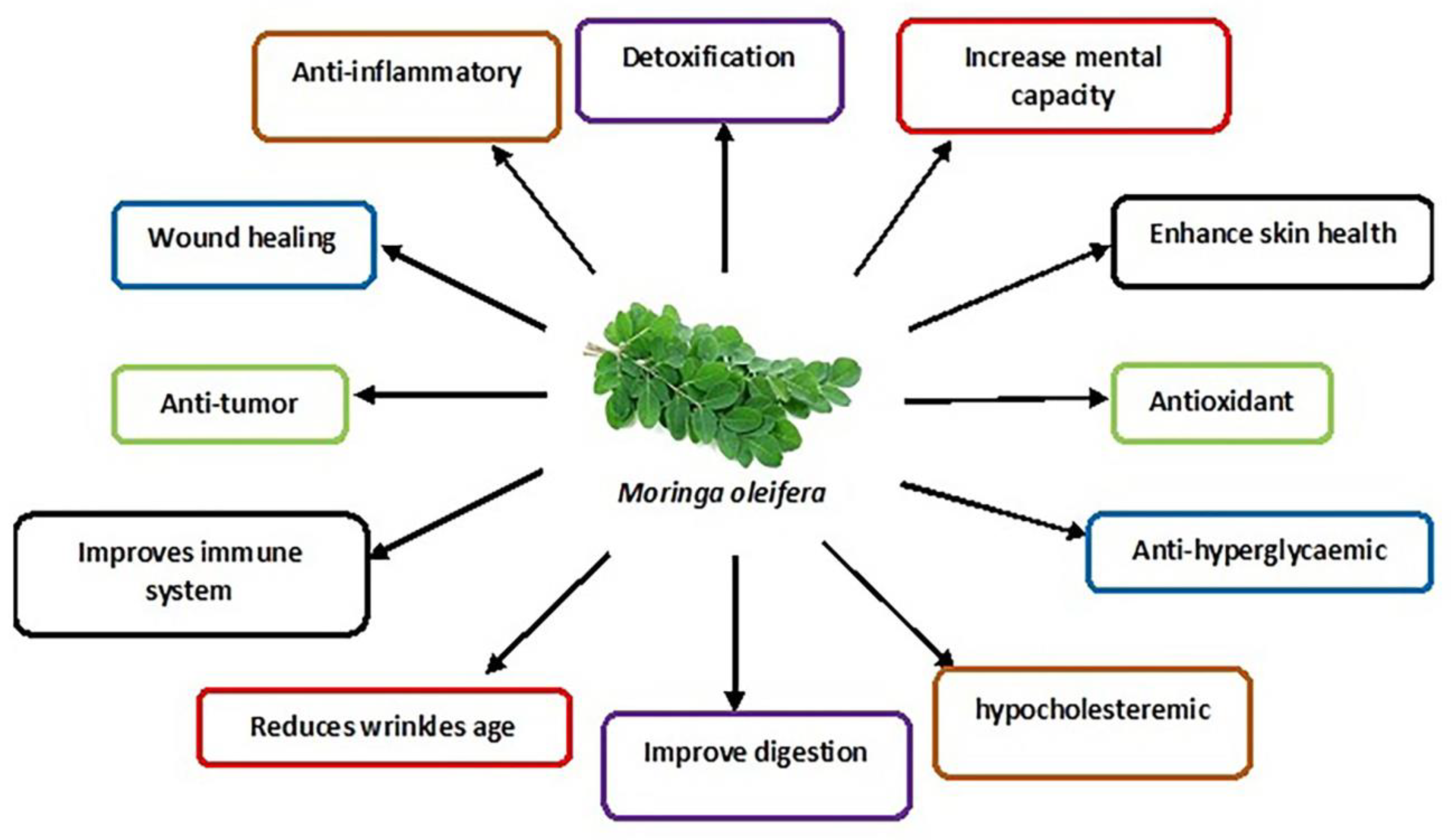
Moringa oleifera (drumstick tree, horseradish tree, ben-oil tree or kelor tree) is of the family ‘Moringaceae’. In Southern Asia and Middle East Africa, the tree is mainly cultivated for its various nutraceutical and medicinal properties contained in different parts of the tree, such as seeds, roots, flowers, pots and leaves. In vivo and in vitro studies have been conducted to determine the effects of M. oleifera leaves on the male reproductive system.
