Active pharmaceutical ingredients (APIs) can be defined as those biological compounds used for the detection, prevention, and treatment of different types of diseases. Asymmetric oxidation processes have constituted a valuable tool for the synthesis of APIs, especially for the preparation of optically active sulfoxides, compounds with interesting biological properties. Biocatalyzed reactions usually occur with high efficiency, excellent selectivity, good yields, environmental sustainability, and lower costs, which make them more attractive from an industrial perspective. However, it must be taken into account that these procedures also present some drawbacks, such as the (relatively) high substrate specificity of biocatalysts or the low substrate loadings required due to their generally low solubility in water.
- active pharmaceutical ingredients
- oxidations
- biocatalysis
- Green Chemistry
1. Introduction
2. Biocatalytic Approaches for the Oxidative Preparation of APIs and Precursors
2.1. Oxidations Catalyzed by Monooxygenases
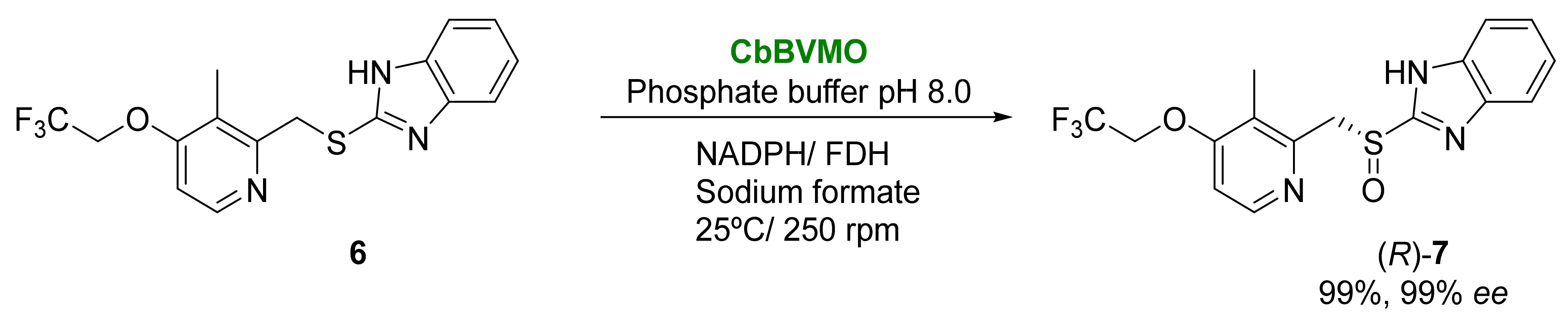
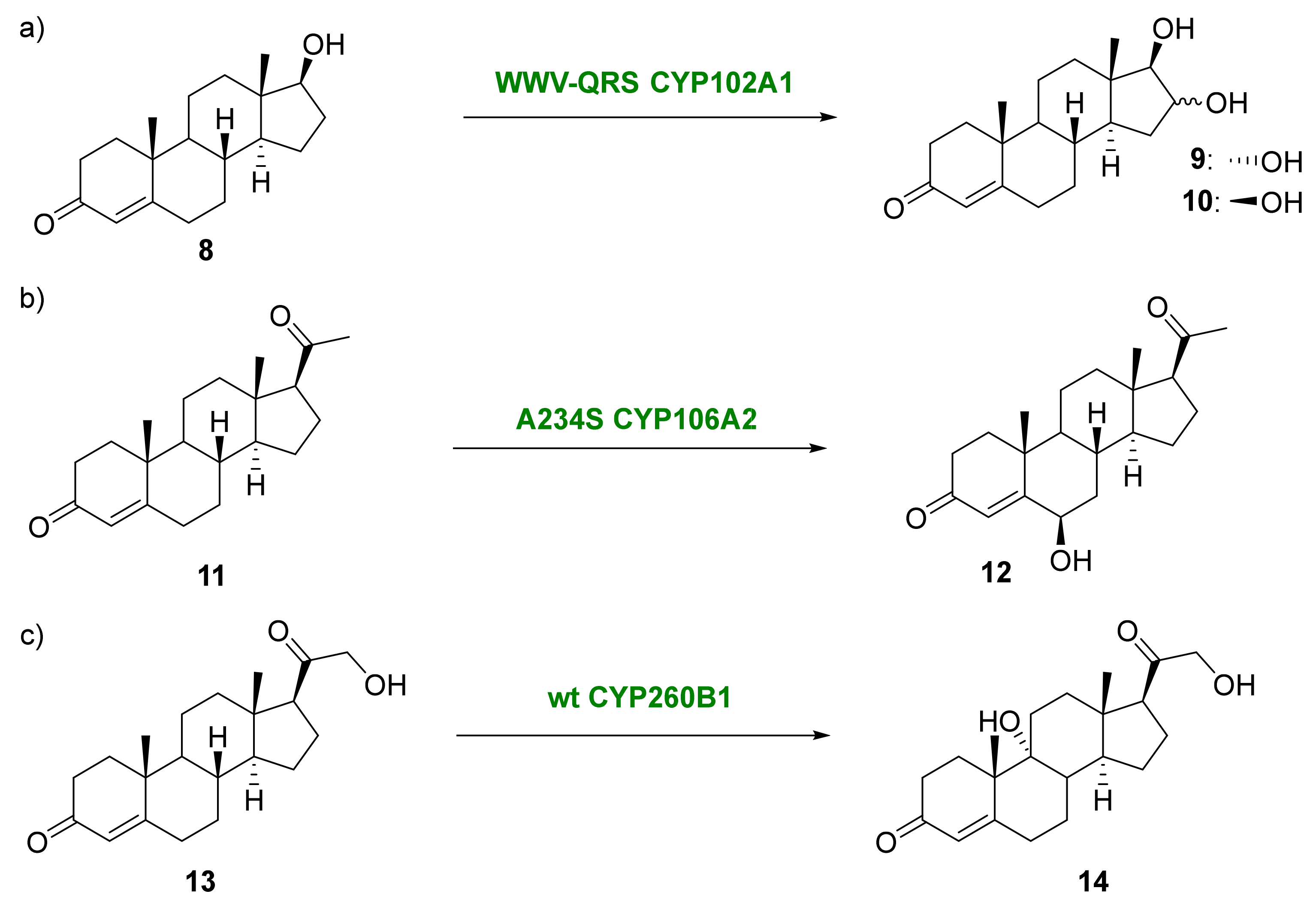
2.2. Other Biocatalyzed Oxidations
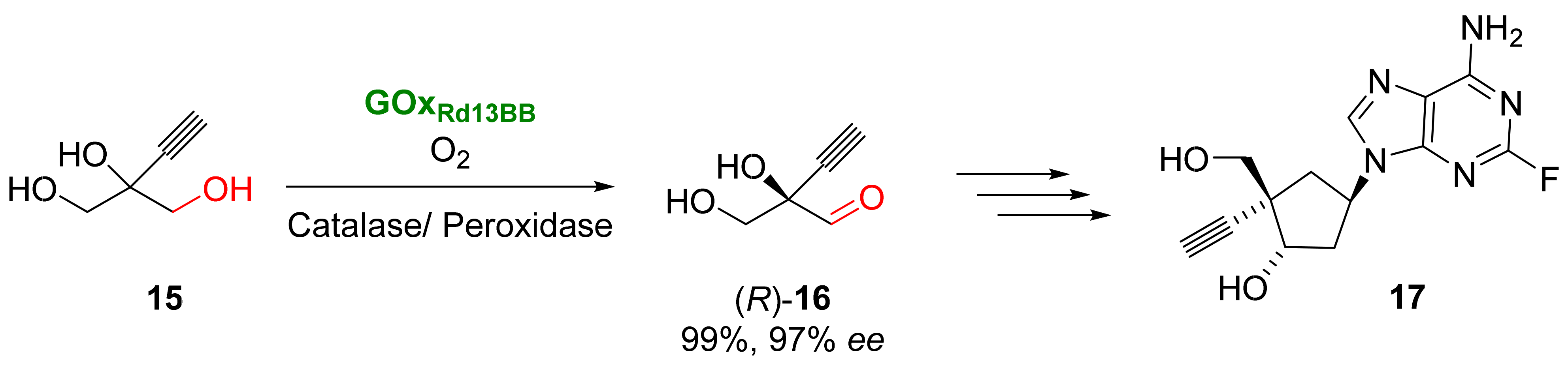
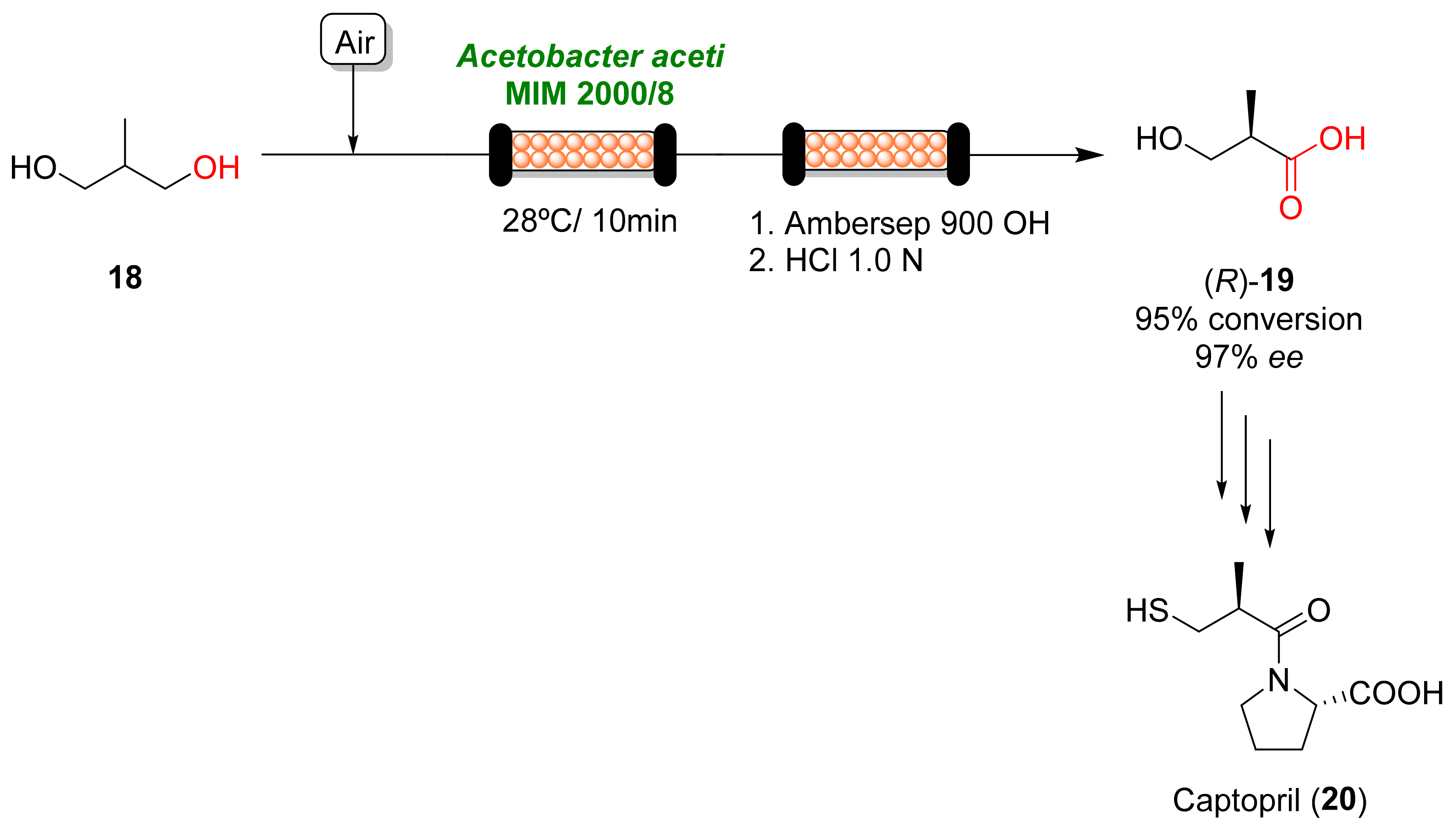
This entry is adapted from the peer-reviewed paper 10.3390/catal13030638
References
- U.S. Food & Drug Administration Glossary of Terms. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/drugsfda-glossary-terms (accessed on 22 January 2023).
