Various deep-learning (DL) methods that utilize a combination of omics data and imaging data have been applied to the diagnosis, prognosis, and treatment options of clinical COVID-19 patients. Even with the emerging deep-learning methods, human intervention is still essential in the clinical diagnosis and treatment of COVID-19 patients.
- artificial intelligence
- deep learning
- multi-modal learning
1. Deep Learning for Diagnosis of COVID-19
Various deep-learning (DL) methods that utilize a combination of omics data and imaging data have been applied to the diagnosis, prognosis, and treatment options of clinical COVID-19 patients. However, even with the emerging deep-learning methods, human intervention is still essential in the clinical diagnosis and treatment of COVID-19 patients. Therefore, the goal of DL is not to surpass or replace humans, but rather to provide decision-support tools to help researchers studying COVID-19 and health professionals in the clinical management of COVID-19 patients.
COVID-19 is traditionally diagnosed by real-time RT-PCR testing of respiratory or blood samples [1] (Figure 1). However, considering the simplicity and sensitivity of the test, CXR radiography has become the mainstay of screening, triaging, and diagnosing varieties of COVID-19. Researchers [2][3] noted that the majority of the COVID-19 positive cases in their study presented bilateral radiographic abnormalities in CXR images, such as ground-glass opacity, bilateral abnormalities, and interstitial abnormalities in CXR and CT images. Indeed, early works on COVID-19 imagery identified the existence of pulmonary lesions in non-severe and even recovered patients [4]. Among various deep-learning classifiers, CNNs, in particular, have been enormously effective in computer vision and medical image analysis tasks. COVID-Net represents one of the earliest convolutional networks designed for detecting COVID-19 cases automatically from CXR images [5]. This architecture design consists of two parts: a human–computer collaborative design approach and a machine-driven design exploration part. A lightweight residual projection–expansion–projection–extension (PEPX) design pattern is used in this architecture. An interpretability-driven check was also performed for decision validation, achieving 87.1 percent recall and 93.3 percent accuracy. To reduce the amount of data and time required for training, transfer learning may be an appropriate solution [6]. Researchers have achieved state-of-the-art performance on pneumonia recognition using ensemble models with pre-trained architectures trained on ImageNet, using AlexNet, DenseNet121, Inception V3, GoogLeNet and ResNet18 to achieve 96.4 percent accuracy and 99.62 percent recall on unseen data from the Guangzhou Women and Children’s Medical Center dataset [7]. COVID CAPS [8], a CNN model based on CapsNets, was able to process small data sets with 95.7 percent accuracy. Most computer vision tasks, such as image classification, semantic segmentation, object recognition, etc., are based on 2D CNN [9]. Since high-dimensional data is difficult for humans to understand, the application of multidimensional CNN in 3D is not common, as described above. Hybrid-COVID [10] is a novel Hybrid 2D/3D framework based on CNN that uses the potential synergy between a pre-trained VGG16 model (i.e., 2D CNN) and a shallow 3D CNN to efficiently and effectively diagnose COVID-19 from CXR images with 96.91 percent accuracy. Researchers proposed a medical image segmentation method for COVID-19 lung CT image segmentation using an advanced high-density GAN dataset combined with a multi-layer attention mechanism approach from U-Net [11].