- Moringa oleifera
- male infertility
- oxidative stress
- reactive oxygen species
- testicular cells
- semen quality
1. Introduction
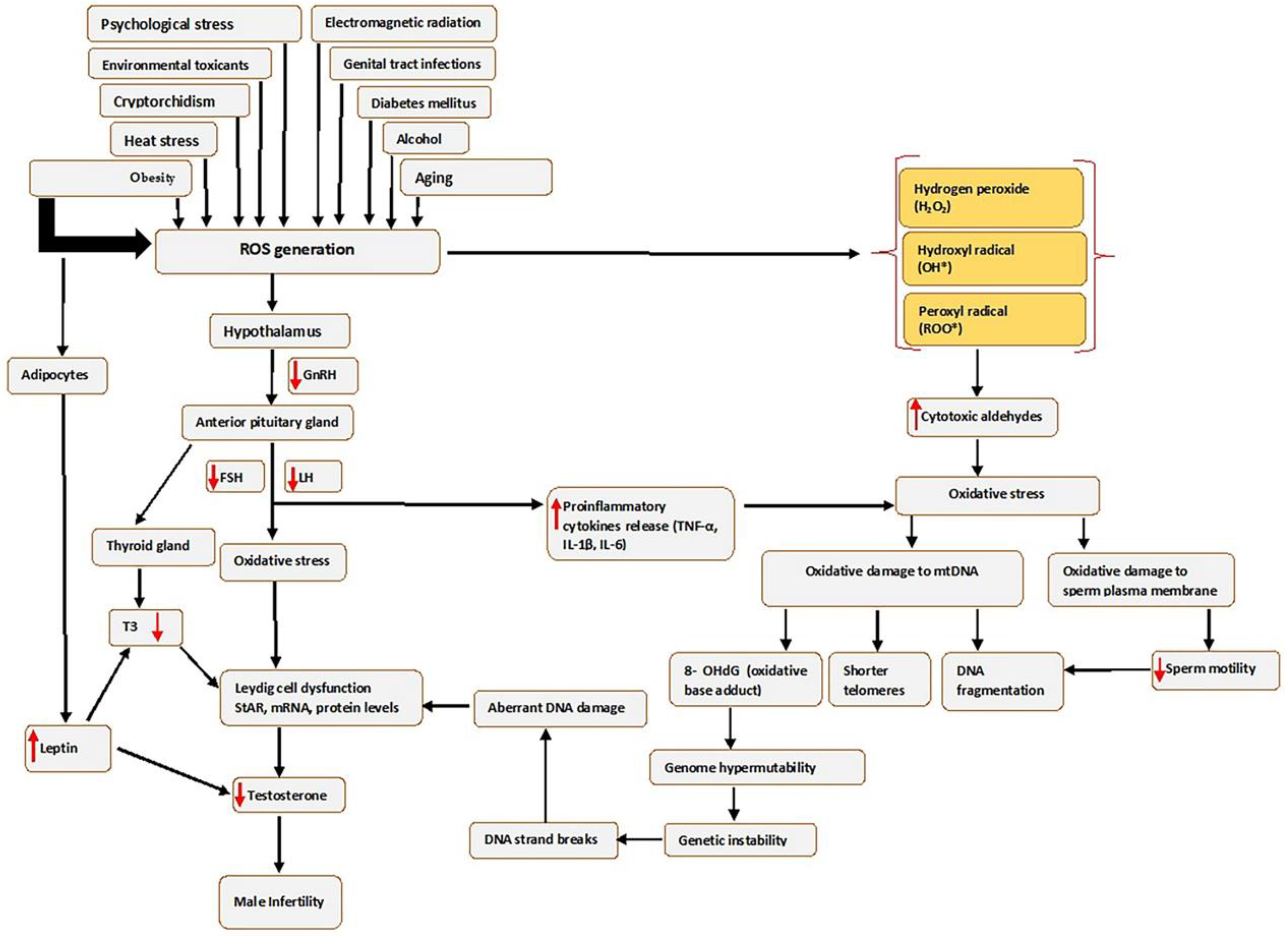
2. Male Infertility
3. Oxidative Stress
4. Moringa oleifera
5. Studies on the Effects of M. oleifera Leaf Extracts on Male Reproductive Function
5.1. Sperm Parameters
5.2. Hormonal Levels
5.3. Testis
5.4. Male Reproductive Cells: Leydig Cells and Sperm Cells
6. Effects of M. oleifera Leaf Extracts on Male Reproductive System Constituents following Exposure to Male Infertility Risk Factors
|
Male Infertility Risk Factors |
Oxidative Stress Parameters |
Hormonal Levels |
Sperm Parameters |
Gene Expression |
Testicular Histology Examination |
Authors |
|---|---|---|---|---|---|---|
|
Heat stress |
↑ TAC, ↓ GST |
↑ Testosterone levels |
↑ sperm quality (sperm concentration with intact acrosome, total sperm output, motility and viability). |
↑ the normal morphology and number of the tubular epithelial cells, germinal Sertoli cells, spermatogonia, spermatocytes, early spermatids, late spermatids and spermatozoa. ↓ Leydig cells and Sertoli cells pyknosis. |
||
|
Electromagnetic radiations |
↓ MDA, ↑ SOD and ↑ CAT |
↑ Serum testosterone levels |
↑ Epididymal sperm count and motility. ↓ sperm defects (pyriform head, detached head, coiled tails and multiple abnormalities). |
↓ degeneration in some parts of the seminiferous tubules and ↑ the number of Leydig cells. |
||
|
Environmental toxicants |
↓ testicular tissue GST activity and MDA. ↑ GPx. ↑ SOD, ↑ CAT |
↑ Testosterone. ↑ serum FSH and LH |
↑ sperm motility, ↑ sperm viability, ↑ sperm count and ↓ sperm abnormalities. |
↑ StAR gene, ↑ cytochrome p450o17 subfamily A (CYP17A), ↑ CYP11A1 and ↑ HSD17B3 genes of the steroidogenic hormones. ↓ expression of CYP19A1 aromatase gene. |
↓ weight of the reproductive organ. ↑ elongated spermatids and spermatozoa, ↑ the epididymal histological integrity, ↑ sperm density and ↓ congestion and interstitial oedema in the seminal vesicle and prostate gland. |
|
|
Obesity |
↓ MDA, ↑ SOD, ↑ CAT and ↑ GSH |
↑ Testosterone, ↑ FSH and ↑ LH |
↑ sperm count, ↑ sperm motility, ↓ immotile spermatozoa, ↓ primary and secondary sperm abnormalities. |
|||
|
Diabetes |
↓ TBARS, ↑ SOD, ↑ CAT, ↑ GSH and ↑ Ascorbic acid |
↑ LH, ↑ FSH and ↑ testosterone |
↑ sperm count and ↑ sperm mobility. |
