Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Organic electronic devices have gained popularity because of their promising tunable electronic properties, flexibility, low-cost, versatile functionalization, and processability. Organic field effect transistors (OFETs) are not only the fundamental building blocks of flexible and large-area electronic devices but are also a useful tool for measuring charge-carrier mobilities of newly organic semiconductors.
- quinoidal π-conjugated materials
- OFETs
- indophenine dyes
- Quinoidal Semiconducting Materials
- Organic Diradical TE Materials
- Photovoltaic
1. Introduction
Organic electronic devices have gained enormous popularity over the past 30 years because of their promising tunable electronic properties, flexibility, low-cost, versatile functionalization, and processability. The critical component of these devices is the organic semiconductors (OSCs) material, which serves as the active layer and determines the performance of the device. Over the last decade, one of the driving forces of development within this field is synthesizing novel, high-performance building blocks and extending the library of organic materials for various optoelectronic and energy applications. However, most of the reported works focus on development of hole-transport (p-type) OSCs, while less research has been conducted on electron-transport (n-type) OSCs materials. One main reason for the lack of high-performance n-type OSCs is the availability of π-conjugated building units with strong electron-deficiency necessary to ensure sufficiently deep-lying LUMO energy and to enable the fabrication of n-type OSCs with stable electron transport features. For electron transport materials, the majority can only undergo stable n-type transport under the conditions of nitrogen or vacuum, as electron carriers can be easily trapped by H2O and O2 in the environment during the manufacturing of the device. It is generally assumed that a LUMO energy level at −4.0 eV is a prerequisite to developing a stable electron transport OSC, as the presence of a high electron affinity to facilitate electron injection and environmental stability enables the acquisition of high-performance electron transport OFETs. Furthermore, the specific building blocks should have selective reaction sites that provide handles for insertion into the π-conjugated system and should be readily compatible with a broad range of chemical reactions [1]. Frontier molecular orbital (FMO) energy levels (both HOMO and LUMO energy levels) can be accurately can be accurately regulated by modifying them to match the work