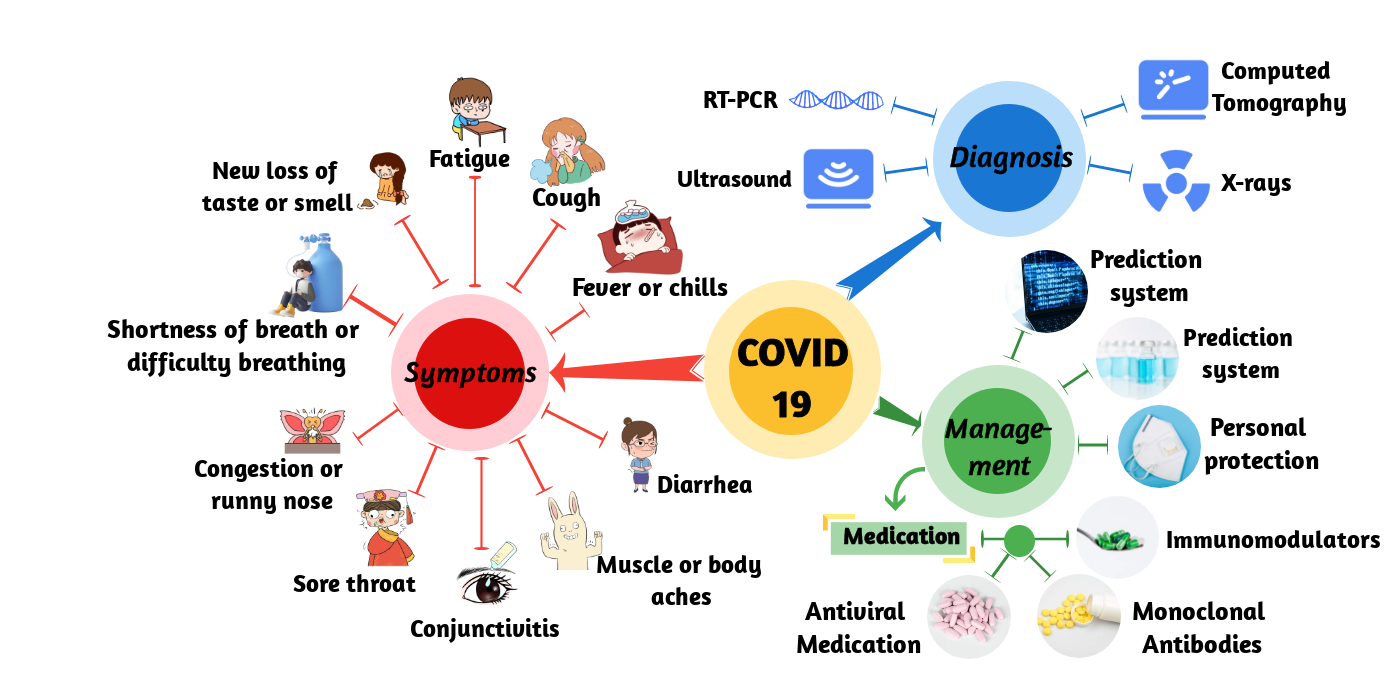
Figure 1. Schematic of COVID-19. It shows the symptoms, diagnosis, and management of COVID-19.
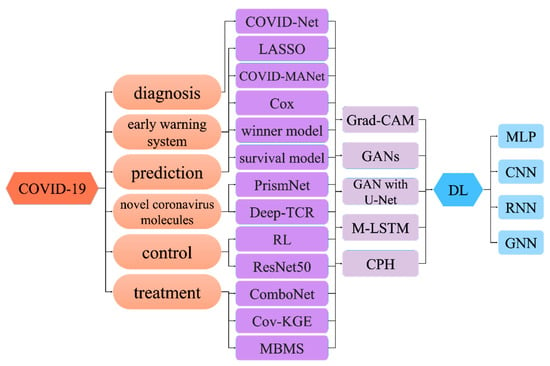
2. Deep Learning for COVID-19 Early Warning System
3. Deep Learning for COVID-19 Prediction
4. Deep Learning for Novel Coronavirus Molecules
5. Deep Learning for COVID-19 Control
6. Deep Learning for COVID-19 Treatment
This entry is adapted from the peer-reviewed paper 10.3390/math11061279
References
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in different types of clinical specimens. Jama 2020, 323, 1843–1844.
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Liu, L.; Gao, J.-Y.; Hu, W.-M.; Zhang, X.-X.; Guo, L.; Liu, C.-Q.; Tang, Y.-W.; Lang, C.-H.; Mou, F.-Z.; Yi, Z.-J.; et al. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in chongqing, China. medRxiv 2020.
