Unicellular eukaryotes of the Trypanosomatidae family include human and animal pathogens that belong to the Trypanosoma and Leishmania genera. Diagnosis of the diseases they caused requires the sampling of body fluids (blood, lymph, peritoneal fluid, cerebrospinal fluid, etc.) or organ biopsies (bone marrow, spleen, etc.), which are mostly obtained through invasive methods. Body fluids or appendages can be alternatives to these invasive biopsies but their appropriateness remains poorly studied. To further address this question, we perform a systematic review on clues evidencing the presence of parasites, genetic material, antibodies, and antigens in body secretions, appendages, or the organs or proximal tissues that produce these materials.
- Trypanosoma
- Trypanosomiasis
- Leishmaniasis
- Chagas disease
- Canine Leishmaniasis
- Human African Trypanosomiasis
- Sleeping sickness
- Animal trypanosomiasis
- Dourine
- Nagana
- Canine visceral leishmaniasis
- Feline leishmaniasis
- Diagnosis
- Noninvasive biopsy
- Urine
- Feces
Systematic Review of Non-invasive Sampling Strategies for the Diagnosis and Detection of Trypanosomatid Pathogens and Infections
1. Introduction
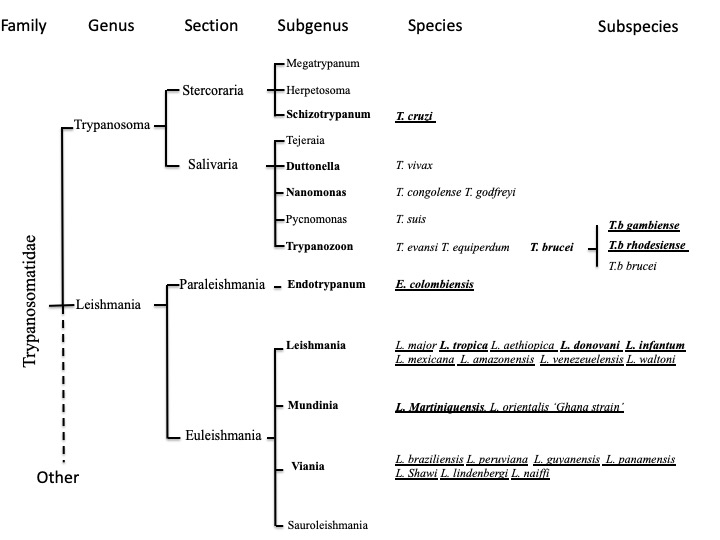
Unicellular eukaryotes of the Trypanosomatidae family include human and animal pathogens that belong to the Trypanosoma and Leishmania genera (including Endotrypanum) (Figure 1). Leishmania and possibly Trypanosoma are probably descended from the parasites of blood-sucking insects that survived accidental transmission to a vertebrate host during feeding [1].
Figure 1. Classification of human and animal pathogenic trypanosomatids. Human pathogenic species are underlined, and pathogens causing systemic infection are in bold.
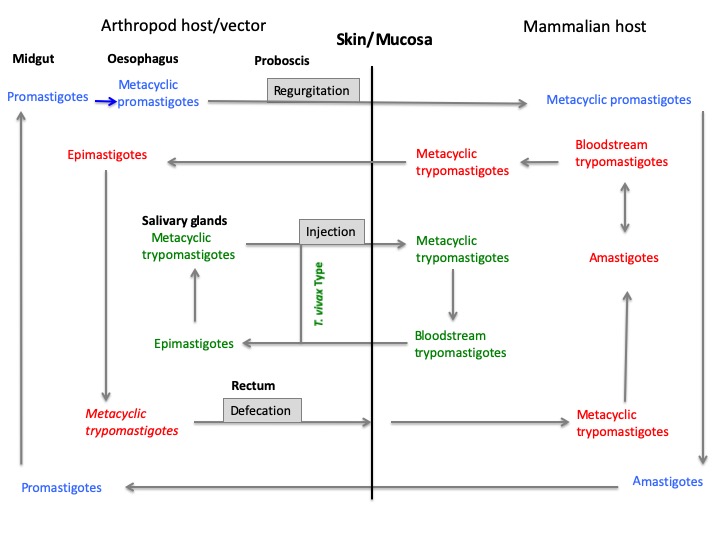
They possess a complex life cycle that includes arthropod vectors belonging to the Hemiptera and Diptera orders (Figure 2). Two Trypanosoma subspecies of T. brucei (i.e., Trypanosoma brucei gambiense, T. brucei rhodesiense) and T. cruzi, along with 21 species of Leishmania, are pathogenic for humans. They cause human African trypanosomiasis (HAT or sleeping sickness), Chagas disease (CD), and cutaneous (CL), muco-cutaneous (MCL) or visceral (VL) leishmaniases [2][3][4][5] (http://leishmania.ird.fr/). Occasional infections in humans with T. evansi, T lewisi, T. brucei brucei or T. congolense have been described, but little is known about the public health importance of these diseases [6].