- Park, J.; Kelly, M.A.; Kang, J.X.; Seemakurti, S.S.; Ramirez, J.L.; Hatzell, M.C.; Sievers, C.; Bommarius, A.S. Production of active pharmaceutical ingredients (APIs) from lignin-derived phenol and catechol. Green Chem. 2021, 23, 7488–7498.
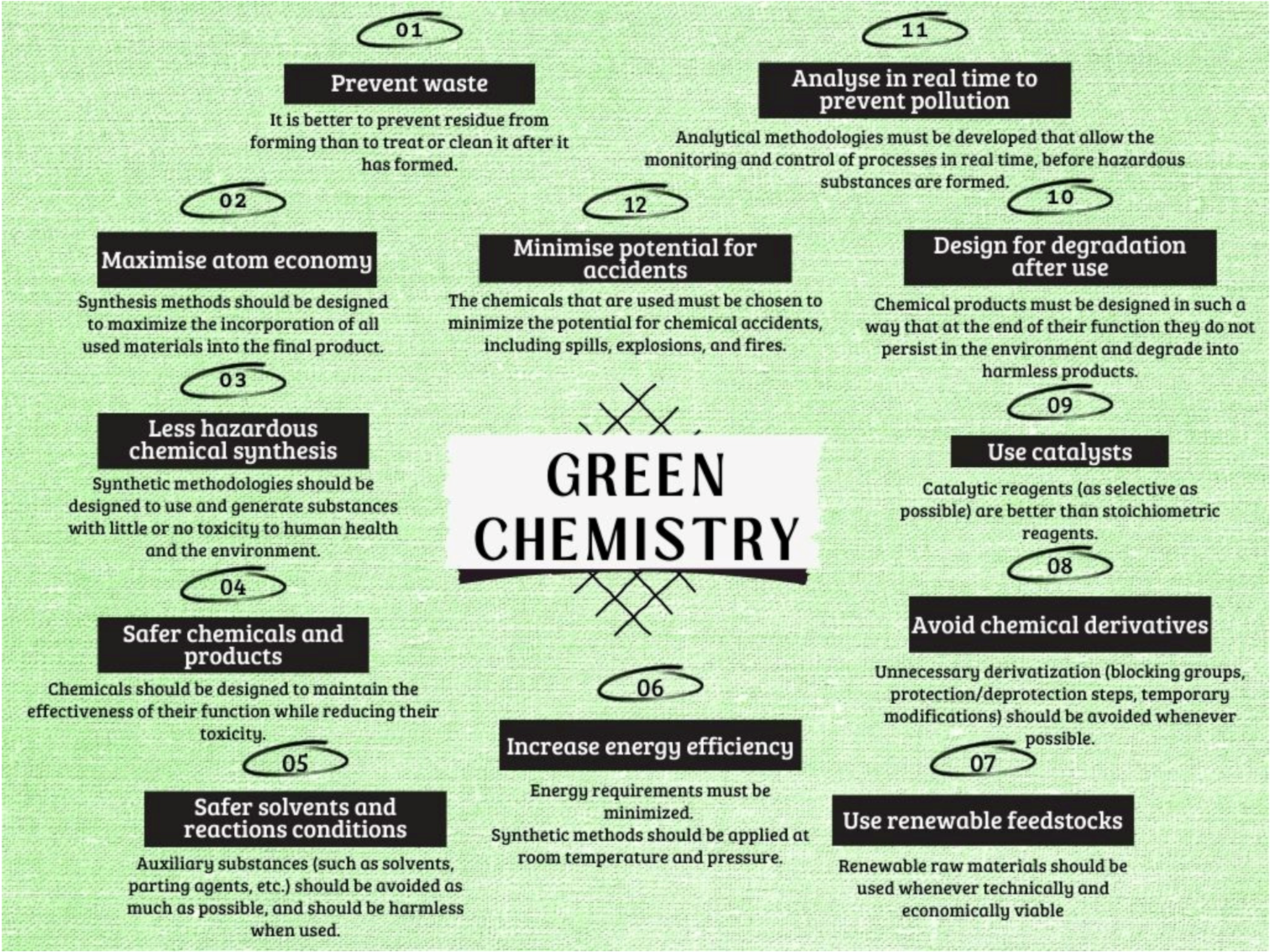
- Horvath, I.T.; Anastas, P.T. Innovations and Green Chemistry. Chem. Rev. 2007, 107, 2169–2173.