- Wang, L.; Lin, Z.Q.; Wong, A. Covid-net: A tailored deep convolutional neural network design for detection of COVID-19 cases from chest x-ray images. Sci. Rep. 2020, 10, 19549.
- Hu, Q.; Gois, F.N.B.; Costa, R.; Zhang, L.; Yin, L.; Magaia, N.; de Albuquerque, V.H.C. Explainable artificial intelligence-based edge fuzzy images for COVID-19 detection and identification. Appl. Soft Comput. 2022, 123, 108966.
- Chouhan, V.; Singh, S.K.; Khamparia, A.; Gupta, D.; Tiwari, P.; Moreira, C.; Damaševičius, R.; de Albuquerque, V.H.C. A novel transfer learning based approach for pneumonia detection in chest x-ray images. Appl. Sci. 2020, 10, 559.
- Afshar, P.; Heidarian, S.; Naderkhani, F.; Oikonomou, A.; Plataniotis, K.N.; Mohammadi, A. Covid-caps: A capsule network-based framework for identification of COVID-19 cases from x-ray images. Pattern Recognit. Lett. 2020, 138, 638–643.
- Li, Z.; Liu, F.; Yang, W.; Peng, S.; Zhou, J. A survey of convolutional neural networks: Analysis, applications, and prospects. IEEE Trans. Neural Netw. Learn. Syst. 2022, 33, 6999–7019.
- Bayoudh, K.; Hamdaoui, F.; Mtibaa, A. Hybrid-covid: A novel hybrid 2d/3d cnn based on cross-domain adaptation approach for COVID-19 screening from chest x-ray images. Phys. Eng. Sci. Med. 2020, 43, 1415–1431.
- Zhang, J.; Yu, L.; Chen, D.; Pan, W.; Shi, C.; Niu, Y.; Yao, X.; Xu, X.; Cheng, Y. Dense gan and multi-layer attention based lesion segmentation method for COVID-19 ct images. Biomed. Signal Process. Control 2021, 69, 102901.
- Shi, W.; Tong, L.; Zhu, Y.; Wang, M.D. COVID-19 automatic diagnosis with radiographic imaging: Explainable attention transfer deep neural networks. IEEE J. Biomed. Health Inform. 2021, 25, 2376–2387.
- Diaz-Escobar, J.; Ordóñez-Guillén, N.E.; Villarreal-Reyes, S.; Galaviz-Mosqueda, A.; Kober, V.; Rivera-Rodriguez, R.; Rizk, J.E.L. Deep-learning based detection of COVID-19 using lung ultrasound imagery. PLoS ONE 2021, 16, e0255886.
- Fang, C.; Bai, S.; Chen, Q.; Zhou, Y.; Xia, L.; Qin, L.; Gong, S.; Xie, X.; Zhou, C.; Tu, D.; et al. Deep learning for predicting COVID-19 malignant progression. Med. Image Anal. 2021, 72, 102096.
- Näppi, J.J.; Uemura, T.; Watari, C.; Hironaka, T.; Kamiya, T.; Yoshida, H. U-survival for prognostic prediction of disease progression and mortality of patients with COVID-19. Sci. Rep. 2021, 11, 9263.
- Sun, C.; Hong, S.; Song, M.; Li, H.; Wang, Z. Predicting COVID-19 disease progression and patient outcomes based on temporal deep learning. BMC Med. Inform. Decis. Mak. 2021, 21, 1–16.
- Uemura, T.; Näppi, J.J.; Watari, C.; Hironaka, T.; Kamiya, T.; Yoshida, H. Weakly unsupervised conditional generative adversarial network for image-based prognostic prediction for COVID-19 patients based on chest ct. Med. Image Anal. 2021, 73, 102159.
- Vaid, S.; Kalantar, R.; Bhandari, M. Deep learning COVID-19 detection bias: Accuracy through artificial intelligence. Int. Orthop. 2020, 44, 1539–1542.
