A severe and well-known threat to the environment, the non-biodegradability of plastics obliges different stakeholders to find legislative and technical solutions for producing valuable polymers which are biodegradable and also exhibit better characteristics for packaging products. Microorganisms are recognized as exciting sources for the production of biopolymers with applications in the food industry, package production, and several other fields. Ubiquitous organisms, lactic acid bacteria (LAB) are well studied for the production of exopolysaccharides (EPS), but much less as producers of polylactic acid (PLA) and polyhydroxyalkanoates (PHAs). Based on their good biodegradability feature, as well as the possibility to be obtained from cheap biomass, PLA and PHAs polymers currently receive increased attention from both research and industry.
- lactic acid bacteria
- polylactic acid
- polyhydroxyalkanoates
- exopolysaccharides
- food application
- food packaging
1. Introduction
2. Classification of Biopolymers Produced by Lactic Acid Bacteria
3. Biopolymers-Producing Lactic Acid Bacteria Strains
4. Polyesters from LAB
4.1. Polylactic Acid (PLA) Production Associated with LAB
4.2. Polyhydroxyalkanoates (PHAs) Production by LAB
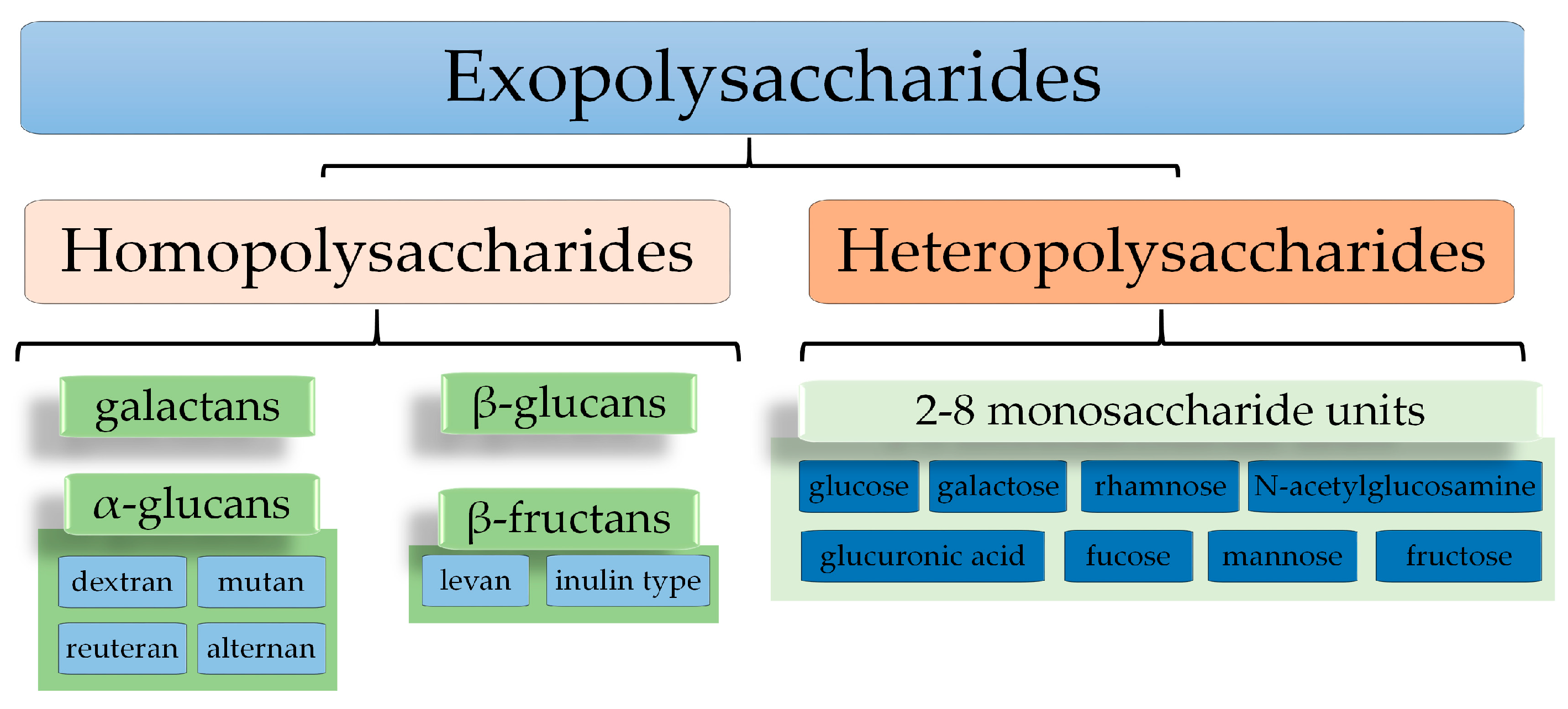
5. Exopolysaccharides from LAB
6. Processing Methods of Biopolymers Produced by LAB
-
for higher flexibility, plasticizers that have the ability to increase the mobility of biopolymer chains due to the reduction of intermolecular forces are added. Thus, to improve flexibility the kefiran films are plasticized with sorbitol, galactitol [85], glycerol, oleic acid, polyols, and sugars (glucose, galactose, sucrose) [83], and levan films, with glycerol [86].