↑ mean number of spermatogonia in the seminiferous tubules, ↑ population of the round (normal) spermatids. ↑ diameter of the seminiferous tubules, ↑ nuclear diameter of the Leydig cells and ↑ weight of the epididymis. |
||
|
Therapy and medications (HAART) |
↑ FSH, ↑ LH and ↑ testosterone |
↓ the sperms with abnormal morphology, ↑ semen quality (sperm progressivity, sperm volume, sperm motility, sperm count and viability). |
↑ testicular weight. ↑ normal testicular morphology. |
[76] |
||
|
Alcohol |
↑ myoid living cells, spermatogenic living cells, spermatogonia, spermatocytes, spermatids and spermatozoa and lumen filled with semen. ↓ Reduced Leydig cells disruption |
[77] |
||||
|
Psychological stress |
↓ PDE-5 activity, ↑ testosterone and ↓ corticosterone |
↑ interstitial Leydig cells and ↑ spermatozoa in the seminiferous tubule lumen. |
[78] |
|||
|
Aging |
↑ sperm count and ↑ normal sperm morphology. |
[79] |
||||
|
Cryptorchidism |
↓ GGT activity, ↑ SOD activity and ↓ MDA |
↑ testicular testosterone |
↑ sperm count, ↑ germ cell count. |
↑ testicular weight, ↓ the abnormal appearance of the testes. ↓ abnormal appearance of the seminiferous epithelium. |
||
|
Food |
↑ sperm motility. |
[82] |
↑ = Increase; ↓ = Decrease. Abbreviations: CAT, catalase; GST, glutathione -S-transferase; GGT, gamma-glutamyl transferase; HAART, highly active antiretroviral therapy; FSH, follicle stimulating hormone; GSH, glutathione; GPx, glutathione peroxidase; LH, luteinizing hormone; MDA, malondialdehyde; PDE-5, Phosphodiesterase-5; SOD, superoxide dismutase; StAR, steroidogenic acute regulatory protein; TAC, total antioxidant capacity; TBARS, thiobarbituric acid reactive substances.
7. Mechanism of Action of M. oleifera Extract on Oxidative Stress and Male Fertility
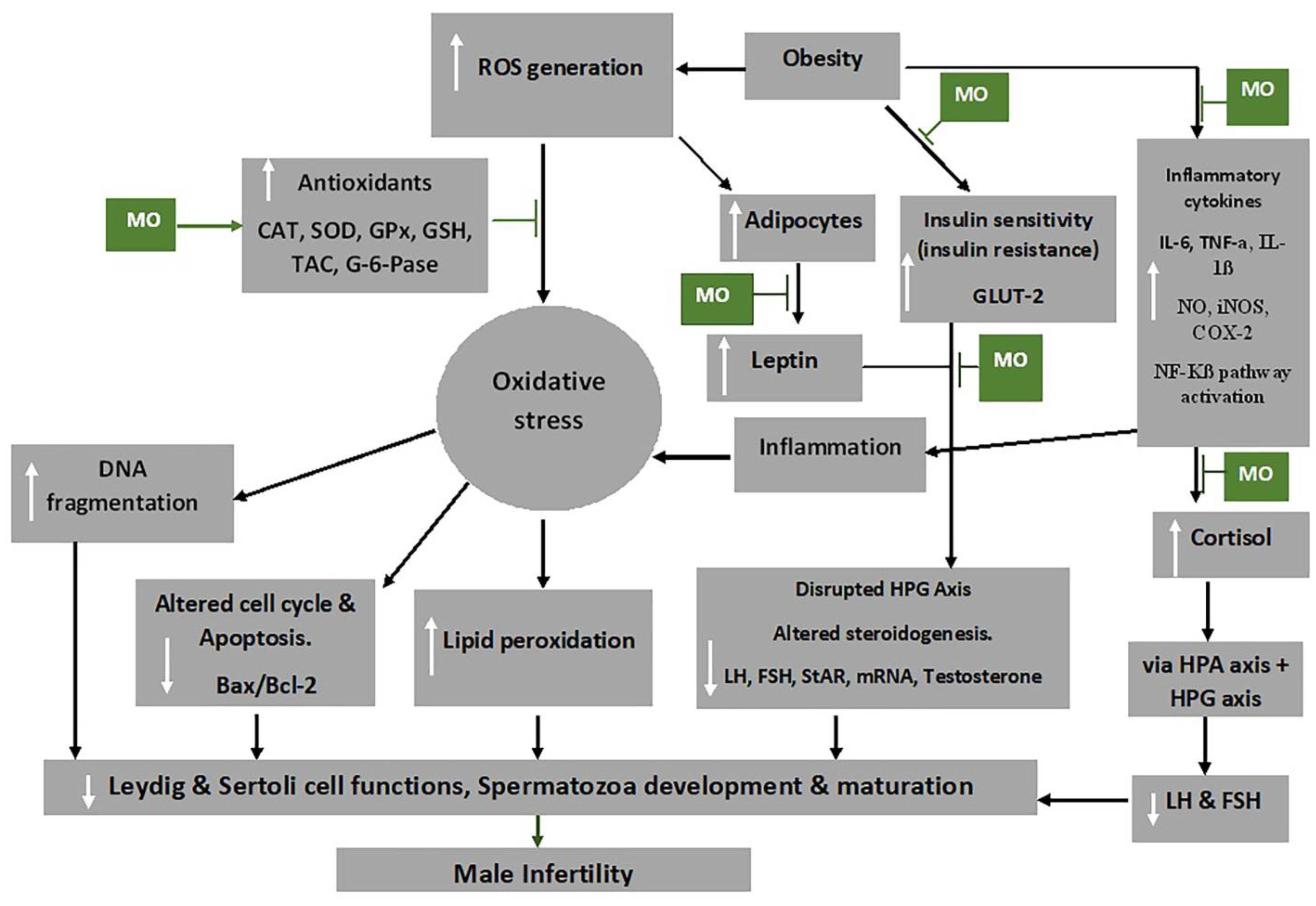
 Indicated the regulatory/inhibitory role of Moringa oleifera extract.
Indicated the regulatory/inhibitory role of Moringa oleifera extract.This entry is adapted from the peer-reviewed paper 10.3390/app13074387
References
- Alahmar, A.T. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18.
- Öztekin, Ü.; Caniklioğlu, M.; Sarı, S.; Selmi, V.; Gürel, A.; Işıkay, L. Evaluation of Male Infertility Prevalence with Clinical Outcomes in Middle Anatolian Region. Cureus 2019, 11, e5122.
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37.
- Deka, P.K.; Sarma, S. Psychological aspects of infertility. Br. J. Med. Pract. 2010, 3, 336.