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312.
- Sheldon, R.A. Fundamentals in Green Chemistry: Efficiency in reaction design. Chem. Soc. Rev. 2012, 41, 1437–1451.
- Anastas, P.T.; Williamson, T.C. Green Chemistry: Frontiers in Benign Chemical Syntheses and Processes; Oxford University Press: Oxford, UK, 1998.
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998.
- United Nation Sustainable Development Goals. Available online: https://sdgs.un.org/goals (accessed on 22 January 2023).
- Campos, K.R.; Coleman, P.J.; Alvárez, J.C.; Dreher, S.D.; Garbaccio, R.M.; Terrett, N.K.; Tillyer, R.D.; Truppo, M.D.; Parmee, E.R. The importance of synthetic chemistry in the pharmaceutical industry. Science 2019, 363, eaat0805.
- Sharma, S.; Das, J.; Braje, W.M.; Dash, A.K.; Handa, S. A glimpse into Green Chemistry practices in the pharmaceutical industry. ChemSusChem 2020, 13, 2859–2875.
- Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Green chemistry in the synthesis of pharmaceuticals. Chem. Rev. 2022, 122, 3637–3710.
- Constable, D.J.C.; Jimenez-Gonzalez, C.; Henderson, R.K. Perspective on solvent use in the pharmaceutical industry. Org. Process Res. Dev. 2007, 11, 133–137.
- Prat, D.; Pardigon, O.; Flemming, H.-W.; Letestu, S.; Ducandas, V.; Isnard, P.; Guntrum, E.; Senac, T.; Ruisseau, S.; Cruciani, P.; et al. Sanofi’s solvent selection guide: A step toward more sustainable processes. Org. Process Res. Dev. 2013, 17, 1517–1525.
- Plechkova, N.V.; Seddon, K.R. Application of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150.
- Liu, Y.; Friesen, J.B.; Lankin, J.B.; Chen, S.-N.; Pauli, G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690.
- Miele, M.; Pillari, V.; Pace, V.; Alcántara, A.R.; de Gonzalo, G. Application of biobased solvents in asymmetric catalysis. Molecules 2022, 27, 6701.
- Miele, M.; Ielo, L.; Pillari, V.; Fernández, M.; Alcántara, A.R.; Pace, V. Biomass-Derived Solvents in Sustainable Organic Synthesis: Tools and Strategies; Protti, S., Palmieri, A., Eds.; Royal Society of Chemistry: Croydon, UK, 2022; pp. 239–279.
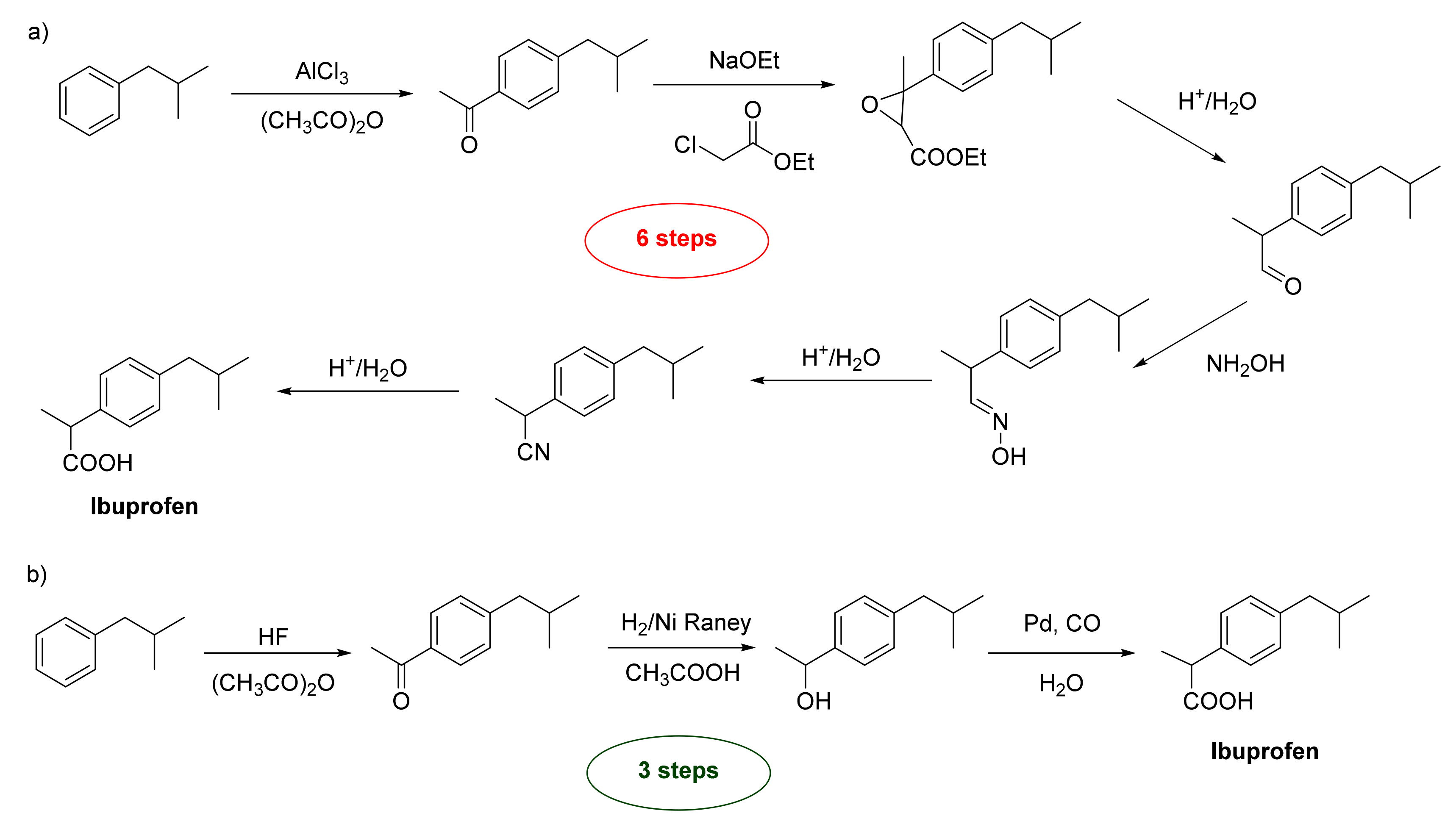
- Stuart, N.J.; Sanders, A.S. Phenyl Propionic Acids. US Patent 3385886, 2 February 1961.
