balance between bone resorption and bone deposition, which is maintained by progenitor cells that can differentiate into osteoclasts (bone resorption) and osteoblasts (bone deposition) [10,11].
The cementum is a hard, avascular connective tissue that covers the roots of teeth. It is located between the dentin and the PDL, as can be seen in Figure 1. The primary function of the cementum is to anchor the PDL fibers. The cementum’s composition is very similar to that of the alveolar bone, namely 65% (w/w) inorganic material, 23% (w/w) organic material, and 12% water [8]. The organic material is constituted by up to 90% of collagen type I. Interestingly, the majority of non-collagenous matrix proteins present in the cementum are also found in bone, namely fibronectin, osteocalcin, osteonectin, and osteopontin [12]. The cementum is produced as repair tissue to fill root fractures and resorptive defects [9,12]. In periodontal regeneration, new cementum is formed from cementoblasts. Reports suggest that the PDL serves as a source of progenitor cells for cementoblasts involved in cementum formation and also for osteoclasts and osteoblasts involved in bone remodeling [10,11].
The PDL is a complex, highly cellular, fibrous connective tissue located between the alveolar bone and the cementum, as can be seen in Figure 1. The width of the PDL ranges between 100 and 400 µm; however, it progressively decreases in thickness with age [10,13]. The extracellular compartment of the PDL is composed of highly aligned and organized collagen fiber bundles and non-collagenous matrix constituents, such as glycoproteins and proteoglycans [14]. The collagen fiber bundles provide the structural strength of the PDL and are mainly composed of collagen type I [8]. The fibers and fibrils present in the PDL are in the scale of nanometers to micrometers [9,13]. The extremities of the collagen fiber bundles are embedded in cementum or alveolar bone. The PDL is primarily responsible for providing support and mechanical stability to the teeth. It connects the cementum covering the tooth to the alveolar bone, ensuring the attachment of the tooth to the bone, while absorbing the shock from the considerable forces associated with mastication [3,14]. When characterized through tensile testing under loads between 1 and 5 N, the PDL demonstrated values of elastic modulus in the range between 0.607 and 4.274 MPa. Its elastic behavior is influenced by the loading rate, type of tooth, root level, and individual variability [15]. Furthermore, the PDL is innervated and can act as a sensory receptor for regulating pressure on the teeth and proper positioning of the jaw during mastication.
The PDL possesses an extensive blood supply and a diversity of cell populations, which include osteoblasts, osteoclasts, cementoblasts, fibroblasts, and progenitor cells [16] Another cell population that is present in the PDL are periodontal ligament stem/stromal cells (PDLSCs), which serve as a source for renewable progenitor cells, which can differentiate into osteoblasts, cementoblasts, and fibroblasts. Due to the presence of these heterogeneous cell populations, the PDL serves as a cell reservoir for tissue homeostasis, repair, and regeneration [3,14]. Blood vessels present in the PDL provide nutrients necessary for the maintenance of the ligament and the hard tissues. The PDL connects the root cementum to the alveolar bone and sustains a balance between formation and maintenance of the hard and soft tissues. The unique structure and composition of the PDL is essential for the physiological functionalities of periodontal tissues [3].
Periodontal disease is characterized by an inflammatory infection of the periodontium. This infection is caused and sustained by bacteria from dental plaque accumulation. In early stages of the disease, there is inflammation only of the gingiva, known as gingivitis, which is reversible with effective oral hygiene [1]. However, if left untreated, gingivitis can progress to periodontitis. Periodontitis in its advanced form is characterized by the loss and destruction of the periodontal tissues, including PDL, root cementum, and alveolar bone, as can be seen in Figure 1 [2,17]. This results in the loss of the tooth attachment to its supporting structures of the periodontium and in the formation of pockets surrounding the tooth. The symptoms of severe periodontitis include pain and discomfort during mastication, drifting and mobility of teeth, and tooth loss [18]. Periodontitis is the main cause of tooth loss, which is a global health problem representing a burden to society and the economy, particularly affecting older people [2]. The economic burden of periodontal disease was estimated to be USD 154.06B in the United States and EUR 158.64B in Europe, in 2018 [19]. Periodontitis is prevalent in adults and elderly populations and can also occur in children and adolescents. The prevalence of periodontal disease, which includes gingivitis and periodontitis, is estimated to range from 20% to 50% worldwide [1,17]. This large range of estimated prevalence is due to the absence of a unique and consensual case definition among different countries and populations [18]. Periodontitis can be characterized by the number of affected teeth, the magnitude of the pocket depth, the loss of tooth attachment capacity, and the loss of alveolar bone. More severe forms of periodontitis are estimated to affect 10% of the population [17].