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.-L.; Henkel, R.; Vij, S.; Arafa, M.; Selvam, M.K.P.; Shah, R. Male infertility. Lancet 2021, 397, 319–333.
- Sharma, A. Male Infertility; Evidences, Risk Factors, Causes, Diagnosis and Management in Human. Ann. Clin. Lab. Res. 2017, 5, 188.
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Sengupta, P.; Durairajanayagam, D.; Henkel, R.; Sadeghi, M.R. Reactive oxygen species and male reproductive hormones. Reprod. Biol. Endocrinol. 2018, 16, 87.
- Abdillahi, H.; Van Staden, J. South African plants and male reproductive healthcare: Conception and contraception. J. Ethnopharmacol. 2012, 143, 475–480.
- Nwajiaku, L.A.; Mbachu, I.I.; Ikeako, L. Prevalence, Clinical Pattern and Major Causes of Male Infertility in Nnewi, South East Nigeria: A Five Year Review. Afrimedic J. 2012, 3, 16–19.
- Retzler, K. Erectile Dysfunction: A Review of Comprehensive Treatment Options for Optimal Outcome. J. Restor. Med. 2019, 8, e20190104.
- Kumar, P.; Kumar, N.; Thakur, D.S.; Patidar, A. Male hypogonadism: Symptoms and treatment. J. Adv. Pharm. Technol. Res. 2010, 1, 297.
- Khodamoradi, K.; Khosravizadeh, Z.; Parmar, M.; Kuchakulla, M.; Ramasamy, R.; Arora, H. Exogenous testosterone replacement therapy versus raising endogenous testosterone levels: Current and future prospects. F&S Rev. 2021, 2, 32–42.
- Opuwari, C.S.; Moundipa, P.F. Herbal medicine used to treat andrological problems: Africa. In Herbal Medicine in Andrology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 83–91.
- Syarifuddin, N.; Toleng, A.; Rahardja, D.; Ismartoyo, I.; Yusuf, M. Improving Libido and Sperm Quality of Bali Bulls by Supplementation of Moringa oleifera Leaves. Media Peternak. 2017, 40, 88–93.
- Eze, U.A.; Okonofua, F.E. High Prevalence of Male Infertility in Africa: Are Mycotoxins to Blame? Afr. J. Reprod. Health 2015, 19, 9–17.
- Zarrabi, A.D.; Kruger, T.F. The challenges of supporting male infertility treatment in South Africa. Nat. Rev. Urol. 2018, 15, 719–720.
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763.
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990.
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19.
- Li, R.; Jia, Z.; Trush, M.A. Defining ROS in biology and medicine. React. Oxyg. Species Apex NC 2016, 1, 9–21.
- Bouchez, C.; Devin, A. Mitochondrial Biogenesis and Mitochondrial Reactive Oxygen Species (ROS): A Complex Relationship Regulated by the cAMP/PKA Signaling Pathway. Cells 2019, 8, 287.