function of the electrode. Despite the impressive work on the synthesis of n-type OSCs, the further development of novel building blocks enabling the production of high-performance materials remains a serious challenge. The exploration in this area has been driven by the development of new applications that require specific molecular design, namely non-fullerene organic solar cells, organic thin film transistors (OTFT), organic electrochemical transistors (OECT), organic thermoelectric (TE) generators, etc.
2. Basic Background of Organic Electronic Devices and Assessment Parameters
This section briefly outlines certain organic electronic devices, which are the primary building blocks for various OSC-based applications, Figure 1. For a more detailed discussion, some excellent reviews focusing on this topic are recommended [2].
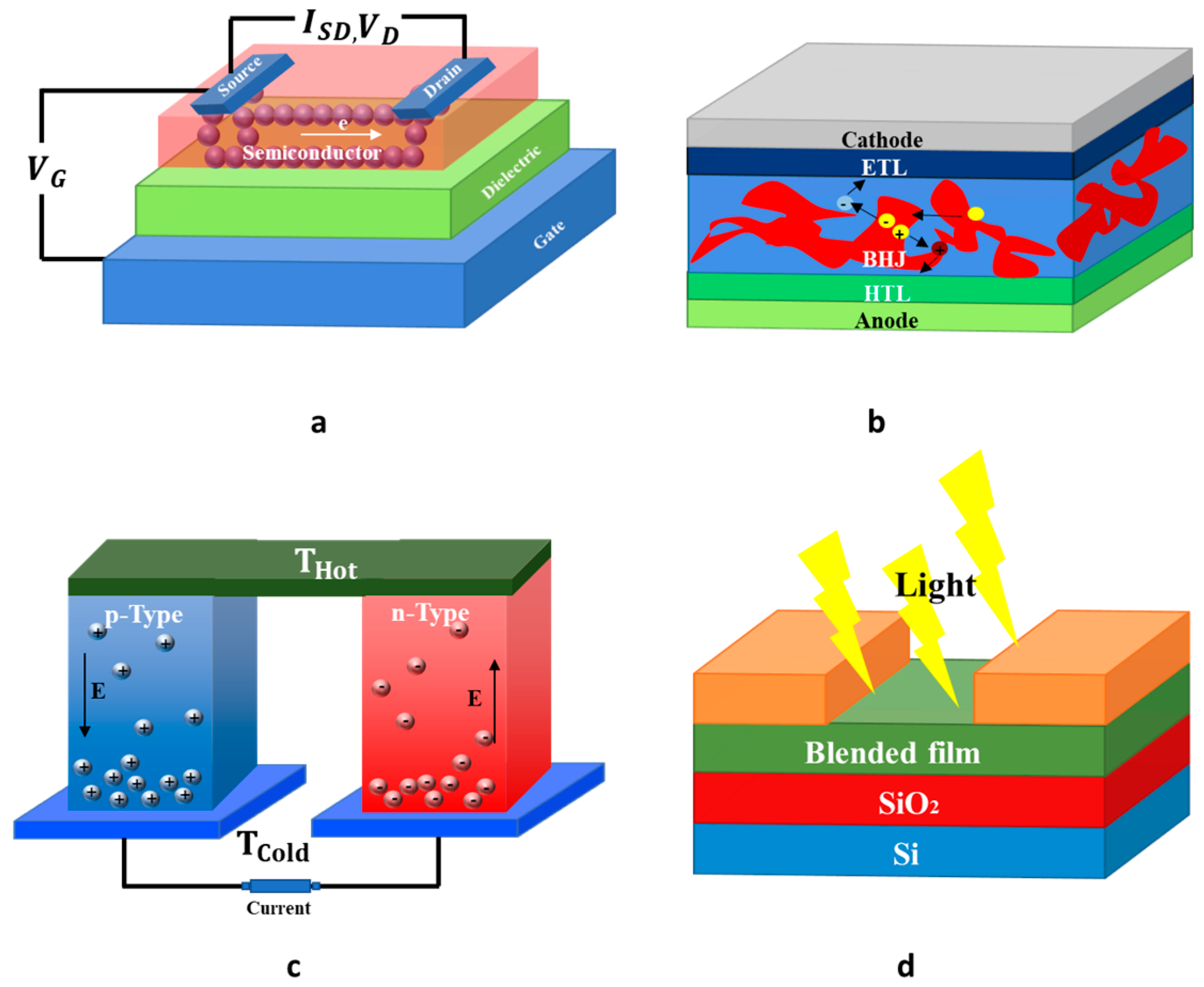
Figure 1. Representative thin-film optoelectronic devices that use OSC including (a) a bottom-gate top-contact OFET; (b) a bulk heterojunction organic solar cell; (c) an organic TE device; (d) a photo transistor.
OFETs are not only the fundamental building blocks of flexible and large area electronic devices but are also a useful tool for measuring charge-carrier mobilities of newly OSCs, and for understanding, assessing charge transport behavior of OSCs. OFETs can be fabricated in different ways, with the most common constructions being the bottom-gate bottom-contact (BGBC) and bottom-gate top-contact (BGTC) structures [3]. For the first case, the source and drain electrodes are placed directly on the dielectric film, where most of the charge carriers are expected to be transported; whereas for the second case, the source and drain electrodes are positioned on top of the semiconducting layer, and the charge carriers must cross the semiconducting layer to arrive at the channel. Therefore, with those straightforward differences, it means that the device configuration is able to affect the extracted charge mobility [4]. OFETs can operate in two regimes: linear and saturation regimes. One figure of merit for OFETs is the charge mobility, which could be extracted by using OFET-equation in linear and/or saturation regimes. Although OFETs were already widely used to evaluate the charge transport characteristics in newly synthetized OSCs; nonetheless, it is challenging to compare charge transport parameters of different materials as the OFET-mobility is governed by several factors such as the OFET-configuration used, the contact resistances, the choice of dielectric through its surface morphology, and the morphology of active layer.