Figure 2: Developmental life cycle of Leishmania (blue), T. cruzi (red), and Trypanosoma sp (green)
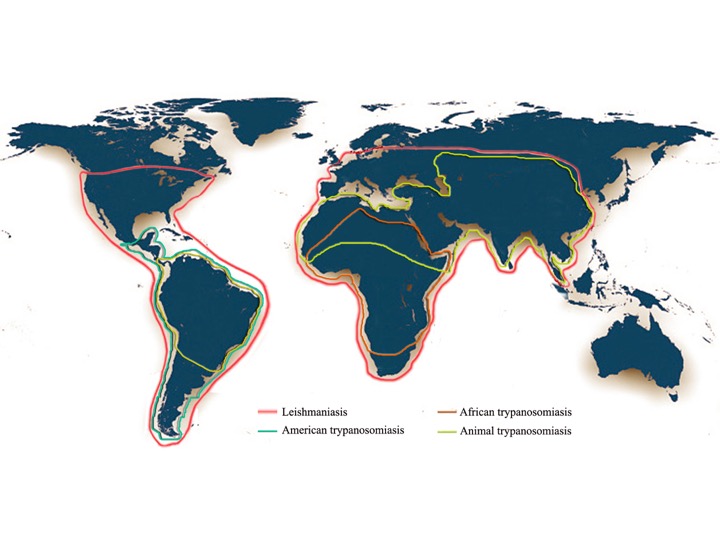
In addition to their impact on human health, these diseases also affect domestic, feral or wild animals. Canine visceral leishmaniases (CVL) are mainly caused by L. infantum infection and occasionally by L. donovani or L. major. Trypanosoma congolense, T. evansi, T. b. brucei, T. vivax, T. simiae, T. suis and more rarely T. godfreyi affect livestock, causing animal trypanosomiasis, and T. equiperdum affects equids [7][8]. Altogether, worldwide, more than 30 million people are infected with these pathogens, and approximately 100,000 persons die every year from Trypanosoma brucei spp., T. cruzi or Leishmania spp. infections [9]. An estimated of 48 million cattle are at risk of contracting Animal Trypanosomiasis in Africa. African Animal Trypanosomiasis (AAT) causes about 3 million deaths in cattle every year (http://www.fao.org/paat/the-programme/the-disease/en/). A map of the worldwide distribution of African and American human trypanosomiasis, animal trypanosomiasis and leishamniasis is given in the figure 3.
Figure 3. Worldwide distribution of African trypanosomiasis (Human African Trypanosomiasis), American Trypanosomiasis (Chagas Disease), Animal Trypanosomiasis, and Leishmaniasis. The map was drawn using adobe photoshop version 5.
2. Diagnosis and detection of Trypanosomatid’s infections
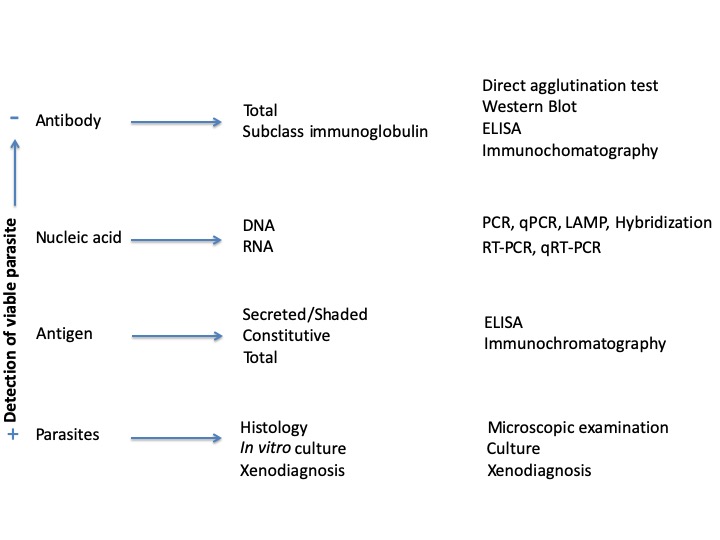
The diagnosis relies on the detection of parasites (parasitological methods), Nucleic acid (Molecular methods), antibody or antigens (Immunological methods) (Figure 4).
Figure 4. Overview of the methodologies in use to detect infections caused by trypanosomatid parasites.PCR: polymerase chain reaction. qPCR: quantitative polymerase chain reaction. RT-PCR: Reverse transcript polymerase chain reaction. qRT-PCR quantitative reveverse transcript polymerase chain reaction. LAMP: loop-mediated isothermal amplification. ELISA: enzyme-linked immunosorbent assay.
Microscopic examination of biopsy represents the simplest methodological approach to diagnose infection and detect pathogens. The hard identification at the species/subspecies level and its low sensitivity are limitations. Like microscopic examination, the in vitro parasites cultivation presents the advantage of being relatively simple to perform but has low sensitivity and requires sophisticated laboratory equipments. Xenodiagnosis is more complex than the other parasitological approaches but do not require biological sampling. Molecular methods involved polymerase chain reaction (PCR) or isothermal amplification of the genetic material. PCR is relatively simple to perform and to visualize. Refinements in PCR technologies included the development of nested PCR and of multiplexed PCR methodologies that have increased sensitivity and discriminative capacity of the test. Other refinement in the detection of the amplified product includes PCR-ELISA (Enzyme-Linked Immunosorbent Assay). PCR-RFLP (Restriction fragment Lenght Polymorphism) allows detecting variation between DNA fragments patterns, generated by restriction enzyme digestion caused by alternative nucleotides at the restriction sites that can be used for Leishmania and trypanosome species discrimination. PCR-HRM (High Resolution Melting) detects dsDNA alternatives by ascertaining changes in the fluorescence intensity, of a DNA-intercalating dye, during the dissociation process of double-stranded DNA (dsDNA) to single-stranded DNA (ssDNA). It was applied with success to Leishmania and T. cruzi detection, and species and DTU delineation [10][11]. Oligochromatography-PCR (OC-PCR) provides a simple and rapid format for detection of PCR or nucleic acid sequence-based amplification (NASBA) products, visualized on a dipstick by hybridization with a gold-conjugated probe. This detection format takes only 5–10 min and requires no equipment other than a water bath and a pipette [12][13]. Loop-mediated isothermal amplification (LAMP) uses the strand displacement activity of a DNA polymerase to amplify the dsDNA target with four primers designed to recognize six distinct regions. Amplification is completed in a single step at an isothermal temperature [14]. LAMP can be more sensitive than conventional PCR for the detection of Leishmania and Trypanosoma species [15][16]. The Dermal diagnostic tests or Leishmanin skin test (LST)/Montenegro test is based on the delayed type hypersensitivity (DTH) reactions raised following intradermal injection of killed Leishmania promastigotes into the skin forearm. It does not require biological sampling. Indirect immunofluorescence (IFAT) relies on the use parasites layered on a fluorescent glass slide that is used to test the presence of anti-parasites antibodies in the patient serum. This