- Ikemura, K.; Bellin, E.; Yagi, Y.; Billett, H.; Saada, M.; Simone, K.; Stahl, L.; Szymanski, J.; Goldstein, D.Y.; Gil, M.R. Using automated machine learning to predict the mortality of patients with covid-19: Prediction model development study. J. Med. Internet Res. 2021, 23, e23458.
- Meng, L.; Dong, D.; Li, L.; Niu, M.; Bai, Y.; Wang, M.; Qiu, X.; Zha, Y.; Tian, J. A deep learning prognosis model help alert for COVID-19 patients at high-risk of death: A multi-center study. IEEE J. Biomed. Health Inform. 2020, 24, 3576–3584.
- Suppakitjanusant, P.; Sungkanuparph, S.; Wongsinin, T.; Virapongsiri, S.; Kasemkosin, N.; Chailurkit, L.; Ongphiphadhanakul, B. Identifying individuals with recent COVID-19 through voice classification using deep learning. Sci. Rep. 2021, 11, 1–7.
- Liang, W.; Yao, J.; Chen, A.; Lv, Q.; Zanin, M.; Liu, J.; Wong, S.; Li, Y.; Lu, J.; Liang, H.; et al. Early triage of critically ill COVID-19 patients using deep learning. Nat. Commun. 2020, 11, 1–7.
- Dong, Y.M.; Sun, J.; Li, Y.X.; Chen, Q.; Liu, Q.Q.; Sun, Z.; Pang, R.; Chen, F.; Xu, B.Y.; Manyande, A.; et al. Development and validation of a nomogram for assessing survival in patients with COVID-19 pneumonia. Clin. Infect. Dis. 2021, 72, 652–660.
- Gao, J.; Sharma, R.; Qian, C.; Glass, L.M.; Spaeder, J.; Romberg, J.; Sun, J.; Xiao, C. Stan: Spatio-temporal attention network for pandemic prediction using real-world evidence. J. Am. Med. Inform. Assoc. 2021, 28, 733–743.
- Dairi, A.; Harrou, F.; Zeroual, A.; Hittawe, M.M.; Sun, Y. Comparative study of machine learning methods for COVID-19 transmission forecasting. J. Biomed. Inform. 2021, 118, 103791.
- Liao, Z.; Song, Y.; Ren, S.; Song, X.; Fan, X.; Liao, Z. Voc-dl: Deep learning prediction model for COVID-19 based on voc virus variants. Comput. Methods Programs Biomed. 2022, 224, 106981.
- Mary, S.R.; Kumar, V.; Venkatesan, K.J.P.; Kumar, R.S.; Jagini, N.P.; Srinivas, A. Vulture-based adaboost-feedforward neural frame work for COVID-19 prediction and severity analysis system. Interdiscip Sci. 2022, 14, 582–595.
- Mansour, R.F.; Escorcia-Gutierrez, J.; Gamarra, M.; Gupta, D.; Castillo, O.; Kumar, S. Unsupervised deep learning based variational autoencoder model for COVID-19 diagnosis and classification. Pattern Recognit. Lett. 2021, 151, 267–274.
- Liao, Z.; Lan, P.; Fan, X.; Kelly, B.; Innes, A.; Liao, Z. Sirvd-dl: A COVID-19 deep learning prediction model based on time-dependent sirvd. Comput. Biol. Med. 2021, 138, 104868.
- Kafieh, R.; Arian, R.; Saeedizadeh, N.; Amini, Z.; Serej, N.D.; Minaee, S.; Yadav, S.K.; Vaezi, A.; Rezaei, N.; Javanmard, S.H. COVID-19 in iran: Forecasting pandemic using deep learning. Comput. Math. Methods Med. 2021, 2021, 1–16.
- Taz, T.A.; Ahmed, K.; Paul, B.K.; Kawsar, M.; Aktar, N.; Mahmud, S.M.H.; Moni, M.A. Network-based identification genetic effect of SARS-CoV-2 infections to idiopathic pulmonary fibrosis (ipf) patients. Brief. Bioinform. 2020, 22, 1254–1266.
