Please note this is an old version of this entry, which may differ significantly from the current revision.
The human nervous system exhibits limited regenerative capabilities following damage to the central nervous system (CNS), leading to a scarcity of effective treatments for nerve function recovery. In contrast, zebrafish demonstrate remarkable regenerative abilities, making them an ideal model for studying the modulation of inflammatory processes after injury.
- TNF/Tnfrsf1a
- HDAC1
- macrophages
- ependymal–radial glia progenitors
- pMN progenitors
- radial glial cells
- spinal cord injury
- zebrafish
1. Introduction
A previous report described that the immune response could promote neuronal regeneration in zebrafish, especially macrophages. Macrophages are immune cells that are important for tissue repair and regeneration. In mammals, axonal repair following traumatic spinal cord injury (SCI) is dependent upon the rapid development of reparative M2-macrophages [1], because sustained recruitment of inflammatory blood-derived macrophages can facilitate extensive secondary axonal dieback and substantially delay the reparative process. However, activated macrophages can also promote axonal regeneration [2], suggesting that the immune response plays complex roles after spinal injury. In zebrafish, which contain two types of macrophages, unpolarized macrophages are recruited to the inflammation site and adopt a M1-like phenotype. Subsequently, the macrophages convert their functional phenotypes from M1-like to M2-like in response to a progressive inflammatory microenvironment [3]. Moreover, microglia are activated after SCI in adult [4] and larval [5] zebrafish, suggesting that innate immune cells have repair functions. In zebrafish, T cells can also mediate organ-specific regenerative programs, suggesting that adaptive immunity is also important for spinal cord regeneration in this species [6]. However, the mechanisms through which macrophages can promote neuronal regeneration in zebrafish, as well as the existence of a pro-regenerative macrophage subpopulation that can promote neuronal regeneration in zebrafish, are unclear.
After injury, the immune response is a significant source of non-developmental signals. Studies have demonstrated the positive effects of macrophages during regenerative neurogenesis in zebrafish. The cytokine tumor necrosis factor (TNF; also known as TNF-α) mediates a broad range of cellular activities, including proliferation, survival, differentiation, and apoptosis, and it is considered essential for inducing and maintaining the inflammatory immune response [7]. Recent studies have demonstrated that TNFα can exhibit dual roles depending on the context, with both pro-inflammatory and pro-regenerative functions. In the context of spinal cord injury, the specific subtypes of macrophages and the balance between TNFα1 and TNFα2 signaling are crucial for determining the regenerative outcome. Activated tnfa+ macrophages have been determined to express a mixture of M1 and M2 markers, which can facilitate axon growth and neurogenesis [8]. The interplay between TNFα1 and TNFα2 signaling is essential for modulating the switch between pro-inflammatory and pro-regenerative functions. TNFα1, typically associated with pro-inflammatory responses, can trigger cell death and impede regeneration. On the other hand, TNFα2 signaling has been linked to increased regenerative outcomes by promoting cell survival and tissue repair [9]. The activation of TNFα2 in the presence of TNFα1 can counteract the detrimental effects of TNFα1 signaling, thereby creating a favorable environment for regeneration. Furthermore, the downstream signaling pathways activated by TNFα1 and TNFα2 also contribute to their distinct cellular responses. While TNFα1 primarily activates the NF-κB pathway, leading to inflammation and cell death, TNFα2 preferentially activates the PI3K/Akt pathway, which promotes cell survival and regeneration. Understanding the delicate balance between TNFα1 and TNFα2 signaling and their distinct mechanisms in the regenerative process could provide insights for the development of targeted therapies to enhance spinal cord repair following injury.