methodology was assayed for the serodiagnosis of Chagas disease, sleeping sickness, leishmaniasis and animal trypanosomiasis [17][18][19][20]. IFAT methodology is more used for surveillance program than for clinical diagnosis. Western blot allows visualize antigens targeted during antibody response. It presents the advantage of being more sensitive and specific than ELISA (see below). The direct agglutination test (DAT), further modified for detection of the agglutination activity on a card (CATT), allows the visualization of the precipitin activity. It uses whole micro-organisms as a means of looking for serum antibodies. CATT is a commonly serological test for HAT and is still in use for AAT serodiagnosis [21][22]. The agglutination methodology can also be performed with antibody coated latex beads to trap antigen. KAtex, a commercialized latex agglutination test, is developed for the diagnosis of visceral leishmaniasis and use a specific Leishmania antibody coated on latex particles [23]. Enzyme linked immunoabsorbent assays (ELISA) can be performed to detect and quantify antibodies or antigens in samples. Alternatively, sandwich ELISA can be used to detect circulating parasite’s antigens, that informs on the ongoing infectious process. Immunochromatography (ICT) or lateral flow test is based on a series of capillary beds that has the capacity to transport fluid spontaneously. The analyte is deposited on the dipstick and then spontaneously migrates to the first element that acts as a sponge to holds an excess of sample fluid. Once soaked, the fluid migrates to the second element in which antibody or antigen is present in conjunction with colored particles. The analyte migrates to the third component of the test, on which antibodies are immobilized to stop the flow. The methodologies used for typing detect trypanosomatidae parasites and diagnosing infections are summarized in the table 1.
Table 1. Methodologies to diagnose Chagas disease (CD), animal trypanosomiases (AT), human African trypanosomiasis (HAT), and leishmaniosis and/or to detect their respective causative agents.
|
Methodologies |
Quantification |
Culture |
CD detection & identification |
AT detection & identification |
HAT detection & identification |
Leishmania detection & identification |
Ref |
|
||
|
|
||||||||||
|
DNA/RNA-based Methods |
PCR |
PCR/qPCR /Multiplex |
yes |
no |
yes/DTU |
yes/sp |
yes/sp |
yes/sp |
[24-26] |
|
|
PCR-OC |
no |
no |
NA/NA |
yes/sp |
yes/sp |
yes/sp |
[12,27][ |
|
||
|
PCR-ELISA |
yes |
no |
NA/NA |
yes/sp |
yes/sp |
yes/sp |
[28-31] |
|
||
|
PCR-HRM |
no |
no |
yes/DTU |
NA/NA |
NA/NA |
yes/sp |
[11,32][ |
|
||
|
PCR-RFLP |
no |
no |
yes/DTU |
yes/sp |
yes/sp |
yes/sp |
[33-35] |
|
||
|
PCR-sequencing |
no |
no |
yes/DTU |
yes/sp |
yes/sp |
yes/sp |
[25,34] |
|
||
|
Other |
PFGE |
no |
yes |
yes/NA |
yes/sp |
yes/sp |
no/sp |
[34,36,37] |
|
|
|
NASBA |
no |
no |
NA/NA |
NA/NA |
yes/sp |
yes/sp |
[27] |
|
||
|
LAMP |
poss |
no |
yes/no |
yes/sp |
yes/sp |
yes/sp |
[16,38] |
|
||
|
Non DNA-based Methods |
Parasitology |
Microscopic examination |
yes |
no |
yes/no |
yes/no |
yes/no |
yes/no |
[2,39-41] |
|
|
In vitro parasite culture |
no |
yes |
yes/no |
yes/no |
yes/no |
yes/no |
/ |
|
||
|
Isolation in experimental animals |
no |
no |
yes/no |
NA/NA |
yes/no |
yes/no |
/ |
|
||
|
Xenodiagnosis |
no |
no |
yes/no |
NA/NA |
yes/no |
yes/no |
[42-45] |
|
||
|
Dermal diagnostic tests |
no |
no |
NA/NA |
NA/NA |
NA/NA |
yes/no |
[46] |
|
||
|
Immunology/Serology |
ELISA Ab |
no |
no |
yes/no |
yes/no |
yes/no |
yes/no |
[47-49] |
|
|
|
ELISA Ag |
no |
no |
yes/no |
yes/no |
NA/NA |
yes/no |
[50] |
|
||
|
IFAT |
no |
no |
yes/no |
yes/no |
yes/no |
yes/gen |
[18-20,51,52] |
|
||
|
ICT Ag |
no |
no |
NA/NA |
NA/NA |
NA/NA |
yes/gen |
[53] |
|
||
|
ICT Ab |
no |
no |
NA/NA |
NA/NA |
yes/no |
yes/no |
[54,55] |
|
||
|
DAT/CATT |
no |
no |
yes/yes |
yes/yes |
yes/yes |
yes/yes |
[21,47,48,56] |
|
||
|
Western blot |
no |
no |
yes/no |
NA/NA |
NA/NA |
yes/sp |
[57,58] |
|
||
|
Protein-based methods |
MLEE |
no |
yes |
no/DTU |
no/sp |
no/sp |
no/sp |
/ |
|
|
|
MALDI-TOF |
no |
yes |
no/DTU |
NA/NA |
no/sp |
no/sp |
[59,60] |
|
||
|
|
||||||||||
gen: genera. sp: species. DTU: discrete typing unit. NA: not available. DAT: direct agglutination test. CATT: card agglutination test for trypanosomiasis. MLEE: multilocus enzymatic electrophoresis. MALDI-TOF: matrix-assisted laser desorption ionization-time of flight. ICT: immunochromatographic test. ELISA: enzyme-linked immunosorbent assay. PCR-OC: polymerase chain reaction with oligochromatography. LAMP: loop-mediated isothermal amplification. Ab: Antibody. Ag: Antigen. PFGE: pulse field gel electrophoresis. NASBA: nucleic acid sequence-based amplification. PCR: polymerase chain reaction. HRM: high melting resolution. RFLP: restriction fragment length polymorphism.