-
to ensure pH and high-temperature stability, EPSs are combined with biosurfactants (lipoproteins, polysaccharide-lipid complex, phospholipid), and PLA with cellulose [83];
-
enhanced mechanical properties can be achieved when composite films made of EPSs, lipids, and hydrocolloids are formed [84]. Moreover, EPSs combined with starch (corn starch, cassava starch) form films with improved mechanical and chemical properties [83], and nanocomposite films composed of starch/kefiran/ZnO [87] or levan and starch have increased tensile strength [84].
-
for improved antimicrobial properties, nanocomposite films are formed by adding essential oils and other active compounds [83].
7. Applications of Biopolymers Produced by LAB in the Food Industry
This entry is adapted from the peer-reviewed paper 10.3390/polym15061539
References
- Awasthi, S.K.; Kumar, M.; Kumar, V.; Sarsaiya, S.; Anerao, P.; Ghosh, P.; Singh, L.; Liu, H.; Zhang, Z.; Awasthi, M.K. A comprehensive review on recent advancements in biodegradation and sustainable management of biopolymers. Environ. Pollut. 2022, 307, 119600.
- Das, A.; Ringu, T.; Ghosh, S.; Pramanik, N. A comprehensive review on recent advances in preparation, physicochemical characterization, and bioengineering applications of biopolymers. Polym. Bull. 2022, 1–66.
- Varghese, S.; Dhanraj, N.D.; Rebello, S.; Sindhu, R.; Binod, P.; Pandey, A.; Jisha, M.S.; Awasthi, M.K. Leads and hurdles to sustainable microbial bioplastic production. Chemosphere 2022, 305, 135390.
- Ali, S.S.; Abdelkarim, E.A.; Elsamahy, T.; Al-Tohamy, R.; Li, F.; Kornaros, M.; Zuorro, A.; Zhu, D.; Sun, J. Bioplastic production in terms of life cycle assessment: A state-of-the-art review. Environ. Sci. Ecotechnol. 2023, 13, 100254.
- Guérin, M.; Silva, C.R.-D.; Garcia, C.; Remize, F. Lactic acid bacterial production of exopolysaccharides from fruit and vegetables and associated benefits. Fermentation 2020, 6, 115.
- Li, D.; Li, J.; Zhao, F.; Wang, G.; Qin, Q.; Hao, Y. The influence of fermentation condition on production and molecular mass of EPS produced by Streptococcus thermophilus 05-34 in milk-based medium. Food Chem. 2016, 197, 367–372.
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A. Physical, mechanical, barrier, and thermal properties of polyol-plasticized biodegradable edible film made from kefiran. Carbohydr. Polym. 2011, 84, 477–483.
- Mazzoli, R.; Bosco, F.; Mizrahi, I.; Bayer, E.A.; Pessione, E. Towards lactic acid bacteria-based biorefineries. Biotechnol. Adv. 2014, 32, 1216–1236.
- European Economic and Social Committee. Available online: https://www.eesc.europa.eu/en/agenda/our-events/events/european-packaging-sector (accessed on 10 February 2023).