Recent research on zebrafish has highlighted the importance of macrophages in promoting axonal regrowth. Specifically, TNF-α has been identified as a key factor in this process [10]. Inhibiting TNF-α has been shown to decrease axonal regrowth, indicating its indispensable role in the process. These findings suggest that TNF-α plays a critical role in promoting regeneration in association with other neuroglia. For instance, macrophages can indirectly affect ependymo-radial glia (ERG) progenitor cells, promoting regenerative neurogenesis in zebrafish after spinal cord injury [11]. Thus, the effects of macrophages could act indirectly by inducing the re-expression of developmental signals from the environment. Indeed, developmental signals such as Wnt, Fgf, Shh, and dopamine are re-expressed after spinal injury in zebrafish, promoting regenerative neurogenesis. For example, Wnt/b-catenin signaling is required for radial glial neurogenesis following SCI [12]. Additionally, Goldshmit et al. (2018) have demonstrated the role of Fgf in driving neural proliferation and neurite outgrowth of different spinal cord neuron populations during both neural development and adult regeneration [13]. Therefore, macrophages can affect different types of neuroglia and promote regeneration via developmental signals or non-developmental signals.
2. Overview of the Response of Macrophages to Spinal Cord Injury in Zebrafish
Zebrafish are a well-established model organism for studying nerve damage regeneration mechanisms, particularly after SCI. This is because of their remarkable ability to regenerate neural tissue after injury [14][15]. Macrophages, which are a type of immune cell, are crucial in the response to SCI in zebrafish. They play a vital role in the regenerative process by clearing debris and promoting tissue repair [16]. As the injury site is cleared of debris, macrophages, the primary immune cells involved in the inflammatory response, shift their phenotype from a pro-inflammatory (M1) to an anti-inflammatory (M2) state [17]. This phenotypic shift is essential for promoting tissue repair and regeneration. M2 macrophages secrete factors such as interleukin-10 (IL-10); transforming growth factor-beta (TGFβ); cytokines IL-4 and IL-10; and surface markers CD206 and CD163 [18][19]. These factors have anti-inflammatory and pro-regenerative effects, supporting the transition from inflammation to tissue repair. Below, researchers describe four different phases, each with distinct macrophage functions after SCI in zebrafish.
2.1. Phase 1: Acute Inflammatory Response
Upon SCI, zebrafish macrophages are rapidly recruited to the injury site and begin phagocytosing cellular debris. The macrophages also secrete pro-inflammatory cytokines, such as TNF-α and IL-1β [10], which attract additional immune cells to the injury site. Macrophages rapidly respond to injury-dependent changes in neuronal activity, alter their morphology to an activated amoeboid state, and remove nonfunctional neuronal cells after injury in a pro-inflammatory response. The response involves molecules such as the complement system, TNF-α, IL-1β, CXC3CL1, CX3CR1, and MHC-1 [20][21].
2.2. Phase 2: Resolution of Inflammation
As the injury site is cleared of debris, the macrophages shift their phenotypes to become anti-inflammatory. They secrete factors such as IL-10, TGFβ, and cytokines IL-4, IL-10, CD206, and CD163, which promote tissue repair and regeneration [22][23]. The macrophages may regulate aspects of appendage regeneration through Wnt/β-catenin signaling [24][25]. This signaling pathway affects macrophage proliferation and cytokine release, which can modulate inflammation and regeneration of the appendage.
2.3. Phase 3: Promotion of Axon Regeneration
Macrophages have been shown to promote axon regeneration in various models by releasing factors that enhance axonal growth, such as brain-derived neurotrophic factor (BDNF) and fibroblast growth factor (FGF) [26][27], although their specific role in zebrafish axon regeneration warrants further investigation. In addition to BDNF and FGF, zebrafish macrophages release other growth factors and cytokines that promote axon regeneration, including nerve growth factor (NGF) and insulin-like growth factor 1 (IGF-1) [28][29]. These factors act in a coordinated manner to stimulate axon growth and re-establish neuronal connections.
2.4. Phase 4: Modulation of Glial Scar Formation
Glial scarring is a major obstacle to neural regeneration in mammals [30]. In zebrafish, macrophages appear to play a role in modulating glial scar formation by promoting the clearance of myelin debris and releasing factors that inhibit excessive scar formation [31]. Additionally, zebrafish macrophages release factors that inhibit excessive scar formation. One such factor is chondroitin sulfate proteoglycans (CSPGs), which are known to be major contributors to the formation of a glial scar [32][33]. Macrophages in zebrafish have been shown to express enzymes that degrade CSPGs, thereby preventing the formation of an excessive glial scar.