3. Systematic review of Non-invasive Sampling Strategies for the Diagnosis and Detection of Trypanosomatid Pathogens and Infections
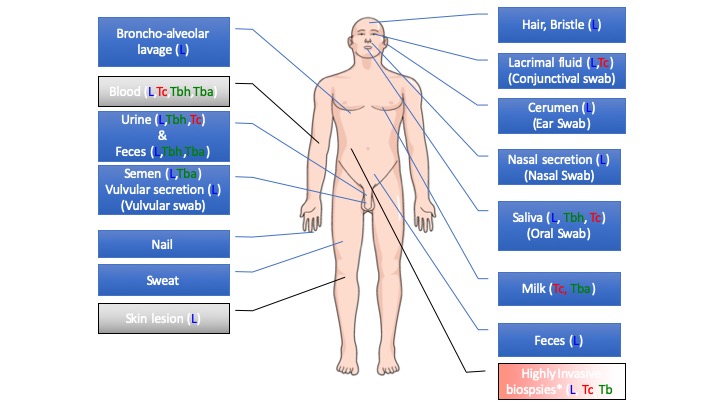
The selection of the appropriate biopsy for diagnoses relates to the physiopathology of the diseases, reflecting the disseminative capacity (tissue or organ tropism) of these pathogens within its host. Therefore, the diagnosis of these diseases requires the sampling of body fluids (blood, lymph, peritoneal fluid, cerebrospinal fluid, etc.) or organ biopsies (bone marrow, spleen, etc.), which are mostly obtained through invasive methods. Alternative biological samples, such as body secretions (milk, saliva, urine, semen, nasal secretion, lacrimal fluid, earwax, sweat, feces, etc.) or appendages (nail, hair, bristles, etc.) that are constantly produced, might be an interesting alternative to invasive biopsies. No- invasive biological sampling that do not require trained professional and are easy and safe to collect would render the diagnosis more convenient. We address the interest of such biological material, via a systematic review of the published literature and meta-analysis on data extracted from a defined pool of published paper. A schematic overview is given in the figure 3.
Figure 4: Schematic representation of the compiled evidence on the presence of Trypanosoma and Leishmania parasites in body secretions or appendages. Tba: Trypanosoma species responsible for animal trypanosomiasis; Tbh: Trypanosoma species responsible for human African trypanosomiasis; Tc: Trypanosoma cruzi (American trypanosomiasis, Chagas disease); L: Leishmania spp. Blue boxes represent material obtained through noninvasive methods, while gray and red boxes represent materials obtained through invasive or highly invasive methods.
3.1 Urine
Urine is an easy-to-collect secretion that is produced daily. Therefore, a large amount of information has been gathered on the presence of trypanosomatid parasites within this liquid.
Human and Animal Leishmaniases. The survival capacity of Leishmania, and cruzi, in urine is known since 1966 [24]. In vitro, urine can promote the growth of the Leishmania promastigote and can be used as a low-cost culture adjuvant alternative to serum [25]. The first evidence of the presence of Leishmania in the urine of patients infected by L. donovani came in the 1930s through the detection of Leishman-Donovan bodies in the urine of infected patients [26]. The presence of viable Leishmania parasites in the urine of infected individuals is documented [27][28][29]. The crossing of the glomerular barrier by Leishmania is thought to be a consequence of VL renal lesions and renal failure [30][31]. Tubulointerstitial involvement and glomerulonephritis are main causative of the proteinuria disorder[32][33][34]. Urine represents a fluid from which parasite DNA is easily extracted for detection and species identification [35], and has been probed in patients [36][27][37][38], and in animal reservoirs [39][40]. These searches were performed in VL caused by L. infantum [35][26][27][41]; in CL and VL-HIV+ patients infected by L. martiniquensis [42]; in CL due to L. major or L. tropica [36]; in South American cutaneous and mucocutaneous leishmaniasis caused by L. braziliensis, L. guyanensis or L. peruviana and in canine visceral leishmaniasis [39][40][43]. The presence, in urine, of precipitin activities directed against several microorganisms is known since 1948 [44]. The nature of these activities was formerly attributed to antibodies in 1965 [45]. In 1983, the presence of anti-Leishmania antibodies in urine was demonstrated [46][47]. Since then, the anti-Leishmania antibody response in patient urine to diagnose VL has been further investigated. ELISA, which uses recombinant antigens or whole antigen preparations as well as the direct agglutination test (DAT), were used to test for disease diagnosis using patient urine [35][46][48][49][47][50][51][52][53][54]. Immunochromatographic tests to detect rk39 antibodies are currently commercialized and have been thoroughly tested in urine [35][50][55][56][57]. Antibodies present in urine directed against rKP42, a kinesin-related protein and a homolog of rK39, also showed remarkable sensitivity and specificity for VL [58]. This specificity and sensitivity were comparable to those obtained with ELISA performed using acetone-treated L. donovani promastigote antigens or DAT. The detection of the antibody response against Leishmania infection, due to L. major, L. tropica or L. infantum, was also investigated using Western blot [46][47]. IgA or IgG are detected in the urine of dogs suffering from, leishmaniasis [59], where antibodies directed against L. infantum are present [60][61][62]. Because of the persistence of antibodies after cure, these tests cannot be used to diagnose VL in people with a past history of VL. A search for Leishmania proteins in urine has therefore been undertaken [45][63][64][65][66], as well as for changes in the urinary proteome of infected individuals [67]. In L. infantum-infected patients, iron superoxide dismutase, L. infantum tryparedoxin, and L. infantum nuclear transport factor 2 (Li-ntf2) were identified by mass spectrometry analysis [63][64]. When used in a multiplex ELISA test, these biomarkers show a sensitivity superior to 80% for VL diagnosis caused by L. infantum but fail to accurately diagnose VL due to L. donovani [63][64]. In L. donovani-infected patients, two biomarkers showing a sensitivity of approximately 82% were characterized [68]. A low-molecular-mass heat-stable leishmanial carbohydrate antigen has allowed the development of a latex agglutination test (KAtex) to be commercialized [69][70]. Its efficiency was thoroughly tested in various VL endemic areas [67][35][37][54][71][72][67][73][74]. The KAtex test in urine might be useful for the detection of VL within the clinical case definition: fever for more than two weeks, splenomegaly and no previous history of VL [75].