- Mahmud, S.M.H.; Al-Mustanjid, M.; Akter, F.; Rahman, M.S.; Ahmed, K.; Rahman, M.H.; Chen, W.; Moni, M.A. Bioinformatics and system biology approach to identify the influences of SARS-CoV-2 infections to idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease patients. Brief. Bioinform. 2021, 22, bbab115.
- Sun, L.; Li, P.; Ju, X.; Rao, J.; Huang, W.; Ren, L.; Zhang, S.; Xiong, T.; Xu, K.; Zhou, X.; et al. In vivo structural characterization of the SARS-CoV-2 rna genome identifies host proteins vulnerable to repurposed drugs. Cell 2021, 184, 1865–1883.e1820.
- Sidhom, J.W.; Baras, A.S. Deep learning identifies antigenic determinants of severe SARS-CoV-2 infection within t-cell repertoires. Sci. Rep. 2021, 11, 14275.
- Yang, D.; Yurtsever, E.; Renganathan, V.; Redmill, K.A.; Özgüner, Ü. A vision-based social distancing and critical density detection system for COVID-19. Sensors 2021, 21, 4608.
- Zhang, T.; Li, J. Understanding and predicting the spatio-temporal spread of COVID-19 via integrating diffusive graph embedding and compartmental models. Trans GIS 2021, 25, 3025–3047.
- Jin, W.; Stokes, J.M.; Eastman, R.T.; Itkin, Z.; Zakharov, A.V.; Collins, J.J.; Jaakkola, T.S.; Barzilay, R. Deep learning identifies synergistic drug combinations for treating COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2105070118.
- Ottakath, N.; Elharrouss, O.; Almaadeed, N.; Al-Maadeed, S.; Mohamed, A.; Khattab, T.; Abualsaud, K. Vidmask dataset for face mask detection with social distance measurement. Displays 2022, 73, 102235.
- Siah, C.R.; Lau, S.T.; Tng, S.S.; Chua, C.H.M. Using infrared imaging and deep learning in fit-checking of respiratory protective devices among healthcare professionals. J. Nurs. Sch. 2022, 54, 345–354.
- Sethi, S.; Kathuria, M.; Kaushik, T. Face mask detection using deep learning: An approach to reduce risk of coronavirus spread. J. Biomed. Inform. 2021, 120, 103848.
- Zeng, Y.; Chen, X.; Luo, Y.; Li, X.; Peng, D. Deep drug-target binding affinity prediction with multiple attention blocks. Brief. Bioinform. 2021, 22, bbab117.
- Nguyen, D.D.; Gao, K.; Chen, J.; Wang, R.; Wei, G.W. Unveiling the molecular mechanism of SARS-CoV-2 main protease inhibition from 137 crystal structures using algebraic topology and deep learning. Chem. Sci. 2020, 11, 12036–12046.
- Zeng, X.; Song, X.; Ma, T.; Pan, X.; Zhou, Y.; Hou, Y.; Zhang, Z.; Li, K.; Karypis, G.; Cheng, F. Repurpose open data to discover therapeutics for COVID-19 using deep learning. J. Proteome Res. 2020, 19, 4624–4636.
- Roh, H.; Shin, S.; Han, J.; Lim, S. A deep learning-based medication behavior monitoring system. Math. Biosci. Eng. 2021, 18, 1513–1528.
- Santos, M.A.G.; Munoz, R.; Olivares, R.; Filho, P.P.R.; Ser, J.D.; de Albuquerque, V.H.C. Online heart monitoring systems on the internet of health things environments: A survey, a reference model and an outlook. Inf. Fusion 2020, 53, 222–239.
- Parah, S.A.; Kaw, J.A.; Bellavista, P.; Loan, N.A.; Bhat, G.M.; Muhammad, K.; de Albuquerque, V.H.C. Efficient security and authentication for edge-based internet of medical things. IEEE Internet Things J. 2021, 8, 15652–15662.