- Beattie, M.C.; Chen, H.; Fan, J.; Papadopoulos, V.; Miller, P.; Zirkin, B.R. Aging and Luteinizing Hormone Effects on Reactive Oxygen Species Production and DNA Damage in Rat Leydig Cells1. Biol. Reprod. 2013, 88, 100.
- Wang, Y.; Chen, F.; Ye, L.; Zirkin, B.; Chen, H. Steroidogenesis in Leydig cells: Effects of aging and environmental factors. Reproduction 2017, 154, R111–R122.
- Baskaran, S.; Finelli, R.; Agarwal, A.; Henkel, R. Reactive oxygen species in male reproduction: A boon or a bane? Andrologia 2020, 53, e13577.
- Agarwal, A.; Durairajanayagam, D.; Du Plessis, S.S. Utility of antioxidants during assisted reproductive techniques: An evidence based review. Reprod. Biol. Endocrinol. 2014, 12, 112.
- Aitken, R.J.; Baker, M.A. Oxidative stress, sperm survival and fertility control. Mol. Cell. Endocrinol. 2006, 250, 66–69.
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293.
- Spiers, J.G.; Chen, H.-J.C.; Sernia, C.; Lavidis, N.A. Activation of the hypothalamic-pituitary-adrenal stress axis induces cellular oxidative stress. Front. Neurosci. 2015, 8, 456.
- Greifová, H.; Jambor, T.; Tokárová, K.; Speváková, I.; Knížatová, N.; Lukáč, N. Resveratrol attenuates hydrogen peroxide-induced oxidative stress in TM3 Leydig cells in vitro. J. Environ. Sci. Health Part A 2020, 55, 585–595.
- Leisegang, K.; Henkel, R. The in vitro modulation of steroidogenesis by inflammatory cytokines and insulin in TM3 Leydig cells. Reprod. Biol. Endocrinol. 2018, 16, 26.
- Tugaeva, K.V.; Sluchanko, N.N. Steroidogenic acute regulatory protein: Structure, functioning, and regulation. Biochem. Mosc. 2019, 84, 233–253.
- Ahima, R.S. Revisiting leptin’s role in obesity and weight loss. J. Clin. Investig. 2008, 118, 2380–2383.
- Caprio, M.; Isidori, A.M.; Carta, A.R.; Moretti, C.; Dufau, M.L.; Fabbri, A. Expression of Functional Leptin Receptors in Rodent Leydig Cells1. Endocrinology 1999, 140, 4939–4947.
- Agarwal, A.; Dutta, S. Obesity. In Male Infertility; Parekattil, S.J., Esteves, S.C., Agarwal, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 497–508.
- Panner Selvam, M.K.; Agarwal, A.; Henkel, R.; Finelli, R.; Robert, K.A.; Iovine, C.; Baskaran, S. The effect of oxidative and reductive stress on semen parameters and functions of physiologically normal human spermatozoa. Free Radic. Biol. Med. 2020, 152, 375–385.
- Clark, B.J.; Stocco, D.M. The steroidogenic acute regulatory protein (StAR). In Cholesterol Transporters of the START Domain Protein Family in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2014; pp. 15–47.
- Takeshima, T.; Kuroda, S.; Yumura, Y. Reactive Oxygen Species and Sperm Cells. In Reactive Oxygen Species (ROS) in Living Cells; Filip, C., Albu, E., Eds.; InTech: London, UK, 2018.
- Kurkowska, W.; Bogacz, A.; Janiszewska, M.; Gabryś, E.; Tiszler, M.; Bellanti, F.; Kasperczyk, S.; Machoń-Grecka, A.; Dobrakowski, M.; Kasperczyk, A. Oxidative Stress is Associated with Reduced Sperm Motility in Normal Semen. Am. J. Men’s Health 2020, 14, 1557988320939731.
- Pujianto, D.A.; Oktarina, M.; Sharaswati, I.A.S.; Yulhasri, Y. Hydrogen peroxide has adverse effects on human sperm quality parameters, induces apoptosis, and reduces survival. J. Hum. Reprod. Sci. 2021, 14, 121–128.
- Jr, S.T.P.; Gummow, B.; Parker, A.J.; Paris, D.B.B.P. Antioxidant supplementation mitigates DNA damage in boar (Sus scrofa domesticus) spermatozoa induced by tropical summer. PLoS ONE 2019, 14, e0216143.
- Juan, C.; de la Lastra, J.P.; Plou, F.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642.
- Abd, H.H.; Ahmed, H.A.; Mutar, T.F. Moringa oleifera leaves extract modulates toxicity, sperms alterations, oxidative stress, and testicular damage induced by tramadol in male rats. Toxicol. Res. 2020, 9, 101–106.
- Ishola, I.O.; Yemitan, K.O.; Afolayan, O.O.; Anunobi, C.C.; Durojaiye, T.E. Potential of Moringa oleifera in the Treatment of Benign Prostate Hyperplasia: Role of Antioxidant Defence Systems. Med. Princ. Pract. 2018, 27, 15–22.
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive Components in Moringa oleifera Leaves Protect against Chronic Disease. Antioxidants 2017, 6, 91.
- Sun, M.C.; Ruhomally, Z.B.; Boojhawon, R.; Neergheen-Bhujun, V.S. Consumption of Moringa oleifera Lam Leaves Lowers Postprandial Blood Pressure. J. Am. Coll. Nutr. 2020, 39, 54–62.