Human African Trypanosomiasis & Animal Trypanosomiases. No information on the presence of DNA, antibodies or antigens in the urine of human individuals affected by sleeping sickness was collected during the systematic review. The sole evidence on the presence of genetic material in urine comes from an experimental infection of vervet monkeys by brucei. In this model, Trypanosoma DNA could be amplified from urine, with LAMP as early as 17 days postinfection [76]. Biochemical changes associated with trypanosome infection are published on animal models infected by various Trypanosoma species. Rabbits infected by parasites of the T. brucei subgroup showed a progressive increase in proteins released in the urine [77][78]. The presence of fibrinogen and fibrin degradation products in the urine of rabbits infected by T. brucei is suggestive of a glomerular permeability change [77]. Mice or Microtus montanus infected by T. b. gambiense showed an increase in the excretion of aromatic amino acid catabolites [79][80][81][82]. In mice infected with T. evansi, the concentration in phenylpyruvic acid, 4-hydroxyphenylpyruvic acid, and indole-3-pyruvic acid correlates with parasitemia and returns to normal following suramin treatment [83]. These metabolites are also detected in dogs and donkeys experimentally infected [83]. The high rate of aromatic amino acid catabolism by African trypanosomes was associated with the large decrease in free serum levels of aromatic amino acids and with alterations in host tyrosine and phenylalanine metabolism. These events correlate with the pathology of sleeping sickness and the depletion reported in certain amino acids (tryptophan), which would lead to the depletion of essential metabolites such as serotonin and the toxicity of end products such as phenylpyruvate reviewed in [84]. Changes in the urinary proteome of patients suffering from sleeping sickness were observed, notably, in proteins related to several infectious processes. These changes can be the rationale for developing noninvasive tools aimed at tracking the disease stage [85]. In animal models, T. brucei parasites are observed in the kidney glomeruli of infected rats, and T. lewisi in the kidney capillaries [86][87]. T. musculi, a parasite specific to mice, resides in the blood and lacks intracellular stages. After immune clearance of the flagellates from the general circulation, mice became resistant to reinfection. However, long after parasites are no longer detected in the peripheral blood, they still persist in the vasa recta of the kidneys in a peculiar biological stage [88], releasing molecular determinants in the urine as potential diagnostic biomarkers.
Chagas Disease. Evidence on the capacity of cruzi to survive in urine came along with those on Leishmania [23], and T. cruzi amastigotes have been occasionally detected in the kidney [89]. Parasite DNA was detected in the urine of experimentally infected pigs (Sus scrofa) or mice [90][91][92]. The crossing of T. cruzi to urine in experimentally infected mice appars independent of renal injuries [92]. The presence of DNA in urine is associated with the presence of parasite DNA in blood and heart and with a high level of parasite DNA in blood, but not with the presence of parasites in kidney or kidney injury [92]. The detection of antigens within the urine of patients suffering from acute or chronic CD [93][94][95] has opened up some new innovative approaches for diagnosis. The presence of these urinary antigens is generally associated with active or recent infections. A number of T. cruzi urinary antigens can be identified and classified according to their molecular weight, such as the 80 kDa iron binding protein or the 150-160 kDa antigen. These antigens were detected by the use of antibodies raised against an immunodominant epitope of T. cruzi. In addition, parasite tubulin was also detected in urine as well as a set of immunoreactive antigens [96][97][98]. To develop a diagnostic test based on a capture ELISA system, a panel of polyclonal antibodies was produced against membrane antigens or trypomastigote excreted/secreted antigens. The test performed on urine from patients positive for ELISA capture against sera demonstrated a 100% positivity [99]. Antigens are present in urine at low concentrations and are susceptible to degradation after collection. These characteristics limit the sensitivity and the reliability of all urinary-based antigen detection. The use of nanoporous hydrogel particles produced with poly(N-isopropylacrylamide) (poly(NIPAm)) and N,N9-methylenebisacrylamide (BAAm) coupled to chemical baits via amidation reaction has the potential to concentrate and preserve the antigens [99] for its application using urine [100]. The test, called Chunap (Chagas urine nanoparticle test), has been further developed and evaluated for congenital transmission of T. cruzi. In this condition, it showed more than 90% sensitivity and more than 95% specificity [99]. It also demonstrated good sensitivity in HIV-T. cruzi coinfected cases [99].