- European Parliament. Available online: https://www.europarl.europa.eu/RegData/etudes/BRIE/2022/734698/EPRS_BRIE_734698_Revision_Directive_Packaging.pdf (accessed on 10 February 2023).
- Jacob, J.; Gopi, S. Chapter 3. Isolation and physicochemical characterization of biopolymers. In Biopolymers and Their Industrial Applications; Thomas, S., Gopi, S., Amalraj, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 45–79.
- Kanmani, K.; Aravind, J.; Kamaraj, M.; Sureshbabub, S.; Karthikeyan, S. Environmental applications of chitosan and cellulosic biopolymers: A comprehensive outlook. Bioresour. Technol. 2017, 242, 295–303.
- Monilola, W.S.; Makinde, O.E. Production and Characterization of Polyhydroxyalkanoates from Lactic Acid Bacteria Isolated from Dairy Wastewater, Fermented Cow Milk and ‘Ogi’. J. Adv. Microbiol. 2020, 20, 31–46.
- Ahmed, Z.; Ahmad, A. Biopolymer produced by the lactic acid bacteria: Production and practical application. In Microbial Production of Food Ingredients and Additives; Academic Press: Cambridge, MA, USA, 2017; pp. 217–257.
- Zhou, K. Engineering microbes to synthesize functionalized biopolymers. J. Mater. Chem. B 2022, 10, 7132–7135.
- Rajoka, M.S.R.; Wu, Y.; Mehwish, H.M.; Bansal, M.; Zhao, L. Lactobacillus exopolysaccharides: New perspectives on engineering strategies, physiochemical functions, and immunomodulatory effects on host health. Trends Food Sci. Technol. 2020, 103, 36–48.
- Torino, M.I.; Font de Valdez, G.; Mozzi, F. Biopolymers from lactic acid bacteria. Novel applications in foods and beverages. Front. Microbiol. 2015, 6, 834.
- Mozzi, F.; Vaningelgem, F.; Hébert, E.M.; Van der Meulen, R.; Moreno, M.R.F.; Font de Valdez, G.; De Vuyst, L. Diversity of Heteropolysaccharide-Producing Lactic Acid Bacterium Strains and Their Biopolymers. Appl. Environ. Microbiol. 2006, 72, 4431–4435.
- Welman, A.D.; Maddox, I.S. Exopolysaccharides from lactic acid bacteria: Perspectives and challenges Review. Trends Biotechnol. 2003, 21, 269–274.
- Ruas-Madiedo, P. Methods for the Screening, Isolation, and Characterization of Exopolysaccharides Produced by Lactic Acid Bacteria. J. Dairy Sci. 2005, 88, 843–856.
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C.G. Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties. Foods 2022, 11, 156.
- Nabot, M.; Guérin, M.; Sivakumar, D.; Remize, F.; Garcia, C. Variability of Bacterial Homopolysaccharide Production and Properties during Food Processing. Biology 2022, 11, 171.
- Sørensen, M.S.; Rochfort, K.D.; Maye, S.; MacLeod, G.; Brabazon, D.; Loscher, C.; Freeland, B. Exopolysaccharides of Lactic Acid Bacteria: Production, Purification and Health Benefits towards Functional Food. Nutrients 2022, 14, 2938.
- Leemhuis, H.; Pijning, T.; Dobruchowska, J.M.; Dijkstra, B.W.; Dijkhuizen, L. Glycosidic bond specifi city of glucansucrases: On the role of acceptor substrate binding residues. Biocatal. Biotransform. 2012, 30, 366–376.
- De Vuyst, L.; Degeest, B. Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol. Rev. 1999, 23, 153–177.
- Berthold-Pluta, A.M.; Pluta, A.S.; Garbowska, M.; Stasiak-Różańska, L. Exopolysaccharide-producing lactic acid bacteria—Health-promoting properties and application in the dairy industry. Advancements of Microbiology. Postępy Mikrobiol.—Adv. Microbiol. 2019, 58, 191–204.
- Díaz-Montes, E. Dextran: Sources, Structures, and Properties. Polysaccharides 2021, 2, 33.