- Andraos, J. Designing a green organic chemistry lecture course. In Green Organic Chemistry in Lecture and Laboratory; Dicks, A.P., Ed.; CRC Press: Boca Raton, FL, USA, 2016; Chapter 2; pp. 29–68.
- Papadogianakis, G.; Maat, L.; Sheldon, R.A. Catalytic conversions in water. Part 5: Carbonylation of 1-(4-isobutylphenyl)- ethanol to ibuprofen catalysed by water-soluble palladium-phosphine complexes in a two-phase system. J. Chem. Technol. Biotechnol. 1997, 70, 83–91.
- Trost, B.M. Atom Economy: A challenge for organic synthesis. Angew. Chem. Int. Ed. 1995, 34, 259–281.
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 108, 801–838.
- Domínguez de María, P.; de Gonzalo, G.; Alcántara, A.R. Biocatalysis as useful tool in asymmetric synthesis: An assessment of recently granted patents (2014–2019). Catalysts 2019, 9, 802.
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic synthesis for industrial applications. Angew. Chem. Int. Ed. 2021, 60, 88–119.
- Domínguez de María, P. Biocatalysis, sustainability, and industrial applications: Show me the metrics. Curr. Opin. Green Sustain. Chem. 2021, 31, 100514.
- Patel, R.N. Biocatalysis for synthesis of pharmaceuticals. Bioorg. Med. Chem. 2018, 26, 1252–1274.
- Simíc, S.; Zukíc, E.; Schmermund, L.; Faber, K.; Winkler, C.K.; Kroutil, W. Shortening synthetic routes to small molecule active pharmaceutical ingredients employing biocatalytic methods. Chem. Rev. 2022, 122, 1052–1126.
- Rossini, G.; Robescu, M.S.; Licastro, E.; Tedesco, C.; Martello, I.; Maffei, L.; Vincenti, G.; Bavaro, T.; Collina, S. Biocatalysis: A smart and green tool for the preparation of chiral drugs. Chirality 2022, 34, 1403–1418.
- De Gonzalo, G.; Alcántara, A.R.; Domínguez de María, P.; Sánchez-Montero, J.M. Biocatalysis for the asymmetric synthesis of Active Pharmaceutical Ingredients (APIs): This time is for real. Expert Opin. Drug Discover. 2022, 17, 1159–1171.
- Zawodny, W.; Montgomery, S.L. Evolving new chemistry: Biocatalysis for the synthesis of amine-containing pharmaceuticals. Catalysts 2022, 12, 595.
- Colonna, S.; Del Sordo, S.; Gaggero, N.; Carrea, G.; Pasta, P. Enzyme-mediated catalytic asymmetric oxidations. Heteroat. Chem. 2002, 13, 467–473.
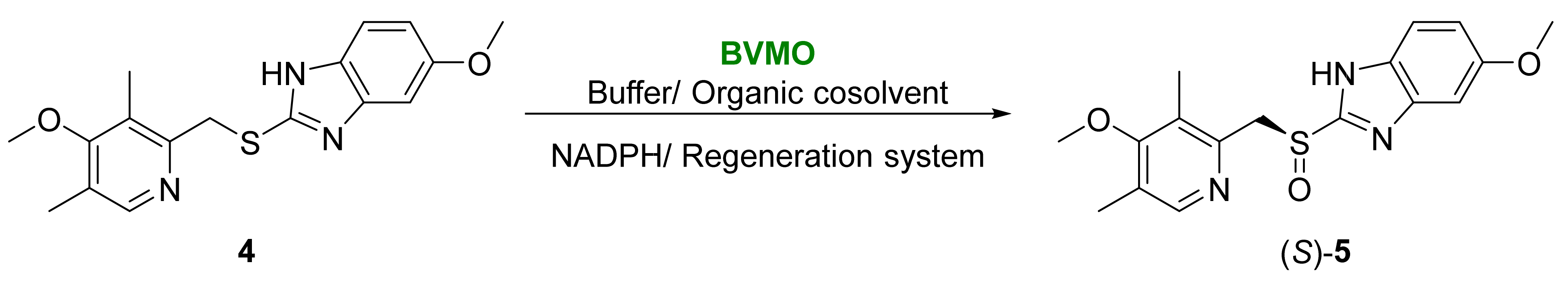
- Dong, J.J.; Fernández-Fueyo, E.; Hollmann, F.; Paul, C.E.; Pesic, M.; Schmidt, S.; Wang, Y.; Younes, S.; Zhang, W. Biocatalytic oxidation reactions: A chemist’s perspective. Angew. Chem. Int. Ed. 2018, 57, 9238–9261.