Although bacterial plaque accumulation is the initiator of gingivitis, the host’s susceptibility to disease progression plays an important role. In patients not susceptible to periodontitis, the primary defense mechanisms are able to control the infection, and the inflammation of the gingiva may persist indefinitely without progressing to periodontitis. On the other hand, the primary defenses of patients susceptible to periodontitis cannot contain the infection of the gingiva, and the infection spreads to the periodontium [17]. The destruction of the periodontal tissues is in fact caused by host-derived mediators and enzymes from inflammatory cells in response to the bacterial infection of the periodontium. The inability to control the infection allows it to further progress into the tooth root, deepening the pockets and resulting in tooth attachment loss and alveolar bone loss [1].
Patient susceptibility is significantly affected by risk factors that increase the probability of periodontitis development. The risk factors can be genetic or environmental. Genetic risk factors that increase patient susceptibility to disease include defects of phagocytosis, which leads to an insufficient response to the bacterial infection, and enhanced enzyme production for a bacterial challenge, resulting in an excessive response with increased tissue damage [1]. Environmental or acquired risk factors include smoking, which is associated with decreased wound healing and reduced bacterial killing. Studies show that smokers are more likely to have severe periodontitis, present increased loss of alveolar bone, and have higher prevalence of tooth loss when compared to non-smokers [1,2]. Poor oral hygiene is another risk factor, as it allows accumulation of dental plaque and is linked to increased severity of periodontitis [2].
Furthermore, there is a link between systemic diseases and periodontitis. Periodontitis poses a risk of systemic complications associated with cardiovascular disease, cancer, lung diseases, and diabetes [20]. In fact, diabetes has a bi-directional relationship with periodontitis. Diabetic patients show higher concentrations of inflammatory mediators compared to non-diabetic individuals. The severity and extent of periodontitis is directly influenced by the metabolic control of diabetic individuals [17,21]. Age is a potential risk factor, as the risk of periodontitis increases with the advancing age, with a higher prevalence of the disease in elderly populations [2]. Poor oral health has been shown to be associated with disability and poor physical function in older populations [22]. In addition to motor disability, intellectual or developmental disability can also have an effect on the state of periodontal health [23]. For example, patients suffering from autism have difficulties in correctly applying oral hygiene rules and undergoing dental visits and therapies, which expose these patients to a greater risk in developing periodontitis [24].
3. Currently Available Treatments for Periodontal Disease
Initial stages of periodontitis can be treated with non-surgical procedures such as dental plaque and tartar removal with scaling and root planing [1,18]. The main goal of these treatments is to control and reduce bacterial plaque accumulation. After the clinical removal of the dental plaque, the patient should practice adequate oral hygiene to achieve a good clinical outcome [18]. Non-surgical treatments can be combined with adjunctive therapies, such as local drug delivery, systemic antibiotics, and systemic host response modulation to improve treatment outcomes. Adjunctive drugs include antibiotics and antimicrobials that are directly administered to the periodontal pocket via a gel or fiber delivery system. Examples of systemic antibiotics are amoxicillin and metronidazole, which in combination result in pronounced clinical improvements [18]. Host response modulation can be particularly beneficial for patients susceptible to disease development. Host modulatory therapies influence the destructive components of the host response to reduce periodontal tissue destruction. These therapies include non-steroidal anti-inflammatory drugs: doxycycline that downregulates collagenases in inflamed periodontal tissues; and bisphosphonates, which reduce osteoclast activity and bone resorption [1].
Non-surgical treatments have been shown to reduce pocket depth and allow formation of new tooth attachment, which can be sufficient for early to moderate stages of periodontitis. However, in some cases and in advanced stages of the disease, surgical therapy is necessary to access sites deeper in the tooth root to control the inflammation, to fully eliminate bacterial plaque, and to stimulate the regeneration of lost periodontal tissues [18]. Pocket reduction surgery is a procedure that involves resecting soft and hard necrotic tissues. Regenerative surgery includes GTR membranes and bone grafts, which are illustrated in Figure 2. An innovative adjuvant is the use of laser treatment in non-surgical or surgical procedures [25]. The surgical and non-surgical currently available methods used to treat periodontitis are summarized in Table 1.