Overall, macrophages are crucial for clearing debris, secreting pro-inflammatory cytokines, promoting tissue repair and regeneration, releasing factors that enhance axonal growth, and inhibiting excessive scar formation. The ability of macrophages to perform these functions is essential for the remarkable regenerative capacity of zebrafish after SCI. Further understanding of the mechanisms underlying this response may lead to new therapeutic approaches to treating SCI in humans.
3. The Role of TNF-Activated Macrophages in Pro-Regenerative Neurogenesis
The immune response plays a crucial role in the regenerative process of the spinal cord after injury [34]. Among the various immune cells involved, macrophages have been identified as key players in promoting neurogenesis [35]. Recent studies have shown that activated tnfa+ macrophages represent a subtype of pro-regenerative macrophages that act on spinal progenitor cells to promote axon growth and neurogenesis [11]. However, the mechanisms by which these macrophages promote regeneration are not fully understood. The TNF signaling appears to be regeneration-specific and not necessary for developmental neurogenesis; its upregulation after injury could be part of the reactivated developmental program for neurogenesis [36]. Therefore, a better understanding of the intracellular signaling that occurs in spinal progenitor cells after injury could lead to new translational approaches for spinal cord repair.
TNF is primarily produced by activated macrophages and is known to promote inflammation and cell death in certain contexts [37]. However, recent studies have shown that TNF can also play a pro-regenerative role by promoting axon growth and neurogenesis in the spinal cord [38][39]. One key subtype of pro-regenerative macrophages are the activated tnfa+ macrophages, which have been shown to express a mixture of M1 and M2 markers, including heparin-binding epidermal growth factor (HB-EGF), a pro-regenerative factor [40]. These macrophages can act on spinal progenitor cells to promote neurogenesis and axon growth [41][42], both directly and indirectly, through other cell types and signals in the injury site. Downstream effectors of TNF signaling include the AP-1 complex, which is involved in promoting pro-regenerative responses in salamanders [11] and has been shown to be important for TNF-induced neurogenesis in mouse SVZ neurospheres [43]. Additionally, TNF signaling upregulates key neurogenic factors such as histone deacetylase 1 (HDAC1), which is involved in the reactivated developmental program for neurogenesis after injury [44].
TNF signaling appears to be regeneration-specific and not necessary for developmental neurogenesis [10]. This may be due to the fact that TNF is primarily produced by reactive macrophages, which are only present after injury. In addition, expression of tnfrsf1a, the receptor for TNF, is dispensable for developmental neurogenesis in unlesioned animals [45]. While TNF signaling shows promise as a potential therapeutic target for promoting spinal cord regeneration, simply enhancing extracellular Tnf signaling may negatively affect other aspects of spinal repair. Therefore, a better understanding of the intracellular signaling in spinal progenitor cells after injury is needed to develop more targeted approaches for spinal cord repair.