- Torres-Pazmiño, D.E.; Winkler, M.; Glieder, A.; Fraaije, M.W. Monooxygenases as biocatalysts: Classification, mechanistic aspects, and biotechnological applications. J. Biotechnol. 2010, 146, 9–24.
- Leisch, H.; Morley, K.; Lau, P.C.K. Baeyer-Villiger monooxygenases: More than just green chemistry. Chem. Rev. 2011, 111, 4165–4222.
- Fürst, M.J.L.J.; Gran-Scheuch, A.; Aalbers, F.S.; Fraaije, M.W. Baeyer–Villiger monooxygenases: Tunable oxidative biocatalysts. ACS Catal. 2019, 9, 11207–11241.
- De Gonzalo, G.; Alcántara, A.R. Multienzymatic processes involving Baeyer–Villiger monooxygenases. Catalysts 2021, 11, 605.
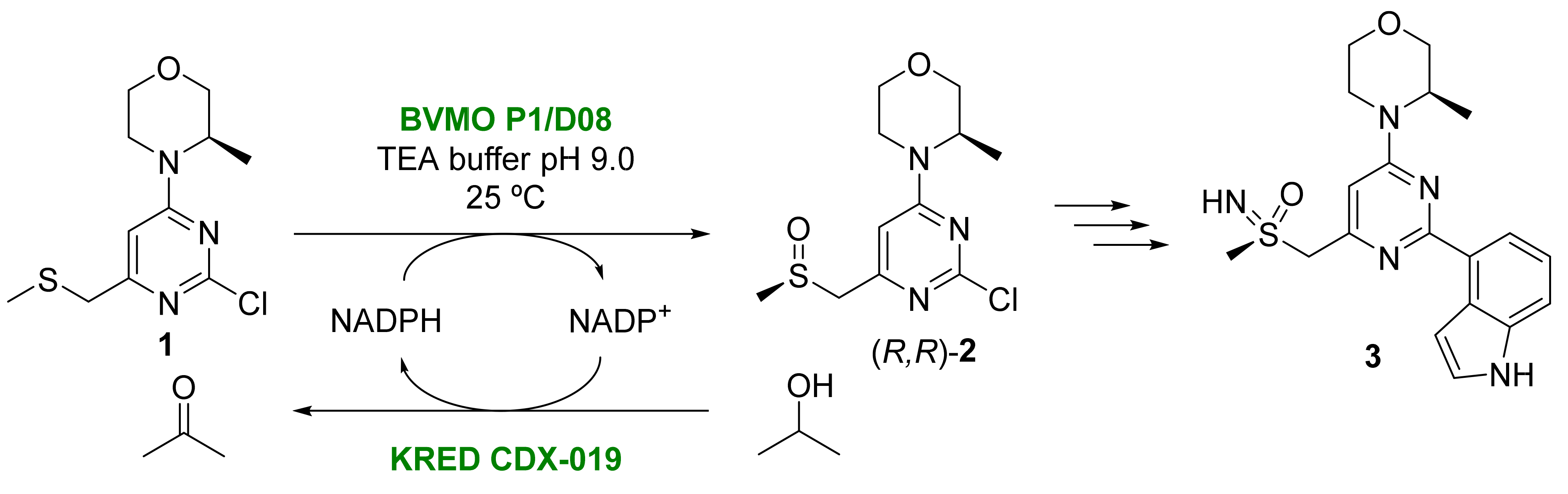
- Foote, K.M.; Nissink, J.W.M.; Turner, P. Morpholinio Pyrimidines and Their Use in Therapy. Patent WO2011154737A, 11 June 2011.
- Goundry, W.R.F.; Adams, B.; Benson, H.; Demeritt, J.; McKown, S.; Mullholland, K.; Robertson, A.; Siedlecki, P.; Tomlin, P.; Vare, K. Development and scale-up of a biocatalytic process to form a chiral sulfoxide. Org. Process Res. Develop. 2017, 21, 107–113.
- Lindberg, P.; Brändström, A.; Wallmark, B.; Mattsson, H.; Rikner, L.; Hoffman, K.-J. Omeprazole: The first proton pump inhibitor. Med. Res. Rev. 1990, 10, 1–54.
- Baker, D.E. Esomeprazole magnesium (Nexium). Rev. Gastroenterol. Disord. 2001, 1, 32–41.
- Cotton, H.; Elebring, T.; Larsson, M.; Li, L.; Sörensen, H.; von Unge, S. Asymmetric synthesis of esomeprazole. Tetrahedron: Asymmetry 2000, 11, 3819–3825.
- Bong, Y.K.; Song, S.; Nazor, J.; Vogel, M.; Widegren, M.; Smith, D.; Collier, S.J.; Wilson, R.; Palanivel, S.M.; Narayanaswamy, K.; et al. Baeyer-Villiger monooxygenase-mediated synthesis of esomeprazole as an alternative for Kagan sulfoxidation. J. Org. Chem. 2018, 83, 7453–7458.
- Stewart, J.D. Cyclohexanone monooxygenase: A useful reagent for asymmetric Baeyer-Villiger reactions. Curr. Org. Chem. 1998, 2, 195–216.
- Zhang, Y.; Wu, Y.-Q.; Xu, N.; Zhao, Q.; Yu, H.-L.; Xu, J.-H. Engineering of cyclohexanone monooxygenase for the enantioselective synthesis of (S)-omeprazole. ACS Sustain. Chem. Eng. 2019, 7, 7218–7226.