- Hu, Q.; Lu, Y.; Luo, Y. Recent advances in dextran-based drug delivery systems: From fabrication strategies to applications. Carbohydr. Polym. 2021, 264, 117999.
- Xu, Y.; Cui, Y.; Yue, F.; Liu, L.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Exopolysaccharides produced by lactic acid bacteria and Bifidobacteria. Structures, physiochemical functions and applications in the food industry. Food Hydrocoll. 2019, 94, 475–499.
- Sanlibaba, P.; Çakmak, G.A. Exopolysaccharides Production by Lactic Acid Bacteria. Appl. Microbiol. 2016, 2, 1000115.
- Aburas, H.; İspirli, H.; Taylan, O.; Yilmaz, M.T.; Dertli, E. Structural and physicochemical characterisation and antioxidant activity of an α-D-Glucan produced by sourdough isolate Weissellacibaria MED17. Int. J. Biol. Macromol. 2020, 161, 648–655.
- Korcz, E.; Varga, L. Exopolysaccharides from lactic acid bacteria: Techno-functional application in the food industry. Trends Food Sci. Technol. 2021, 110, 375–384.
- Monsan, P.; Remaud-Siméon, M.; Andre, I. Transglucosidases as efficient tools for oligosaccharide and glucoconjugate synthesis. Curr. Opin. Microbiol. 2010, 13, 293–300.
- Boddapati, S.; Rai, R.; Gummadi, S.N. Structural analysis and antioxidative properties of mutan (water-insoluble glucan) and carboxymethyl mutan from Streptococcus mutans. Process Biochem. 2020, 97, 130–139.
- Daba, G.M.; Elnahas, M.O.; Elkhateeb, W.A. Contributions of exopolysaccharides from lactic acid bacteria as biotechnological tools in food, pharmaceutical, and medical applications. Int. J. Biol. Macromol. 2021, 173, 79–89.
- Xu, Y.; Coda, R.; Holopainen-Mantila, U.; Laitila, A.; Katina, K.; Tenkanen, M. Impact of in situ produced exopolysaccharides on rheology and texture of fava bean protein concentrate. Food Res. Int. 2018, 115, 191–199.
- Korakli, M.; Vogel, R.F. Structure/function relationship of homopolysaccharide producing glycansucrases and therapeutic potential of their synthesised glycans. Appl. Microbiol. Biotechnol. 2006, 71, 790–803.
- Dimopoulou, M.; Dols-Lafargue, M. Exopolysaccharides Producing Lactic Acid Bacteria in Wine and Other Fermented Beverages: For Better or for Worse? Foods 2021, 10, 2204.
- Hundschell, C.S.; Wagemans, A.M. Rheology of common uncharged exopolysaccharides for food applications. Curr. Opin. Food Sci. 2019, 27, 1–7.
- Zannini, E.; Waters, D.M.; Coffey, A.; Arendt, E.K. Production, properties, and industrial food application of lactic acid bacteria-derived exopolysaccharides. Appl. Microbiol. Biotechnol. 2016, 100, 1121–1135.
- Kavitake, D.; Devi, P.B.; Singh, S.P.; Shetty, P.H. Characterization of a novel galactan produced by Weissella confusa KR780676 from an acidic fermented foodInt. J. Biol. Macromol. 2016, 86, 681–689.
- Abarquero, D.; Renes, E.; Fresno, J.M.; Tornadijo, M.E. Study of exopolysaccharides from lactic acid bacteria and their industrial applications: A review. Int. J. Food Sci. Technol. 2021, 57, 16–26.
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78.
- Coelho, M.C.; Malcata, F.X.; Silva, C.C.G. Lactic acid Bacteria in raw-milk cheeses: From starter cultures to probiotic functions. Foods 2022, 11, 2276.
- The European Food and Safety Authority (EFSA). Available online: https://www.efsa.europa.eu/en/applications/qps-assessment (accessed on 15 February 2023).
- US Food and Drug Administration (FDA). Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 15 February 2023).