4. The TNF/Tnfrsf1a Mediated AP-1 Signaling Pathway Increases Hdac1 after Injury
The TNF-mediated signaling pathway has been shown to be involved in regeneration processes. For instance, in the case of fin regeneration in zebrafish, Tnfrsf1a-mediated sensitivity to exogenous TNF is necessary [46]. This demonstrates that the TNF/Tnfrsf1a signaling pathway plays a role in promoting regeneration in certain tissues. However, it has been reported that the TNF/Tnfrsf1a signaling pathway is not crucial for regeneration in the zebrafish retina [47]. This finding suggests that the role of the TNF/Tnfrsf1a signaling pathway in promoting regeneration might be tissue- or cell-specific, rather than universally applicable across all tissues and cells. Therefore, the TNF-mediated signaling pathway is involved in regeneration, and its role appears to be dependent on the specific tissue or cell type being considered. Further research is needed to better understand the underlying mechanisms and the varying roles of the TNF/Tnfrsf1a signaling pathway in different tissues and cells. Cavone et al. (2021) reported Tnfrsf1a-mediated sensitivity to exogenous TNF in spinal progenitor cells after SCI. The TNF/Tnfrsf1a signaling pathway clearly increased fin and spinal cord cell regeneration, but the retina was not regenerated [11]. Notably, soluble TNF exerts its biological functions by binding to special target cell surface receptors, which have been identified as Tnfrsf1a and Tnfrsf1b [48]. After TNF binding, Tnfrsf1a or Tnfrsf1b can be bound by TNFR-associated protein-2 and the serine/threonine kinase receptor-interacting protein, which mediates survival and proliferating signals through the transcription factor NFκB and activation of the c-jun N-terminus kinase, which in turn mediates new gene transcription via AP-1 [49][50]. Raivich et al. (2004) discovered that the AP-1 transcription factor c-Jun plays a crucial role in axonal growth in the injured CNS. The study also revealed that reduced expression of CD44, galanin, and α7β1 integrin, molecules involved in regeneration, suggests a mechanism for c-Jun-mediated axonal growth, highlighting c-Jun’s significance in axonal regeneration in the injured central nervous system (CNS) [51]. Cavone et al. (2021) found that Tnfrsf1a-mediated exogenous TNF and activation of AP-1 are able to increase regenerative neurogenesis after SCI [11]. It is known that neuronal progenitors lacking HDAC1 and HDAC2 are unable to differentiate into mature neurons and undergo cell death [52]. In zebrafish, HDAC1 represses Notch target gene expression during neurogenesis and favors the generation of motor neurons in response to hedgehog signaling [53] to promote retinal neurogenesis [54]. These findings suggest that the activity of HDAC1 can regulate neural differentiation. TNF-α from macrophages induces Tnfrsf1a-mediated AP-1 activity in progenitors to increase regeneration-promoting expression of HDAC1 and neurogenesis [11] (Figure 1). Overall, the TNF/Tnfrsf1a-mediated AP-1 signaling pathway increases HDAC1 expression and promotes regeneration in zebrafish after injury.