- Xu, N.; Zhu, J.; Wu, Y.-Q.; Zhang, Y.; Xia, J.-Y.; Zhao, Q.; Lin, G.-Q.; Yu, H.-L. Enzymatic preparation of the chiral (S)-sulfoxide drug esomeprazole at pilot-scale levels. Org. Process Res. Develop. 2020, 24, 1124–1130.
- Zhang, Y.; Liu, F.; Xu, N.; Wu, Y.-Q.; Zheng, Y.C.; Zhao, Q.; Lin, G.-Q.; Yu, H.-L.; Xu, J.-H. Discovery of two native Baeyer-Villiger monooxygenases for asymmetric synthesis of bulky chiral sulfoxides. Appl. Environ. Microbiol. 2018, 84, e00638-18.
- Liu, F.; Shou, C.; Geng, Q.; Zhao, C.; Xu, J.; Yu, H. A Baeyer-Villiger monooxygenase from Cupriavidus basilensis catalyzes asymmetric synthesis of (R)-lansoprazole and other pharmaco-sulfoxides. Appl. Microbiol. Biotechnol. 2021, 105, 3169–3180.
- Urlacher, V.; Girhard, M. Cytochrome P450 monooxygenases: An update on perspectives for synthetic applications. Trends Biotechnol. 2012, 30, 26–36.
- Zhang, X.; Peng, Y.; Zhao, J.; Li, Q.; Yu, X.; Acevedo-Rocha, C.G.; Li, A. Bacterial cytochrome P450-catalyzed regio and stereoselective steroid hydroxylation enabled by directed evolution and rational design. Bioresur. Bioprocess. 2020, 7, 2.
- Nahri, L.O.; Fulco, A.J. Characterization of a catalytically self-sufficient 119,000 dalton cytochrome P450 monooxygenase induced by barbiturates in Bacillus megaterium. J. Biol. Chem. 1986, 26, 7160–7169.
- Acevedo-Rocha, C.G.; Gamble, C.; Lonsdale, R.; Li, A.; Nett, N.; Hoebenreich, S.; Deege, A. P450-catalyzed regio- and diastereoselective steroid hydroxylation: Efficient directed evolution enabled by mutability landscaping. ACS Catal. 2018, 8, 3395–3410.
- Schmitz, D.; Janocha, S.; Kiss, F.M.; Bernhardt, R. CYP106A2—A versatile biocatalyst with high potential for biotechnological production of selectively hydroxylated steroid and terpenoid compounds. BBA-Proteins Proteom. 2018, 1866, 11–22.
- Nikolaus, J.; Nguyen, K.T.; Virus, C.; Riehm, J.L.; Hutter, M.; Bernhardt, R. Engineering of CYP106A2 for steroid 9α- and 6β-hydroxylation. Steroids 2017, 120, 41–48.
- Litzenburger, M.; Kern, F.; Khatri, Y.; Bernhardt, R. Conversions of tricyclic antidepressants and antipsychotics with selected P450s from Sorangium cellulosum So ce56. Drug Metab. Dispos. 2015, 43, 392–399.
- Litzenburger, M.; Bernhardt, R. CYP260B1 acts as 9α-hydroxylase for 11-deoxycorticosterone. Steroids 2017, 127, 40–45.
- Ohrui, H.; Kohgo, S.; Hayakawa, H.; Kodama, E.; Matsuoka, M.; Nakata, T.; Mitsuya, H. 2′-Deoxy-4′-C-ethynyl-2-fluoroadenosine: A nucleoside reverse transcriptase inhibitor with highly potent activity against wide spectrum of HIV-1 strains, favorable toxic profiles, and stability in plasma. Nucl. Nucl. Nucleic Acids 2007, 26, 1543–1546.
- Huffman, M.A.; Fryszkowska, A.; Alvizo, O.; Borra-Garske, M.; Campos, K.R.; Canada, K.A.; Devine, P.N.; Duan, D.; Forstater, J.H.; Grosser, S.T.; et al. Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science 2019, 366, 1255–1259.
- Dijkman, W.P.; de Gonzalo, G.; Mattevi, A.; Fraaije, M.W. Flavoprotein oxidases: Classification and applications. Appl. Microbiol. Biotechnol. 2013, 97, 5177–5188.
- Pickl, M.; Fuchs, M.; Glueck, S.M.; Faber, K. The substrate tolerance of alcohol oxidases. Appl. Microbiol. Biotechnol. 2015, 16, 6617–6642.
- Wahart, A.J.C.; Staniland, J.; Miller, G.J.; Cosgrove, S.C. Oxidase enzymes as sustainable oxidation catalysts. R. Soc. Open Sci. 2022, 9, 211572.
- Wiles, C.; Watts, P. Improving chemical synthesis using flow reactors. Expert Opin. Drug Discover. 2007, 2, 1487–1503.
- De Santis, P.; Meyer, L.-E.; Kara, S. The rise of continuous flow biocatalysis—Fundamentals, very recent developments and future perspectives. React. Chem. Eng. 2020, 5, 2155–2184.
- Ötvos, S.B.; Kappe, O. Continuous flow asymmetric synthesis of chiral active pharmaceutical ingredients and their advance interemediates. Green Chem. 2021, 23, 6117–6138.
- Santi, M.; Sancineto, L.; Nascimento, V.; Braun Azeredo, J.; Orozco, E.V.M.; Andrade, L.H.; Gröger, H.; Santi, C. Flow biocatalysis: A challenging alternative for the synthesis of APIs and natural compounds. Int. J. Mol. Sci. 2021, 22, 990.