- George, F.; Daniel, C.; Thomas, M.; Singer, E.; Guilbaud, A.; Tessier, F.J.; Revol-Junelles, A.-M.; Borges, F.; Foligné, B. Occurrence and Dynamism of Lactic Acid Bacteria in Distinct Ecological Niches: A Multifaceted Functional Health Perspective. Front. Microbiol. 2018, 9, 2899.
- Mokoena, M.P. Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Molecules 2017, 22, 1255.
- Salminen, S.; von Wright, A.; Morelli, L.; Marteau, P.; Brassart, D.; de Vos, W.M.; Fondén, R.; Saxelin, M.; Collins, K.; Mogensen, G.; et al. Demonstration of safety of probiotics—A review. Int. J. Food Microbiol. 1998, 44, 93–106.
- Authority, E.F.S. Introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA-Opinion of the Scientific Committee. EFSA J. 2007, 5, 587.
- Werning, M.L.; Hernández-Alcántara, A.M.; Ruiz, M.J.; Soto, L.P.; Dueñas, M.T.; López, P.; Frizzo, L.S. Biological functions of exopolysaccharides from lactic acid bacteria and their potential benefits for humans and farmed animals. Foods 2022, 11, 1284.
- Settanni, L.; Moschetti, G. Non-starter lactic acid bacteria used to improve cheese quality and provide health benefits. Food Microbiol. 2010, 27, 691–697.
- Liu, W.; Pang, H.; Zhang, H.; Cai, Y. Biodiversity of lactic acid bacteria. In Lactic Acid Bacteria; Springer: Berlin/Heidelberg, Germany, 2014; pp. 103–203.
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665–684.
- Jem, K.J.; Tan, B. The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70.
- Nduko, J.M.; Taguchi, S. Microbial production of biodegradable lactate-based polymers and oligomeric building blocks from renewable and waste resources. Front. Bioeng. Biotechnol. 2021, 8, 618077.
- Huang, S.; Xue, Y.; Yu, B.; Wang, L.; Zhou, C.; Ma, Y. A review of the recent developments in the bioproduction of polylactic acid and its precursors optically pure lactic acids. Molecules 2021, 26, 6446.
- Chozhavendhan, S.; Usha, P.; Sowmiya, G.; Rohini, G. A review on bioplastic production—A need to the society. Int. J. Pharm. Sci. Rev. Res. 2020, 62, 27–32.
- Taguchi, S.; Yamada, M.; Matsumoto, K.; Tajima, K.; Satoh, Y.; Munekata, M.; Ohno, K.; Kohda, K.; Shimamura, T.; Kambe, H.; et al. A microbial factory for lactate-based polyesters using a lactate polymerizing enzyme. Proc. Natl. Acad. Sci. USA 2008, 105, 17323–17327.
- Bosco, F.; Cirrincione, S.; Carletto, R.; Marmo, L.; Chiesa, F.; Mazzoli, R.; Pessione, E. PHA production from Cheese Whey and “Scotta”: Comparison between a consortium and a pure culture of Leuconostoc mesenteroides. Microorganisms 2021, 9, 2426.
- Khatami, K.; Perez-Zabaleta, M.; Owusu-Agyeman, I.; Cetecioglu, Z. Waste to bioplastics: How close are we to sustainable polyhydroxyalkanoates production? Waste Manag. 2021, 119, 374–388.
- Wade, M.E.; Strickland, M.T.; Osborne, J.P.; Edwards, C.G. Role of Pediococcus in winemaking. Aust. J. Grape Wine Res. 2018, 25, 7–24.
- Aslim, B.; Caliskan, F.; Beyath, Y.; Guënduëz, U. Poly-L-hydroxybutyrate production by lactic acid bacteria. FEMS Microbiol. Lett. 1998, 159, 293–297.
- Albuquerque, G.E.; Torres, C.A.V.; Reis, M.A.M. Polyhydroxyalkanoate (PHA) production by a mixed microbial culture using sugar molasses: Effect of the influent substrate concentration on culture selection. Water Res. 2010, 44, 3419–3433.
- Amaro, T.M.M.M.; Rosa, D.; Comi, G.; Iacumin, L. Prospects for the Use of Whey for Polyhydroxyalkanoate (PHA) Production. Front. Microbiol. 2019, 10, 992.