- Susanto, H.; Taufiq, A.; Sunaryono, S.; Soontaranon, S.; Hariyanto, Y.A.; Mawardi, A.I.; Adreyanto, N.G.; Yunisa, D.T.; Rufiandita, F.; Nizarghazi, F.; et al. Moringa oleifera Leaf Powder Madura Variety: Characterization and Biomaterials Property for Biomedical and Nanotechnology Application. J. Phys. Conf. Ser. 2018, 1093, 012007.
- Manisha, N.; Rajak, R.; Jat, D. Oxidative stress and antioxidants: An overview. Int. J. Adv. Res. Rev. 2017, 2, 110–119.
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 2016, 5, 49–56.
- El-Sheikh, S.; Khairy, M.; Fadil, H.A.; Abo-Elmaaty, A. Ameliorative Effect of Moringa oleifera Extract on Male Fertility in Paroxetine Treated Rats. Zagazig Vet. J. 2016, 44, 244–250.
- Mohamed, M.A.; Ahmed, M.A.; El Sayed, R.A. Molecular effects of Moringa leaf extract on insulin resistance and reproductive function in hyperinsulinemic male rats. J. Diabetes Metab. Disord. 2019, 18, 487–494.
- Sharmila; Prabsattroo, T.; Wattanathorn, J.; Iamsa-Ard, S.; Muchimapura, S.; Thukhammee, W. Moringa oleifera Leaves Extract Attenuates Male Sexual Dysfunction. Am. J. Neurosci. 2012, 3, 17–24.
- Wafa, W.M.; El-Nagar, H.A.; Gabr, A.A.; Rezk, M.M. Impact of Dietary Moringa oleifera Leaves Supplementation on Semen Characteristics, Oxidative Stress, Physiological Response and Blood Parameters of Heat Stressed Buffalo Bulls. J. Anim. Poult. Prod. 2017, 8, 367–379.
- Zeng, B.; Luo, J.; Wang, P.; Yang, L.; Chen, T.; Sun, J.; Xie, M.; Li, M.; Zhang, H.; He, J.; et al. The beneficial effects of Moringa oleifera leaf on reproductive performance in mice. Food Sci. Nutr. 2019, 7, 738–746.
- Khalifa, W.H.; Ibrahim, F.M.; El Makawy, A.I.; Sharaf, H.A.; Khalil, W.K.B.; Maghraby, N.A. Safety And Fertility Enhancing Role Of Moringa Oleifera Leaves Aqueous Extract In New Zealand Rabbit Bucks. Int. J. Pharm. 2016, 6, 156–168.
- Ajuogu, P.K.; Mgbere, O.O.; Bila, D.S.; McFarlane, J.R. Hormonal changes, semen quality and variance in reproductive activity outcomes of post pubertal rabbits fed Moringa oleifera Lam. leaf powder. J. Ethnopharmacol. 2018, 233, 80–86.
- Laoung-On, J.; Saenphet, K.; Jaikang, C.; Sudwan, P. Effect of Moringa oleifera Lam. Leaf Tea on Sexual Behavior and Reproductive Function in Male Rats. Plants 2021, 10, 2019.
- Cajuday, L.A.; Pocsidio, G.L. Effects of Moringa oleifera Lam. (Moringaceae) on the reproduction of male mice (Mus musculus). J. Med. Plants Res. 2010, 4, 1115–1121.
- Opuwari, C.S.; Matshipi, M.N.; Phaahla, M.K.; Setumo, M.A.; Moraswi, R.T.; Zitha, A.A.; Offor, U.; Choma, S.S.R. Androgenic effect of aqueous leaf extract of Moringa oleifera on Leydig TM3 cells in vitro. Andrologia 2020, 52, e13825.
- Moichela, F.T.; Adefolaju, G.A.; Henkel, R.R.; Opuwari, C.S. Aqueous leaf extract of Moringa oleifera reduced intracellular ROS production, DNA fragmentation and acrosome reaction in Human spermatozoa in vitro. Andrologia 2021, 53, e13903.
- El-Desoky, N.; Hashem, N.; Elkomy, A.; Abo-Elezz, Z. Physiological response and semen quality of rabbit bucks supplemented with Moringa leaves ethanolic extract during summer season. Animal 2017, 11, 1549–1557.
- Ewuola, E.O.; Adeyemi, A.A.; Adeyinka, A.D.; Akabuike, C.F. Potential of Moringa oleifera leaf meal in improving reproductive efficiency of rabbit bucks in hot climate. Niger. J. Anim. Sci. 2019, 21, 80–86.
- Hanafi, A.; Fadholly, A.; Utomo, B.; Sudjarwo, S.A.; Yunus, M.; Hariadi, M.; Legowo, D. Effects of Moringa oleifera L. Extract on leydig and sertoli cells induced high Temperature on Rattus norvegicus. Res. J. Pharm. Technol. 2020, 13, 3361–3364.
- Hidayat, N.; Utomo, B.; Budiarto, R.K.; Legowo, D.; Safitri, E. Effect of grant leaf extract (Moringa oleifera lam) on histopathological featureof white rat (Rattus Norvegicus) testis exposed hot temperature. Pollut. Res. 2020, 39, 1251–1255.