3.2 Feces
Human and Animal Leishmaniases. Most of the references that document the findings of Leishmania in human feces were published during the 1920s and l930s [101][102]. The detection of Leishmania amastigotes and its DNA in the feces of a dog infected by infantum is documented [103]. More recently, a screening of wild gorilla fecal samples revealed the presence of promastigotes and amastigotes of L. major within these samples [104]. Nevertheless, this finding has been a matter of debate [105][99]. More recently, a large diversity of trypanosomatid parasites in the feces of great apes, but no Leishmania DNA, was evidenced [106]. Since the 1920s, at the time Donovan bodies were detected in human feces, no additional information on the detection of parasites or the DNA of Leishmania in human feces has been published. The only other clues on the presence of Leishmania DNA in the human gut come from studies performed on pre-Columbian mummies using next-generation sequencing. These analyses highlight the presence of DNA related to Leishmania and T. cruzi, without being able to firmly identify Leishmania at the species level [107][108].
Human African Trypanosomiasis & Animal Trypanosomiases. The ITS1 region of b. brucei, T. b. gambiense, T. b. rhodesiense and T. b. evansi was successfully amplified from DNA isolated from fecal samples of experimentally infected mice [99] and T. b. rhodesiense and/or T. b. gambiense DNA was detected in the feces of wild gorillas [106][99].
Chagas Disease. Megacolon is a pathological affliction that occurs in chagasic patients [109]. Evidence of the presence of cruzi DNA in the gut of pre-Columbian mummies is documented, depicting that the disease has a long evolutionary history with humans in South America [107][108][110][111]. The tissue tropism of various T. cruzi isolates was investigated in a mouse model of infection. In these experiments, parasite DNA was detected in the small intestine and rectum of the animals [112][113]. In infected mice, the gut is the primary site of parasite persistence in the BALB/c model of chronic Chagas disease and is associated with a perturbation in the gut microbiome [114][115]. In opossums (Didelphis marsupialis), one of the multiple wild reservoirs of T. cruzi, the developmental cycle that usually occurs in the intestine of the triatomine vector can take place in the anal odoriferous glands [116]. In human feces, to our knowledge, no information is currently published.
3.3 Saliva/Oral Swab/Sputum
Oral swab, saliva, and sputum are the easiest and least-invasive sampling methodology for the detection of infectious pathogens. Although bronchoalveolar lavage is not considered a nonintrusive method to collect biological samples, it does not cause damage to tissues.
Human and animal leishmaniases. The presence of viable Leishmania parasites in the saliva of infected patients was demonstrated in 1934 by Forkner [117]. More recently, braziliensis was recovered from the saliva of a person suffering from cutaneous leishmaniasis [118]. A large number of studies describe the successful detection and identification of Leishmania DNA in saliva or oral swabs, with PCR or other methodologies of DNA amplification (LAMP). The DNA was amplified in L. martiniquensis-HIV positive and negative patients [41][119][120][121][99] but also in kala-azar patients infected by L. donovani [122][123] and in dogs suffering from CVL [124][125]. In 1994, a report discussed the presence of agglutinating anti-leishmania activity, an antibody, in the saliva of kala-azar patients [126]. The capacity of anti-leishmania antibodies present in the saliva to be used to diagnose CVL and VL was investigated more recently. For CVL, the detection of IgG2 and IgA antibodies targeting specific recombinant K39 protein (rK39) in saliva demonstrated the usefulness of this test to diagnose CVL and to differentiate between seropositive and seronegative dogs [127]. In humans, a preliminary experiment involving the detection of rK39 antibodies demonstrated 99.2% sensitivity and 100% specificity for diagnosis using patient sputum [55]. Interestingly, KAtex shows higher sensitivity to diagnose Mediterranean visceral leishmaniasis with oral fluid than with urine, even if this test was originally conceived to be used with urine [128].
Human African Trypanosomiasis & Animal Trypanosomiases. Trypanosome-specific IgG can be detected in the saliva of b. gambiense-infected HAT patients using ELISA. Nevertheless, the antibody concentration is at least 250-fold lower in saliva than in serum [129]. The ELISA performed on the saliva of a cohort of 208 individuals, including 78 parasitologically confirmed patients, demonstrated a robust sensitivity and specificity (>90%) comparable with CATT performed on sera [99]. Since then, no additional experiments have been performed.
Chagas Disease. The first evidence on the presence of cruzi in the saliva of experimentally infected dogs dates from 1966 [130][131]. More recently, an ELISA that detected and quantified the IgG response to T. cruzi was developed using saliva from infected patients. The methodology was tested with success on saliva from patients with chronic infection, which is characterized by the absence of blood circulating parasites [132][133]. The oral swab was also tested to detect fragments of Trypanosoma DNA (Trypanosoma dionisii, T. rangeli and T. cruzi) to evaluate the potential reservoirs for T. cruzi in gallery forest bats [134].
3.4 Conjunctival Swab/Lacrimal Fluid/Occular
A swab is a small piece of soft material used for taking a small amount of substance from a body. The conjunctival or corneal swab, a routine practice to perform biological sampling to diagnose eye infection, has been applied to detect trypanosomatid pathogens.