- Abdalla, A.K.; Ayyash, M.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Shah, N.P.; Holley, R. Exopolysaccharides as Antimicrobial Agents: Mechanism and Spectrum of Activity. Front. Microbiol. 2021, 12, 64395.
- Nguyen, P.T.; Nguyen, T.T.; Bui, D.C.; Hong, P.T.; Hoang, Q.K.; Nguyen, H.T. Exopolysaccharide production by lactic acid bacteria: The manipulation of environmental stresses for industrial applications. AIMS Microbiol. 2020, 6, 451–469.
- Van Den Berg, D.; Robijn, G.W.; Janssen, A.C.; Giuseppin, M.; Vreeker, R.; Kamerling, J.P.; Vliegenthart, I.; Ledeboer, A.M.; Verrips, C.T. Production of a novel extracellular polysaccharide by Lactobacillus sake 0-1 and characterization of the polysaccharide. Appl. Environ. Microbiol. 1995, 61, 2840–2844.
- Lule, V.k.; Singh, R.; Pophaly, S.D.; Tomar, S.K. Production and structural characterisation of dextran from an indigenous strain of Leuconostoc mesenteroides BA08 in Whey. Int. J. Dairy Technol. 2016, 69, 520–531.
- Sakr, E.A.E.; Massoud, M.I.; Ragaee, S. Food wastes as natural sources of lactic acid bacterial exopolysaccharides for the functional food industry: A review. Int. J. Biol. Macrom. 2021, 189, 232–241.
- Ruas-Madiedo, P.; Salazar, N.; de los Reyes-Gavilán, G.C. Biosynthesis and chemical composition of exopolysaccharides produced by lactic acid bacteria. In Bacterial Polysaccharides, Current Innovations and Future Trends; Ullrich, M., Ed.; Caister Academic Press: Norfolk, UK, 2009; pp. 279–310.
- Silva, L.A.; Lopes Neto, J.H.P.; Cardarelli, H.R. Exopolysaccharides produced by Lactobacillus plantarum: Technological properties, biological activity, and potential application in the food industry. Ann. Microbiol. 2019, 69, 321–328.
- Xu, Y.; Wang, Y.; Coda, R.; Säde, E.; Tuomainen, P.; Tenkanen, M.; Katina, K. In situ synthesis of exopolysaccharides by Leuconostoc spp. and Weissella spp. and their rheological impacts in fava bean flour. Int. J. Food Microbiol. 2017, 248, 63–71.
- Badel, S.; Bernardi, T.; Michaud, P. New perspectives for Lactobacilli exopolysaccharides. Biotechnol. Adv. 2011, 29, 54–66.
- Zeidan, A.A.; Poulsen, V.K.; Janzen, T.; Buldo, P.; Derkx, P.M.F.; Øregaard, G.; Neves, A.R. Polysaccharide production by lactic acid bacteria: From genes to industrial applications. FEMS Microbiol. Rev. 2017, 41, S168–S200.
- Prete, R.; Alam, M.K.; Perpetuini, G.; Perla, C.; Pittia, P.; Corsetti, A. Lactic acid bacteria exopolysaccharides producers: A sustainable tool for functional foods. Foods 2021, 10, 1653.
- Grobben, G.J.; Van Casteren, W.H.; Schols, H.A.; Oosterveld, A.; Sala, G.; Smith, M.R.; Sikkema, J.; de Bont, A.M. Analysis of the exopolysaccharides produced by Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772 grown in continuous culture on glucose and fructose. Appl. Microbiol. Biotechnol. 1997, 48, 516–521.
- Baruah, R.; Goyal, A. Exopolysaccharides from lactic acid bacteria in fermented foods and beverages. In Applied Biotechnology Reviews, Lactic Acid Bacteria in Food Biotechnology; Ray, R.C., Paramithiotis, S., de Carvalho Azevedo, V.A., Montet, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 305–317.
- Paulo, E.M.; Vasconcelos, M.P.; Oliveira, I.S.; Affe, H.M.d.J.; Nascimento, R.; Melo, I.S.d.; Roque, M.R.d.A.; Assis, S.A.d. An alternative method for screening lactic acid bacteria for the production of exopolysaccharides with rapid confirmation. Food Sci. Technol. 2012, 32, 710–714.