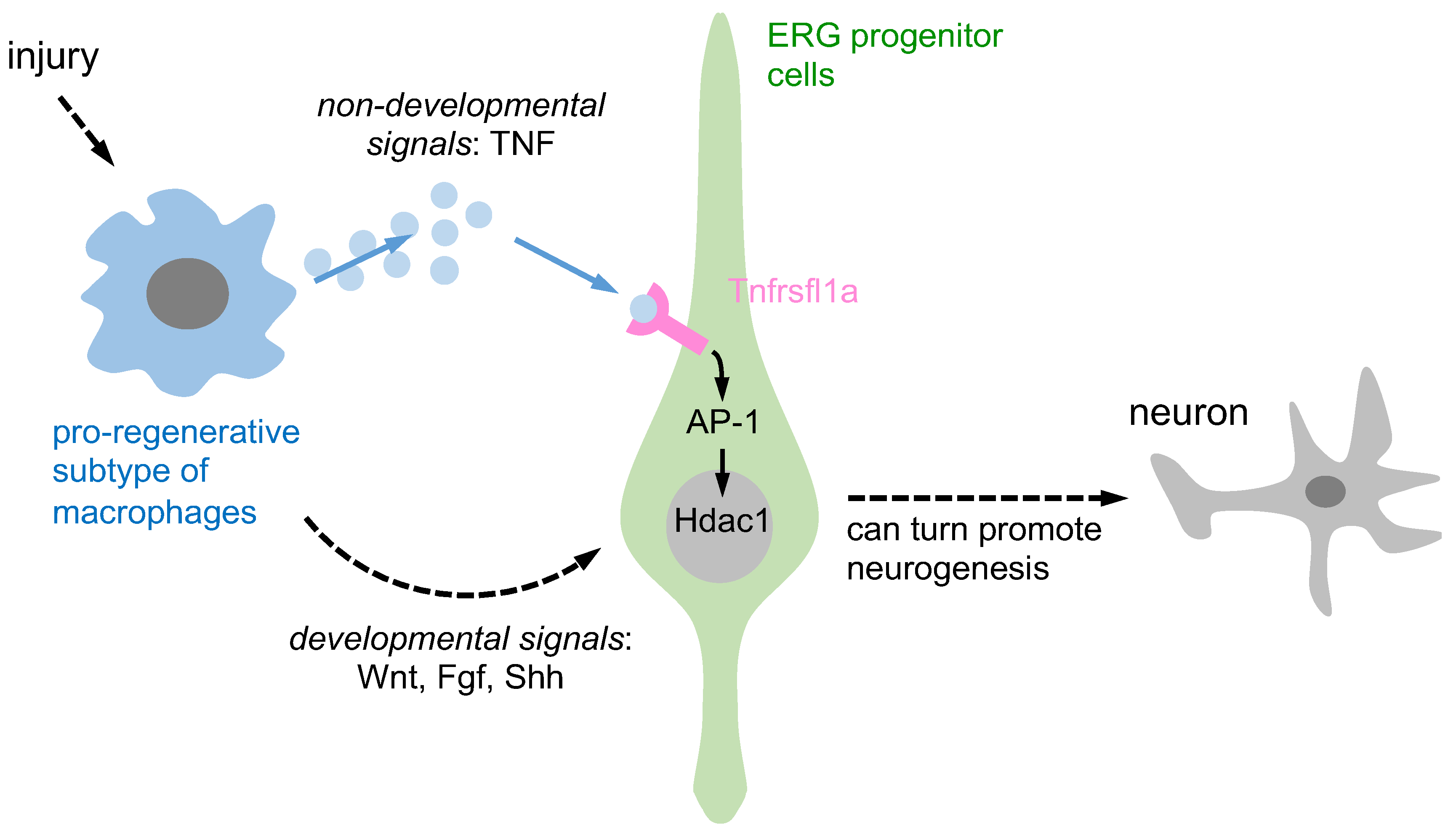
Figure 1. A schematic representation is provided to illustrate the activation of ERG progenitors by pro-regenerative macrophages, which in turn promote neurogenesis through two distinct signaling pathways. Pro-regenerative macrophages utilize two different signaling pathways to induce ependymo-radial glia (ERG) progenitors and promote regenerative neurogenesis. Firstly, through non-developmental signals, macrophages secrete TNF-α, which induces Tnfrsf1a-mediated AP-1 activity in ERG progenitors and promotes Hdac1 expression and neurogenesis. Secondly, through developmental signals, macrophage-secreted TNF-α can indirectly impact ERG progenitor cells by increasing developmental signals such as Wnt, Fgf, and Shh, promoting regenerative neurogenesis.
Past research has shown that macrophages can support regenerative neurogenesis through developmental and non-developmental signaling pathways. They contribute to axonal regeneration through developmental signals while pro-regenerative macrophages release TNF-α, which stimulates ERG progenitors to promote regenerative neurogenesis via Tnfrsf1a-mediated AP-1 activity and Hdac1 expression, representing non-developmental signals. However, the involvement of other neuroglial cell types in this process remains unclear.
This entry is adapted from the peer-reviewed paper 10.3390/ijms24076483
References
- Shechter, R.; Miller, O.; Yovel, G.; Rosenzweig, N.; London, A.; Ruckh, J.; Kim, K.; Klein, E.; Kalchenko, V.; Bendel, P.; et al. Recruitment of beneficial m2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity 2013, 38, 555–569.
- Gensel, J.; Nakamura, S.; Guan, Z.; van Rooijen, N.; Ankeny, D.; Popovich, P. Macrophages promote axon regeneration with concurrent neurotoxicity. J. Neurosci. 2009, 29, 3956–3968.
- Nguyen-Chi, M.; Laplace-Builhe, B.; Travnickova, J.; Luz-Crawford, P.; Tejedor, G.; Phan, Q.; Duroux-Richard, I.; Levraud, J.; Kissa, K.; Lutfalla, G.; et al. Identification of polarized macrophage subsets in zebrafish. eLife 2015, 4, e07288.
- Becker, T.; Becker, C.G. Regenerating descending axons preferentially reroute to the gray matter in the presence of a general macrophage/microglial reaction caudal to a spinal transection in adult zebrafish. J. Comp. Neurol. 2001, 433, 131–147.
- Ohnmacht, J.; Yang, Y.; Maurer, G.W.; Barreiro-Iglesias, A.; Tsarouchas, T.M.; Wehner, D.; Sieger, D.; Becker, C.G.; Becker, T. Spinal motor neurons are regenerated after mechanical lesion and genetic ablation in larval zebrafish. Development 2016, 143, 1464–1474.