Human and animal leishmaniases. In humans, ocular lesions are usually associated with systemic signs [135][136][137]. Ocular pathologies are documented in patients suffering from cutaneous [138][139][140][141][142][143], diffuse cutaneous [144] or post-kala-azar dermal leishmaniasis [135] and in VL [145][146]. In dogs, keratoconjunctivitis and kerato-uveitis are described as the most usual symptoms, occurring in 16–80% of affected dogs [147][148]; keratoconjunctivitis is also observed in feline leishmaniasis [149]. Leishmania has been isolated from the aqueous humor of a patient suffering from leishmaniasis [136]. In naturally infected dogs, anti-Leishmania IgG was detected in the aqueous humor, although at a level not related to the serum level [150][151]. In dogs, histopathological investigations depicted the presence of plasmatic cells and macrophages bearing amastigote forms of Leishmania in the ciliary body, sclerocorneal limbus, iris, and lacrimal duct but also in smooth and striated muscles [151][152][153][154]. Leishmania were observed in squamous carcinoma cells from conjunctival swab samples in an HIV+ patient [155]. Leishmania DNA can be detected and quantified by qPCR in the lacrimal glands of symptomatic dogs [156]. All these clues have prompted testing the efficiency of the conjunctival swab for CVL diagnosis [157][158][159][99] and tracking asymptomatic dog infections [160] but also for diagnosing feline leishmaniasis [99][161][162]. In addition, the detection of Leishmania DNA in conjunctival swabs has also been applied to track infantum wild reservoirs [163][164].
Human African Trypanosomiasis & Animal Trypanosomiases. In humans, eye pathologies associated with trypanosome infections remain unusual [165], and an investigation for the presence of parasites, DNA or antibodies within conjunctival swabs has not been performed. In dogs infected by b. brucei, the eyes are one of the most severely affected organs, and infection by T. evansi can provoke blindness [166][167]. Experimental infections of cats with T. brucei [168] and of cats and goats with T. evansi highlight their disseminative capacity in the eye, with their presence being detected in the aqueous humor [169][170].
Chagas Disease. In 1935, Romana first described the “unilateral schyzotrypanosomic conjunctivitis” associated with acute cruzi infection later known as Romana’s sign [171]. The invasion of the human host by T. cruzi occurs in various ways but mainly via skin lesions or the conjunctival way [172][173]. T. cruzi parasites deposited on the conjunctiva, via the manipulation of contaminated bug feces, are drained with tears into the nasolacrimal duct and nasal cavity. Then, T. cruzi infects the most proximal tissues lined with cuboidal and columnar epithelial cells [173][174]. Surprisingly, reports on eye pathology in CD patients are very scarce. Recently, the first case of Trypanosoma cruzi–associated retinitis was diagnosed [175]. The presence of T. cruzi amastigotes in the conjunctiva, corneal stroma, the adjacent ocular muscle and the interstitial macrophages of Thrichomys apereoides (Rodentia, Echimyidae) experimentally infected with T. cruzi is documented [176].
3.5 Genital Organs: Semen/Vulvular Secretion
Some trypanosomatid infections impact male and female reproductive organs, causing infertility [177]. Leishmania infection provokes a decrease in sperm quality, genital lesions, testicular amyloidosis, chronic prostatitis and epididymal inflammation [177]. Chagas disease is associated with male hormonal changes and a loss in sperm quality due to parasitic load. In females, the invasion of the placenta and hormonal changes are associated with the overproduction of inflammatory cytokines in the oviduct and uterus. In sleeping sickness, an inpairment in the spermatogenic cycle due to damage in the pituitary gland as well as damage to the reproductive organs is reported. In females, impairment in the estrus cycle due to pituitary gland damage is noticed [177].
Human and animal leishmaniases. Leishmaniasis does not belong to the broad list of potential sexually transmitted infections (STIs). Nevertheless, some evidence suggests that venereal transmission of leishmaniasis does occur in dogs and humans [10][178][179][180]. In humans, lesions in the male genitalia are well documented [181][182][183], with the presence of parasites [184][185]. In dogs, genital lesions associated with visceral leishmaniasis and the shedding of Leishmania in the semen of naturally or experimentally infected dogs is described and can lead to infertility [186][187]. In the prepuce and glans of male symptomatic dogs, heavy parasite burden has been detected and is associated with inflammation, testicular degeneration, atrophy, an absence of spermatogenesis, and necrosis [188]. In these dogs, immunohistochromatography showed that 75% of symptomatic dogs and 35% of asymptomatic dogs were positive for Leishmania in the testis. These percentages rose to 95% and 60% for symptomatic and asymptomatic leishmaniasis, respectively, in the epididymal duct. The detection of Leishmania parasites in semen has been evidenced through parasite culture [28], microscopic observation or immunohistology [186][188], and polymerase chain reaction [186][187][189]. A CVL experimental infection of 8 female dogs pinpoints that vulvar swab is at least as sensitive as oral swab for the detection and quantification of Leishmania kDNA, and this methodology is proposed to confirm Leishmania infection in seropositive dogs [190]. The presence of L. infantum amastigotes in the genital tract of naturally infected bitches is documented [191].