- Malang, S.K.; Maina, N.H.; Schwab, C.; Tenkanen, M.; Lacroix, C. Characterization of exopolysaccharide and ropy capsular polysaccharide formation by Weissella. Food Microbiol. 2015, 46, 418–427.
- Mende, S.; Peter, M.; Bartels, K.; Rohm, H.; Jaros, D. Addition of purified exopolysaccharide isolates from S. thermophilus to milk and their impact on the rheology of acid gels. Food Hydrocoll. 2013, 32, 178–185.
- Castro-Bravo, N.; Wells, J.M.; Margolles, A.; Ruas-Madiedo, P. Interactions of surface exopolysaccharides from Bifidobacterium and Lactobacillus within the intestinal environment. Front. Microbiol. 2018, 9, 2426.
- Agarwal, A.; Shaida, B.; Rastogi, M.; Singh, N.B. Food Packaging Materials with Special Reference to Biopolymers-Properties and Applications. Chem. Afr. 2022, 1, 117–144.
- Moradi, M.; Guimarães, J.T.; Sahin, S. Current applications of exopolysaccharides from lactic acid bacteria in the development of food active edible packaging. Curr. Opin. Food Sci. 2021, 40, 33–39.
- Montoille, L.; Vicencio, C.M.; Fontalba, D.; Ortiz, J.O.; Moreno-Serna, V.; Peponi, L.; Matiacevich, S.; Zapata, P.A. Study of the effect of the addition of plasticizers on the physical properties of biodegradable films based on kefiran for potential application as food packaging. Food Chem. 2021, 360, 129966.
- Zikmanis, P.; Juhņeviča-Radenkova, K.; Radenkovs, V.; Segliņa, D.; Krasnova, I.; Kolesovs, S.; Orlovskis, Z.; Šilaks, A.; Semjonovs, P. Microbial Polymers in Edible Films and Coatings of Garden Berry and Grape: Current and Prospective Use. Food Bioprocess Technol. 2021, 14, 1432–1445.
- Babaei-Ghazvini, A.; Shahabi-Ghahfarrokhi, I.; Goudarzi, V. Preparation of UV-protective starch/kefiran/ZnO nanocomposite as a packaging film: Characterization. Food Packag. Shelf Life 2018, 16, 103–111.
- Vivek, N.; Gopalan, N.; Das, S.; Sasikumar, K.; Sindhu, R.; Nampoothiri, K.M.; Pandey, A.; Binod, P. Synthesis and Characterization of Transparent Biodegradable Chitosan: Exopolysaccharide Composite Films Plasticized by Bio-Derived 1,3-Propanediol. Sustain. Chem. 2021, 2, 4.
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.; Mahanti, N.K. Application of biodegradable polymers in food packaging industry: A comprehensive review. J. Package Technol. Res. 2019, 3, 77–96.
- Schmidt Rivera, X.C.; Leadley, C.; Potter, L.; Azapagic, A. Aiding the design of innovative and sustainable food packaging: Integrating techno-environmental and circular economy criteria. Energy Procedia 2019, 161, 190–197.
- Wohner, B.; Pauer, E.; Heinrich, V.; Tacker, M. Packaging-related food losses and waste: An overview of drivers and issues. Sustainability 2019, 11, 264.
- Nilsen-Nygaard, J.; Fernández, N.E.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380.
- Nešić, A.; Cabrera-Barjas, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of Polysaccharide-Based Materials as Advanced Food Packaging. Molecules 2020, 25, 135.
- Wang, J.; Euring, M.; Ostendorf, K.; Zhang, K. Biobased materials for food packaging. J. Bioresour. Bioprod. 2022, 7, 1–13.
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased materials for active food packaging: A review. Food Hydrocoll. 2022, 125, 107419.
- Vinod, A.; Sanjay, M.R.; Suchart, S.; Jyotishkumar, P. Renewable and sustainable biobased materials: An assessment on biofibers, biofilms, biopolymers and biocomposites. J. Clean. Prod. 2020, 258, 120978.