- Bin-Meferij, M.M.; El-Kott, A.F. The radioprotective effects of Moringa oleifera against mobile phone electromagnetic radiation-induced infertility in rats. Int. J. Clin. Exp. Med. 2015, 8, 12487–12497.
- Ramalingam, S.; Suriyakumari, K.V.P.; Philip, X.C. The Effect of Ethanolic Extract of Moringa oleifera Leaves on 4 G-Cell Phone-EMR-Induced Oxidative Stresses Associated with Altered Sperm Count in Pre-Pubertal Wistar Rats. Ann. Rom. Soc. Cell Biol. 2021, 25, 3226–3239.
- Salama, A.A.; Elsaeid, A.A.; Awad, O.M. Effect of Moringa oleifera leaves extract against electromagnetic field impairments on hemoglobin and testes of rat. J. Biosci. Appl. Res. 2020, 6, 132–141.
- Akunna, G.G.; Ogunmodede, O.S.; Saalu, C.L.; Ogunlade, B.; Bello, A.J.; Salawu, E.O. Ameliorative effect of Moringa oleifera (drumstick) leaf extracts on chromium-induced testicular toxicity in rat testes. World J. Life Sci. Med. Res. 2012, 2, 20.
- Elblehi, S.S.; El Euony, O.I.; El-Nahas, A.F. Partial ameliorative effect of Moringa leaf ethanolic extract on the reproductive toxicity and the expression of steroidogenic genes induced by subchronic cadmium in male rats. Environ. Sci. Pollut. Res. 2019, 26, 23306–23318.
- Owolabi, J.O.; Ghazal, O.K.; Williams, F.E.; Ayodele, E.O. Effects of Moringa oleifera (Drumstick) Leaf Extracts on Lead-Induced Testicular Toxicity in Adult Wistar Rat (Rattus Novergicus). Int. J. Biotech. Biomed. Res. 2012, 2, 4003–4009.
- Mansour, M.; Arisha, A.; Algamal, M.; Elsayed, A.; Saad, S.; El Bohi, K. Effect of Moringa oleifera Leaves Extract -SeNPs Conjugate Administration on Testicular Toxicity Induced by Melamine in Rats. 2020. Available online: https://www.semanticscholar.org/paper/Effect-of-Moringa-oleifera-Leaves-Extract-SeNPs-on-Mansour-Arisha/57e9bb5ffdf6942389c0af4f71a127e1df9758d8 (accessed on 18 August 2022).
- Ododo, A.; Ojeka, S.O.; Dapper, V.D. Ameliorative Effect of Aqueous Leaf Extract of Moringa oleifera on Reproductive Function Following Cadmium Chloride Induced Oxidative Stress in Male Wistar Rats. Not. Sci. Biol. 2019, 11, 352–357.
- Alkafafy, M.E.; Sayed, S.M.; El-Shehawi, A.M.; El-Shazly, S.; Farouk, S.; Alotaibi, S.S.; Madkour, D.A.; Orabi, S.H.; Elbaz, H.T.; Ahmed, M.M. Moringa oleifera ethanolic extract ameliorates the testicular dysfunction resulted from HFD-induced obesity rat model. Andrologia 2021, 53, e14126.
- Juan, C.A. Moringa protein drink increases testosterone and anabolic status of men with hyperlipidemia: A randomized controlled study. Turk. J. Kinesiol. 2021, 7, 1–15.
- Jangir, R.N.; Jain, G.C. Ameliorative Effect of Moringa oleifera Lam. Leaves Extract on the Sex Hormone Profile and Testicular Dysfunctions in Streptozotocin-induced Diabetic Wistar Rats. Pharmacogn. Res. 2022, 14, 225–232.
- Priyadarshani, N.; Varma, M.C. Effect of Moringa oleifera leaf powder on sperm count, histology of testis and epididymis of hyperglycaemic mice Mus musculus. Am. Int. J. Res. Form. Appl. Nat. Sci. 2014, 7, 7–13.
- Ogunlade, B.; Jeje, S.O.; Adelakun, S.A.; Akingbade, G.T. Moringa oleifera restored semen quality, hormonal profile, and testicular morphology against Highly Active Antiretroviral Therapy- induced toxicity in adult male Wistar rats. JBRA Assist. Reprod. 2022, 26, 3.
- Bassey, R.B.; Bala, D.N.; Edagha, I.A.; Peter, A.I. The effect of ethanolic extract of Moringa oleifera on alcohol-induced testicular histopathologies in pre-pubertal albino Wistar rats. Biol. Med. 2013, 5, 40.
- Prabsattroo, T.; Wattanathorn, J.; Iamsaard, S.; Somsapt, P.; Sritragool, O.; Thukhummee, W.; Muchimapura, S. Moringa oleifera extract enhances sexual performance in stressed rats. J. Zhejiang Univ. B 2015, 16, 179–190.
- Widiastini, L.P.; Karuniadi, I.G.A.M.; Tangkas, M. Ethanol Extract of Moringa oleifera Increased the Number of Spermatozoa and Improved Sperm Morphology of Old Rattus norvegicus. J. Bioteknol. Biosains Indones. JBBI 2022, 9, 11–19.