- Cushman, D.W.; Cheung, H.S.; Sabo, E.F.; Ondetti, M.A. Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids. Biochemistry 1977, 16, 5484–5491.
- Ondetti, M.A.; Cushman, D.W. Azetidine-2-carboxylic Acid Derivatives. US Patent 4046889, 6 September 1977.
- De Vitis, V.; Dall’Oglio, F.; Pinto, A.; De Micheli, C.; Molinari, F.; Conti, P.; Romano, D.; Tamborini, L. Chemoenzymatic synthesis in flow reactors: A rapid and convenient preparation of captopril. ChemistryOpen 2017, 6, 668–673.
- Romano, A.; Gandolfi, R.; Nitti, P.; Rollini, M.; Molinari, F. Acetic acid bacetria as enantioselective biocatalysts. J. Mol. Catal. B: Enzym. 2002, 17, 235–240.
- Kaur, B.; Chakraborty, D. Biotechnological and molecular approaches for vanillin production: A review. Appl. Biochem. Biotechnol. 2013, 169, 1353–1372.
- Yang, T.-X.; Zhao, L.-Q.; Wang, J.; Song, G.-L.; Liu, H.-M.; Cheng, H.; Yang, Z. Improving whole-cell biocatalysis by addition of deep eutectic solvents and natural deep eutectic solvents. ACS Sustain. Chem. Eng. 2017, 5, 5713–5722.
- Domínguez de María, P.; Guajardo, N.; Kara, S. Enzyme catalysis: In DES. In Deep Eutectic Solvents: Synthesis, Properties and Applications; Ramón, D.J., Guillena, G., Eds.; Wiley-VCH: Weinheim, Germany, 2019; pp. 257–272.
- Hasani, F.Z.I.M.; Amzazi, S.; Lavandera, I. The versatile applications of DES and their influence on oxidoreductase-mediated transformations. Molecules 2019, 24, 2190.
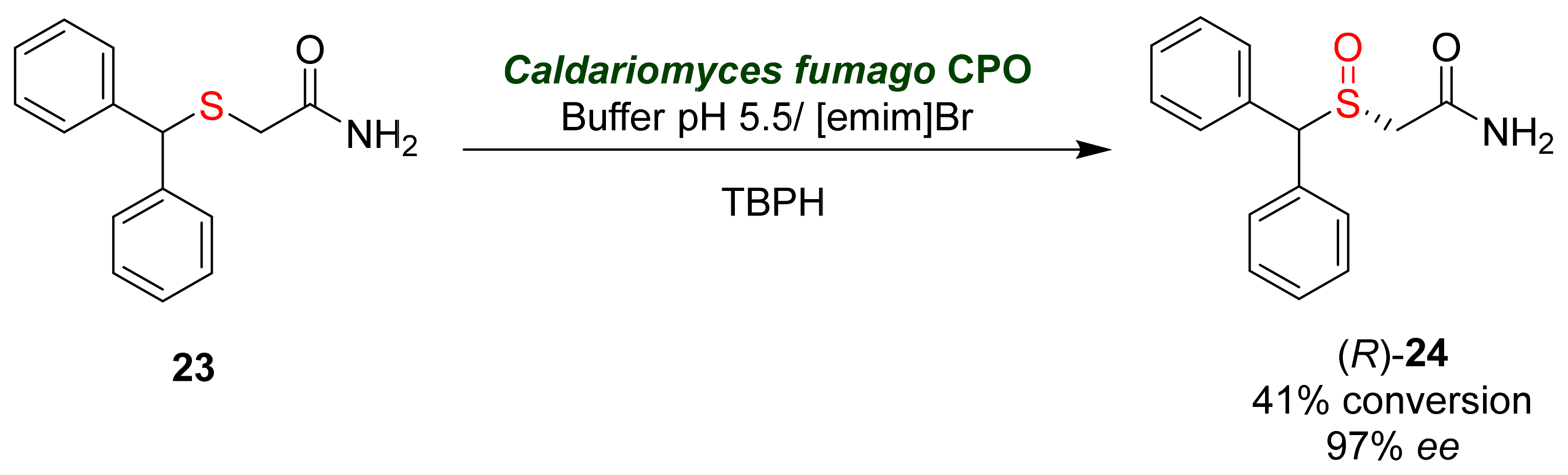
- Czeisler, C.A.; Walsh, J.K.; Roth, T.; Hughes, R.J.; Wright, K.P.; Kingsbury, L.; Arora, S.; Schwartz, J.R.L.; Niebler, G.E.; Dinges, D.F. Modafinil for excessive sleepiness associated with shift-work sleep disorder. N. Engl. J. Med. 2005, 353, 476–486.
- Pliszka, A.G. Modafinil: A review and its potential use in the treatment of long COVID fatigue and neurocognitive deficits. Am. J. Psychiatry Res. J. 2022, 17, 5–7.
- Gao, F.; Wang, L.; Liu, Y.; Wang, S.; Jiang, Y.; Hu, M.; Li, S.; Zhai, Q. Enzymatic synthesis of (R)-modafinil by chloroperoxidase-catalyzed enantioselective sulfoxidation of 2-(diphenylmethylthio) acetamide. Biochem. Eng. J. 2015, 93, 243–249.
- Torres-Duarte, C.; Vazquez-Duhalt, R. Applications and prospective of peroxidase biocatalysis in the environmental field. In Biocatalysis Based on Heme Peroxidases; Torres, E., Ayala, M., Eds.; Springer: Berlin, Germany, 2010.
- Itoh, T. Ionic Liquids as tool to improve enzymatic organic synthesis. Chem. Rev. 2017, 117, 10567–10607.