- Hui, E.; Cheung, J.; Zhu, J.; Su, X.; Taylor, M.J.; Wallweber, H.A.; Sasmal, D.K.; Huang, J.; Kim, J.M.; Mellman, I.; et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 2017, 355, 1428–1433.
- Al-Gayyar, M.M.; Elsherbiny, N.M. Contribution of TNF-α to the development of retinal neurodegenerative disorders. Eur. Cytokine Netw. 2013, 24, 27–36.
- Wan, J.; Ramachandran, R.; Goldman, D. HB-EGF is necessary and sufficient for Müller glia dedifferentiation and retina regeneration. Dev. Cell 2012, 22, 334–347.
- Beldi, G.; Khosravi, M.; Abdelgawad, M.E.; Salomon, B.L.; Uzan, G.; Haouas, H.; Naserian, S. TNFα/TNFR2 signaling pathway: An active immune checkpoint for mesenchymal stem cell immunoregulatory function. Stem Cell Res. Ther. 2020, 11, 281.
- Tsarouchas, T.; Wehner, D.; Cavone, L.; Munir, T.; Keatinge, M.; Lambertus, M.; Underhill, A.; Barrett, T.; Kassapis, E.; Ogryzko, N.; et al. Dynamic control of proinflammatory cytokines Il-1β and Tnf-α by macrophages in zebrafish spinal cord regeneration. Nat. Commun. 2018, 9, 4670.
- Cavone, L.; McCann, T.; Drake, L.K.; Aguzzi, E.A.; Oprişoreanu, A.M.; Pedersen, E.; Sandi, S.; Selvarajah, J.; Tsarouchas, T.M.; Wehner, D.; et al. A unique macrophage subpopulation signals directly to progenitor cells to promote regenerative neurogenesis in the zebrafish spinal cord. Dev. Cell 2021, 56, 1617–1630.
- Briona, L.K.; Poulain, F.E.; Mosimann, C.; Dorsky, R.I. Wnt/ß-catenin signaling is required for radial glial neurogenesis following spinal cord injury. Dev. Biol. 2015, 403, 15–21.
- Goldshmit, Y.; Tang, J.K.K.; Siegel, A.L.; Nguyen, P.D.; Kaslin, J.; Currie, P.D.; Jusuf, P.R. Different Fgfs have distinct roles in regulating neurogenesis after spinal cord injury in zebrafish. Neural Dev. 2018, 13, 24.
- Huang, L.; Fu, C.; Xiong, F.; He, C.; Wei, Q. Stem cell therapy for spinal cord injury. Cell Transplant. 2021, 30, 963689721989266.
- Zeng, C.W.; Kamei, Y.; Shigenobu, S.; Sheu, J.C.; Tsai, H.J. Injury-induced Cavl-expressing cells at lesion rostral side play major roles in spinal cord regeneration. Open Biol. 2021, 11, 200304.
- Var, S.R.; Byrd-Jacobs, C.A. Role of macrophages and microglia in zebrafish regeneration. Int. J. Mol. Sci. 2020, 21, 4768.
- Kanazawa, M.; Ninomiya, I.; Hatakeyama, M.; Takahashi, T.; Shimohata, T. Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. Int. J. Mol. Sci. 2017, 18, 2135.
- Xiong, X.Y.; Liu, L.; Yang, Q.W. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog. Neurobiol. 2016, 142, 23–44.
- Ma, P.F.; Gao, C.C.; Yi, J.; Zhao, J.L.; Liang, S.Q.; Zhao, Y.; Ye, Y.-C.; Bai, J.; Zheng, Q.-J.; Dou, K.-F.; et al. Cytotherapy with M1-polarized macrophages ameliorates liver fibrosis by modulating immune microenvironment in mice. J. Hepatol. 2017, 67, 770–779.
- Sipe, G.O.; Lowery, R.L.; Tremblay, M.È.; Kelly, E.A.; Lamantia, C.E.; Majewska, A.K. Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat. Commun. 2016, 7, 10905.