- Afolabi, A.; Aderoju, H.; Alagbonsi, A. Effects of methanolic extract of Moringa oleifera leave on semen and biochemical parameters in cryptorchid rats. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 230–235.
- Afolabi, A.O.; Olotu, O.O.; Alagbonsi, I.A. Vitamins E and C Alleviate the Germ Cell Loss and Oxidative Stress in Cryptorchidism When Administered Separately but Not When Combined in Rats. ISRN Pharmacol. 2012, 2012, 1–8.
- Nugraha, I.S.; Wibisono, D.S.; Saraswati, I.; Juniarto, A.Z. The Effect of Moringa Leaf Extract (Moringa oleifera L) against Motility of Spermatozoa Mice Exposed to Monosodium Glutamate. Indones. J. Urol. 2022, 29, 41–46.
- Kilany, O.E.; Abdelrazek, H.M.; Aldayel, T.S.; Abdo, S.; Mahmoud, M. Anti-obesity potential of Moringa olifera seed extract and lycopene on high fat diet induced obesity in male Sprauge Dawely rats. Saudi J. Biol. Sci. 2020, 27, 2733–2746.
- Vargas-Sánchez, K.; Garay-Jaramillo, E.; González-Reyes, R.E. Effects of Moringa oleifera on Glycaemia and Insulin Levels: A Review of Animal and Human Studies. Nutrients 2019, 11, 2907.
- Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Dhakarey, R.; Upadhyay, G.; Singh, H.B. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem. Toxicol. 2009, 47, 1109–1116.
- Widiastini, L.P.; Karuniadi, I.A.M.; Karmaya, I.N.M.; Widhiantara, I.G. The potential of a Balinese traditional medicine kelor leaves (Moringa oleifera) for male infertility treatment: A mini review. Indian J. Forensic Med. Toxicol. 2022, 16, 734–742.
- Ojeda, S.R.; Lomniczi, A.; Mastronardi, C.; Heger, S.; Roth, C.; Parent, A.-S.; Matagne, V.; Mungenast, A.E. Minireview: The Neuroendocrine Regulation of Puberty: Is the Time Ripe for a Systems Biology Approach? Endocrinology 2006, 147, 1166–1174.
- Leisegang, K.; Sengupta, P.; Agarwal, A.; Henkel, R. Obesity and male infertility: Mechanisms and management. Andrologia 2020, 53, e13617.
- Metwally, F.M.; Rashad, H.M.; Ahmed, H.H.; Mahmoud, A.A.; Raouf, E.R.A.; Abdalla, A.M. Molecular mechanisms of the anti-obesity potential effect of Moringa oleifera in the experimental model. Asian Pac. J. Trop. Biomed. 2017, 7, 214–221.
- Ceriello, A.; Motz, E. Is Oxidative Stress the Pathogenic Mechanism Underlying Insulin Resistance, Diabetes, and Cardiovascular Disease? The Common Soil Hypothesis Revisited. Arter. Thromb. Vasc. Biol. 2004, 24, 816–823.
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.-H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 366–377.
- Adisakwattana, S.; Chanathong, B. Alpha-glucosidase inhibitory activity and lipid-lowering mechanisms of Moringa oleifera leaf extract. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 803–808.
- Kou, X.; Qi, S.; Dai, W.; Luo, L.; Yin, Z. Arctigenin inhibits lipopolysaccharide-induced iNOS expression in RAW264.7 cells through suppressing JAK-STAT signal pathway. Int. Immunopharmacol. 2011, 11, 1095–1102.
- Kooltheat, N.; Sranujit, R.P.; Chumark, P.; Potup, P.; Laytragoon-Lewin, N.; Usuwanthim, K. An Ethyl Acetate Fraction of Moringa oleifera Lam. Inhibits Human Macrophage Cytokine Production Induced by Cigarette Smoke. Nutrients 2014, 6, 697–710.
- Aschbacher, K.; Rodriguez-Fernandez, M.; Van Wietmarschen, H.; Tomiyama, A.J.; Jain, S.; Epel, E.; Doyle, F.J.; Van Der Greef, J. The hypothalamic–pituitary–adrenal–leptin axis and metabolic health: A systems approach to resilience, robustness and control. Interface Focus 2014, 4, 20140020.
- Aschbacher, K.; O’donovan, A.; Wolkowitz, O.M.; Dhabhar, F.S.; Su, Y.; Epel, E. Good stress, bad stress and oxidative stress: Insights from anticipatory cortisol reactivity. Psychoneuroendocrinology 2013, 38, 1698–1708.
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020, 77, 93–113.
- Makker, K.; Agarwal, A.; Sharma, R. Oxidative stress & male infertility. Indian J. Med. Res. 2009, 129, 357–367.
- Jung, I.L. Soluble Extract from Moringa oleifera Leaves with a New Anticancer Activity. PLoS ONE 2014, 9, e95492.
- Kou, X.; Li, B.; Olayanju, J.B.; Drake, J.M.; Chen, N. Nutraceutical or Pharmacological Potential of Moringa oleifera Lam. Nutrients 2018, 10, 343.
- Orrenius, S.; Nicotera, P.; Zhivotovsky, B. Cell Death Mechanisms and Their Implications in Toxicology. Toxicol. Sci. Off. J. Soc. Toxicol. 2011, 119, 3–19.