The TE effect is a straight conversion of temperature differences into electric voltages and vice versa. Research is focused on novel TE-materials due to increasing energy demand and pollution linked to human activities. It should be remarked that approximately two-thirds of all industrial energy consumption is lost in the form of waste heat [5]. Consequently, it becomes urgent to promote the recovery of this huge waste heat into electrical energy. During the previous ten years, the number of teams active in the research of organic-TE materials has increased significantly. Since they are lightweight, printable, and suitable for large area applications, both p- and n-type TE materials become necessary in practical applications. However, the efficiency of the TE is quite low. The performance of TE is commonly expressed in terms of TE figure of merit, ZT = σS 2T/k, whereby σ, S, T, and k, respectively, represents electrical conductivity, Seebeck coefficient, absolute temperature, and thermal conductivity [6]. The optimal TE-material should have either higher Seebeck coefficient for improved energy conversion, higher electrical conductivity for decreased joule heating, or lower thermal conductivity to conserve the temperature gradient. One of the challenges in the field of TE is the strong interdependence between the σ, S and κ, with optimization of any one of them causing a negative impact on at least one of the others.
In addition to OFET and TE applications, n-type OSCs have also attracted considerable interest as alternative acceptors for non-fullerene solar cells [7] and electron-transporting materials for p-i-n perovskite. Organic and perovskite solar cells offer many benefits: flexibility, printability, low weight, low cost, fashionable designs, the ability to be manufactured by roll-to-roll production, etc. For both types of solar cells, fullerenes and their derivatives have been broadly applied as an acceptor for OPV. However, they suffer from a number of drawbacks including weak light absorption in the visible-near infrared region, high-cost efficiency, etc. To address those limitations, OSCs have been extended to non-fullerene acceptor materials.
The past decade has witnessed tremendous development in molecular design guidelines for the discovery and synthesis of novel quinoidal π-conjugated materials. End-capped quinoidal π-conjugated molecules are a subfamily, with π-extended core having two terminal groups. They possess high electron acceptability and low LUMO levels, which has made them excellent n-type semiconductor materials, with electron mobilities well in excess of 1 cm2 V−1 s−1. The nature of π-extended core and terminal group have a profound effect on the electrical and optoelectronic properties in those materials.
3. Synthetic Tactics of π-Extended Quinoidal Acceptors
π-Conjugated quinoidal molecules are emerging materials for energy and optoelectronic applications. Two main strategies have been developed by chemists for their synthesis. These approaches lead to two different classes of quinoidal materials. The first approach involves embedding the quinoidal moiety into the core of an aromatic π-conjugation. The second approach, known as end-group functionalization, involves terminal capping of the terminal methylene sites by electron-withdrawing functional group (EWG). The functional groups, cyano, ester groups, or aryl groups, contribute to quinoidal character by blocking the reaction sites and delocalizing the spin. A π-extended core and terminal group have a profound effect on the electrical and optoelectronic performance of the resulting material. The general approach of the synthesis of quinoidal families with various terminal units will be briefly mentioned. Four structural modification tactics are discussed in detail, involving the introduction of the dicyanomethylene functionality at the terminal positions of a π-conjugated system, indandione-terminated and triphenylmethane π-conjugated quinoids, and finally indophenine family. Generally, there are two main synthesis routes for the preparation of end-capped π-conjugated quinoidal molecules according to the reactivity and the functionality of the end-group. For quinoidal dicyanomethylene-end capped molecules, the Takahashi reaction is the most efficient way for their synthesis. In this route, the dibrominated aromatic compounds allow for a Takahashi coupling and then for an oxidative dehydrogenation reaction to obtain the desired quinoidal forms (Route A, Figure 2). A quinoidal skeleton composed of four aryl groups bridged by a π-conjugated linker (Thiele’s hydrocarbon) is obtained via lithium–halogen exchange, followed by nucleophilic addition and reduction (route B). The rationale behind the design of these molecules and the methodology developed for their synthesis will be discussed based on the different precursors of forming quinoidal forms. In addition to the above routes, new methods have been reported, such as intra- or inter-molecular radical–radical coupling reaction [8].