- Lee, C.H.; Chun, T. Anti-inflammatory role of TAM family of receptor tyrosine kinases via modulating macrophage function. Mol. Cells 2019, 42, 1.
- Chang, F.; Wang, Y.; Liu, P.; Peng, J.; Han, G.H.; Ding, X.; Wei, S.; Gao, G.; Huang, K. Role of macrophages in peripheral nerve injury and repair. Neural Regen. Res. 2019, 14, 1335.
- Yu, Y.; Yue, Z.; Xu, M.; Zhang, M.; Shen, X.; Ma, Z.; Li, J.; Xie, X. Macrophages play a key role in tissue repair and regeneration. PeerJ 2022, 10, e14053.
- Petrie, T.A.; Strand, N.S.; Tsung-Yang, C.; Rabinowitz, J.S.; Moon, R.T. Macrophages modulate adult zebrafish tail fin regeneration. Development 2014, 141, 2581–2591.
- Majidinia, M.; Aghazadeh, J.; Jahanban-Esfahlani, R.; Yousefi, B. The roles of Wnt/β-catenin pathway in tissue development and regenerative medicine. J. Cell. Physiol. 2018, 2338, 5598–5612.
- Lucini, C.; D’Angelo, L.; Cacialli, P.; Palladino, A.; De Girolamo, P. BDNF, brain, and regeneration: Insights from zebrafish. Int. J. Mol. Sci. 2018, 19, 3155.
- Tayanloo-Beik, A.; Rabbani, Z.; Soveyzi, F.; Alavi-Moghadam, S.; Rezaei-Tavirani, M.; Goodarzi, P.; Arjmand, B.; Larijani, B. Cellular therapy for treatment of spinal cord injury in Zebrafish model. Mol. Biol. Rep. 2021, 48, 1787–1800.
- Li, R.; Li, D.H.; Zhang, H.Y.; Wang, J.; Li, X.K.; Xiao, J. Growth factors-based therapeutic strategies and their underlying signaling mechanisms for peripheral nerve regeneration. Acta Pharmacol. Sin. 2020, 41, 1289–1300.
- Tarnawski, A.S.; Ahluwalia, A. The critical role of growth factors in gastric ulcer healing: The cellular and molecular mechanisms and potential clinical implications. Cells 2021, 10, 1964.
- Adams, K.L.; Gallo, V. The diversity and disparity of the glial scar. Nat. Neurosci. 2018, 21, 9–15.
- Aurora, A.B.; Olson, E.N. Immune modulation of stem cells and regeneration. Cell Stem Cell 2014, 15, 14–25.
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156.
- Liddelow, S.A.; Barres, B.A. Not everything is scary about a glial scar. Nature 2016, 532, 182–183.
- Sabin, K.Z.; Echeverri, K. The role of the immune system during regeneration of the central nervous system. J. Immunol. Regen. Med. 2020, 7, 100023.
- Lyu, J.; Xie, D.; Bhatia, T.N.; Leak, R.K.; Hu, X.; Jiang, X. Microglial/Macrophage polarization and function in brain injury and repair after stroke. CNS Neurosci. Ther. 2021, 27, 515–527.
- Hunyara, J.L.; Kolodkin, A.L. Repurposing developmental mechanisms in the adult nervous system. Curr. Opin. Genet. Dev. 2020, 65, 14–21.
- Cruceriu, D.; Baldasici, O.; Balacescu, O.; Berindan-Neagoe, I. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: Molecular insights and therapeutic approaches. Cell. Oncol. 2020, 43, 1–18.
- Bosak, V.; Murata, K.; Bludau, O.; Brand, M. Role of the immune response in initiating central nervous system regeneration in vertebrates: Learning from the fish. Int. J. Dev. Biol. 2018, 62, 403–417.
- Yong, H.Y.; Rawji, K.S.; Ghorbani, S.; Xue, M.; Yong, V.W. The benefits of neuroinflammation for the repair of the injured central nervous system. Cell. Mol. Immunol. 2019, 16, 540–546.
- Wen, X.; Jiao, L.; Tan, H. MAPK/ERK pathway as a central regulator in vertebrate organ regeneration. Int. J. Mol. Sci. 2022, 23, 1464.