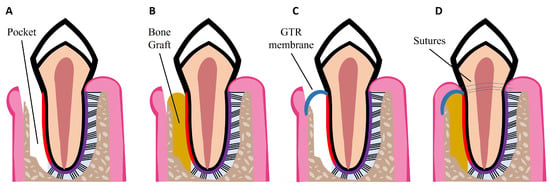
Figure 2. Schematic illustrations of procedures for periodontal regeneration. (A) Loss of PDL and alveolar bone, resulting in periodontal pocket formation. (B) Bone graft placed in the defect site. (C) GTR membrane placed over the defect site. (D) Combination of GTR membrane and bone graft. Wound closure with sutures.
Table 1. Currently available non-surgical and surgical treatments for periodontal disease.
GTR is based on the use of a mechanical barrier membrane that prevents epithelial cells and fibroblasts from migrating into the defect site while maintaining sufficient space for the regeneration of all the periodontal tissues, namely alveolar bone, cementum, and PDL [34]. There are two types of membranes already commercially available that can be used for periodontal regeneration: non-degradable and degradable membranes. Currently available non-degradable membranes include polytetrafluoroethylene membranes, such as CytoplastTM TXT-200; however, a second surgery is required for their removal. To avoid additional surgeries, there are degradable membranes on the market, which are composed of synthetic polymers such as polycaprolactone (PCL), polylactic acid (PLA), and polyglycolic acid (PGA), and of natural polymers such as collagen, for example from porcine collagen, which is used in the Bio-Gide® commercially available membrane. However, current GTR membranes have limitations such as low attachment to the adjacent tissues, which can lead to an early exposure of the defect site and allow bacteria infiltration; lack of antibacterial properties; and poor ability to enhance the regeneration of all the periodontal tissues [3,34]. Thus, new improved membranes need to be developed aiming to meet all the criteria for an ideal GTR membrane, namely: biocompatibility; non-immunogenicity as to not trigger adverse reactions; biodegradability without release of toxic byproducts; cell-occlusivity to exclude specific cell types; and ease of use in a clinical setting [35]. They should also possess appropriate surface area and high porosity for cell attachment, proliferation, and differentiation, as well as mechanical strength to stay in place for at least 4–6 weeks and to maintain space for the slow regenerating periodontium. Finally, GTR membranes should present bioactivity to accelerate tissue repair and induce a coordinated regeneration of all the periodontal tissues [35,36]. Considering the slowly regenerating alveolar bone, bone grafts can be used to fill the defect site. GTR membranes can be combined with bone grafts to prevent membrane collapse, as illustrated in Figure 2D.
Bone grafts are transplanted into bone defects, where they promote bone healing either alone or in combination with other materials. Their main functions are to provide mechanical support and enhance bone regeneration [33]. Bone grafts need to have four essential properties for achieving successful bone regeneration: osseointegration, which refers to the graft’s capability to bind to the bone’s surface; osteogenesis, which is the formation of new bone through osteoblasts present in the graft; osteoconductivity, which is the graft’s potential to generate a scaffold on which host cells can grow; and osteoinductivity, which translates to the graft’s ability to recruit host stem cells into it and induce their differentiation into osteoblasts through local proteins and growth factors [37]. Unfortunately, current bone grafts mainly fulfill only the osteoconductivity property by serving as a structure for regeneration processes to occur [38]. Although some bone grafts might present almost all four essential properties for successful bone regeneration, their success is also influenced by the grafts biocompatibility, biodegradability, structural integrity, and porosity [39]. Moreover, the grafts are envisaged for bone formation, thus neglecting PDL regeneration. It is important to note that not only is osseointegration important, but so also is the attachment of newly formed bone to a regenerated PDL, which in turn connects the newly formed bone to the cementum of the tooth. In addition to bone grafts, GTR membranes also fail to achieve PDL regeneration and integration of soft (PDL) and hard tissues (alveolar bone, cementum) [3]. If the PDL is not regenerated, there is no connection between cementum and alveolar bone, and the tooth will eventually be lost due to the lack of attachment to the bone. These regenerative procedures are still exposed to clinical failures and do not effectively promote periodontal regeneration. Therefore, innovative strategies that promote the regeneration of the entire hierarchical structure of the periodontium are needed to improve clinical outcomes.
This entry is adapted from the peer-reviewed paper 10.3390/nano13081307
This entry is offline, you can click here to edit this entry!