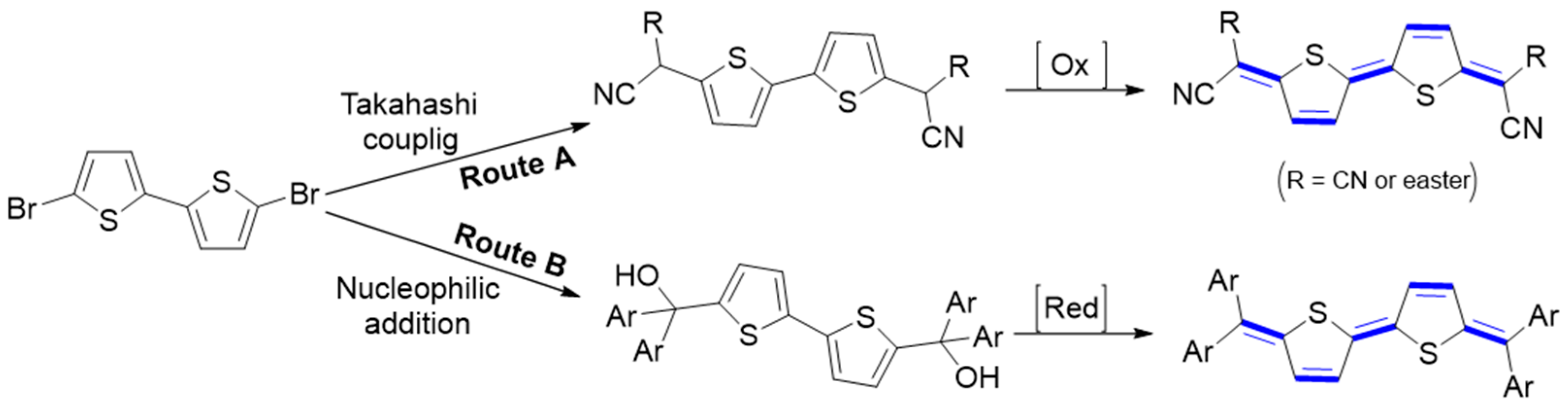
Figure 2. Typical synthetic strategies for the preparation of quinoidal π-conjugated molecules.
This entry is adapted from the peer-reviewed paper 10.3390/ma16062474
References
- Takimiya, K.; Nakano, M. Thiophene-Fused Naphthalene Diimides: New Building Blocks for Electron Deficient π-Functional Materials. Bull. Chem. Soc. Jpn. 2018, 91, 121–140.
- Fan, Y.W.; Liu, J.; Hu, W.P.; Liu, Y.Q.; Jiang, L. The effect of thickness on the optoelectronic properties of organic field-effect transistors: Towards molecular crystals at monolayer limit. J. Mater. Chem. C 2020, 8, 13154–13168.
- Sirringhaus, H. 25th Anniversary Article: Organic Field-Effect Transistors: The Path Beyond Amorphous Silicon. Adv. Mater. 2014, 26, 1319–1335.
- Li, H.; Brédas, J.-L. Developing molecular-level models for organic field-effect transistors. Natl. Sci. Rev. 2020.
- Choi, J.; Gordon, M.P.; Yuan, P.; Kang, H.; Zaia, E.W.; Urban, J.J. CHAPTER 1 Introduction. In Organic Thermoelectric Materials; The Royal Society of Chemistry: London, UK, 2020; pp. 1–20.
- Russ, B.; Glaudell, A.; Urban, J.J.; Chabinyc, M.L.; Segalman, R.A. Organic thermoelectric materials for energy harvesting and temperature control. Nat. Rev. Mater. 2016, 1, 16050.
- Zhang, J.; Tan, H.S.; Guo, X.; Facchetti, A.; Yan, H. Material insights and challenges for non-fullerene organic solar cells based on small molecular acceptors. Nat. Energy 2018, 3, 720–731.
- Li, G.; Matsuno, T.; Han, Y.; Wu, S.; Zou, Y.; Jiang, Q.; Isobe, H.; Wu, J. Fused Quinoidal Dithiophene-Based Helicenes: Synthesis by Intramolecular Radical–Radical Coupling Reactions and Dynamics of Interconversion of Enantiomers. Angew. Chem. Int. Ed. 2021, 60, 10326–10333.
This entry is offline, you can click here to edit this entry!