- Jin, M.; Opalek, J.M.; Marsh, C.B.; Wu, H.M. Proteome comparison of alveolar macrophages with monocytes reveals distinct protein characteristics. Am. J. Respir. Cell Mol. Biol. 2004, 31, 322–329.
- Amiel, A.R.; Tsai, S.L.; Wehner, D. Embracing the diversity of model systems to deconstruct the basis of regeneration and tissue repair. Development 2023, 150, dev201579.
- Bernardino, L.; Agasse, F.; Silva, B.; Ferreira, R.; Grade, S.; Malva, J.O. Tumor necrosis factor-α modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cells 2008, 26, 2361–2371.
- Xu, L.; Xing, Q.; Huang, T.; Zhou, J.; Liu, T.; Cui, Y.; Cheng, T.; Wang, Y.; Zhou, X.; Yang, B.; et al. derived mesenchymal stem cells in a mouse model of traumatic brain injury via PI3K/AKT pathway. Front. Cell. Neurosci. 2019, 12, 498.
- Belenguer, G.; Duart-Abadia, P.; Jordán-Pla, A.; Domingo-Muelas, A.; Blasco-Chamarro, L.; Ferrón, S.R.; Morante-Redolat, J.M.; Fariñas, I. Adult neural stem cells are alerted by systemic inflammation through TNF-α receptor signaling. Cell Stem Cell 2021, 28, 285–299.
- Nguyen-Chi, M.; Laplace-Builhé, B.; Travnickova, J.; Luz-Crawford, P.; Tejedor, G.; Lutfalla, G.; Kissa, K.; Jorgensen, C.; Djouad, F. TNF signaling and macrophages govern fin regeneration in zebrafish larvae. Cell Death Dis. 2017, 8, e2979.
- Lei, X.D.; Sun, Y.; Cai, S.J.; Fang, Y.W.; Cui, J.L.; Li, Y.H. Role of tumor necrosis factor-alpha in zebrafish retinal neurogenesis and myelination. Int. J. Ophthalmol. 2016, 9, 831–837.
- Himmler, A.; Maurer-Fogy, I.; Krönke, M.; Scheurich, P.; Pfizenmaier, K.; Lantz, M.; Olsson, I.; Hauptmann, R.; Stratowa, C.; Adolf, G. Molecular cloning and expression of human and rat tumor necrosis factor receptor chain (p60) and its soluble derivative, tumor necrosis factor-binding protein. DNA Cell Biol. 1990, 9, 705–715.
- Sakurai, H.; Sugita, T. C-Jun N-terminal kinase-mediated AP-1 activation in experimental glomerulonephritis in rats. IUBMB Life 1998, 45, 831–839.
- Choi, H.; Dikalova, A.; Stark, R.J.; Lamb, F.S. c-Jun N-terminal kinase attenuates TNFα signaling by reducing Nox1-dependent endosomal ROS production in vascular smooth muscle cells. Free Radic. Biol. Med. 2015, 86, 219–227.
- Raivich, G.; Bohatschek, M.; Da Costa, C.; Iwata, O.; Galiano, M.; Hristova, M.; Nateri, A.S.; Makwana, M.; Riera-Sans, L.; Wolfer, D.P.; et al. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron 2004, 43, 57–67.
- Montgomery, R.; Hsieh, J.; Barbosa, A.; Richardson, J.; Olson, E. Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development. Proc. Natl. Acad. Sci. USA 2009, 106, 7876–7881.
- Cunliffe, V.T. Histone deacetylase 1 is required to repress Notch target gene expression during zebrafish neurogenesis and to maintain the production of motoneurones in response to hedgehog signalling. Development 2004, 131, 2983–2995.
- Yamaguchi, M.; Tonou-Fujimori, N.; Komori, A.; Maeda, R.; Nojima, Y.; Li, H.; Okamoto, H.; Masai, I. Histone deacetylase 1 regulates retinal neurogenesis in zebrafish by suppressing Wnt and Notch signaling pathways. Development 2005, 132, 3027–3043.
 Encyclopedia
Encyclopedia