Human African Trypanosomiasis & Animal Trypanosomiases. In humans suffering from sleeping sickness, sterility or infertility, menstrual disorder, a loss of libido, impotence, and amenorrhea are reported [192]. Testicular damage and clinical manifestation are described [193], and sexual transmission is very occasionally observed [194]. Trypanosoma equiperdum, responsible for dourine, is a sexually transmitted disease of Equidae [195][196][197]. A loss of fertility is observed in infected animals and is associated with the detection of parasites in semen [198][199]. For Trypanosoma vivax, in addition to tsetse flies, transmission routes include transplacental and sexual routes, and parasites were detected in the semen of infected animals [194][200]. In naturally acquired or experimentally induced animal trypanosomiasis caused by brucei or T. congolense, a decrease in semen production associated with an alteration in spermatogenesis is recorded [201][202][203][204][205]. Histological lesions characterized by testicular degeneration, epididymitis and epididymal epithelial hyperplasia were detected in the same animals and suggested the participation of the parasite in the pathogenic mechanism of reproductive damage, frequently reported in infected animals [200][202][206]. In experimentally infected mice, bioluminescent imaging confirmed the localization of viable trypanosomes in infected mice [207] with an accumulation in the epididymal adipose tissue and in the epididymis [208].
Chagas Disease. Sexual and transplacental transmission are described and have epidemiological relevance [99][209]. In 1911, Vianna described testis lesions in experimentally cruzi-infected guinea pigs [210]. Human orchitis was described in 1916 [211]. The first evidence on the infection of the testis by T. cruzi during the acute phase of the disease dates from 1982 [212]. Since then, experimental infection has further shed light on the disseminative capability of this organism during the acute phase of the disease into the male and female genital organs. In a mouse model of infection, T. cruzi was detected in the preputial glands and skin, penis, testicular albuginea, epididymis, vas deferens, seminal vesicles, prostate and urethral glands [213][214]. In females, T. cruzi invades cells of the vagina, uterus, oviduct, ovary, and clitoris [205][207]. In addition, T. cruzi DNA has been detected in the semen of patients suffering from Chagas disease [99][215]. Limited data exist for humans, but the presence of T. cruzi was reported in seminiferous tubules and ovarian cells of children who succumbed to Chagas disease and in menstrual blood of infected patients [216].
3.6 Milk
The presence of parasites of the Trypanosomatidae family in milk has been probed in view of a maternal transmission risk and food contamination.
Human and animal leishmaniases. Attempts to test the capacity of Leishmania to survive and proliferate in milk were undertaken as early as the 1930s; some evidence on the adequacy of this medium to support Leishmania survival was published [217]. Histopathological investigation of female dogs suffering from CVL probed the presence of Leishmania amastigotes in the mammary glands [188]. Nevertheless, the presence of Leishmania in milk has not yet been reported in patients suffering from leishmaniasis.
Human African Trypanosomiasis & Animal Trypanosomiases. The investigation of trypanosomes in milk has a long history of research and has first focused on the risk of the transmission of pathogens, mainly the risk of transmission of evansi. Evidence on the presence of T. evansi in the milk of lactating cow comes from the work of Zwick and Fisher and described by Henry and Guilhon in 1944 [218]. During the 1910-1930 period, a set of experimental procedures was employed to detect the presence of various species of Trypanosoma species in the milk of experimentally or naturally infected animals [218]. Nathan-Larrier reported that mice and rats experimentally infected by T. equiperdum show trypanosomes in their milk [219].
Chagas Disease. Because cruzi, originally named Schyzotrypanum cruzi, possesses the capacity to cross the epithelium and to infect via the oral route [218][99][220], the presence of this pathogen in the milk has been searched. T. cruzi was found in the milk of experimentally infected mice [219][221][222], and several reports describe the presence of this pathogen in the milk from pregnant women [223][224][225][226], as reviewed by Norman and Lopez-Vélez [227]. In most cases, the presence of T. cruzi in the milk of pregnant women has been attributed to contamination by infected blood due to nipple bleeding [227]. Therefore, the capacity of T. cruzi to invade the mammary gland of infected females has been undertaken. These histological investigations on mice demonstrate the presence of T. cruzi amastigotes in the mammary gland alveoli, excretory ducts, the connective tissue envelope of the ducts, inter- and intralobular connective tissue, histiocytes, adipose tissue, the sebaceous glands of the nipple, striated muscle fibers beneath the nipple, and inside the duct lumen [228]. Such proximity of T. cruzi parasites with colostrum or milk argues for the inactivation of T. cruzi by pasteurization or microwave treatment [229][230].
3.7 Nasal Secretion
Human and animal leishmaniases. The presence of donovani parasites has been detected in the nasal secretions of patients as early as 1936 and reconfirmed sixty years later [25][117]. Parasite DNA can efficiently be detected in this secretion [125]. Parasite DNA has also been detected in the clinically unaffected nasal mucosa of patients infected by L. braziliensis [99]. Among the clinical presentation of human leishmaniasis, mucocutaneous alterations are described. They are mainly present in South America and are caused by a restricted number of Leishmania species [4]. Nevertheless, this uncommon presentation is also reported to be caused by some Old World species [231], suggesting that nasal secretion deserves further investigation to be confirmed as a positive fluid for Leishmania detection.
Human African Trypanosomiasis & Animal Trypanosomiases. No information gathered during the study.
Chagas Disease. No information gathered during the study.
3.8 Ear Swab/Cerumen
Human and animal leishmaniases. Leishmania DNA has been detected and quantified in the cerumen of infected dogs [99]. A recent publication demonstrates that cerumen-qPCR expresses the highest sensitivity (87.5%) to detect genetic materials, followed by hair (lesions: 78.57%, healthy skin: 62.5%), and blood (68.75%) [99]. The ear skin of infected dogs bears a high parasite load compared to other corporal zones and tends to be more infective to sand flies than that of the abdomen [232]. The usefulness of ear swab was investigated in CVL-positive dogs, and a positivity of 43% was recorded [125]. In addition, ear lesions caused by mexicana (Chiclero’s ulcer) are known, but the lesions at this site can be caused by other Leishmania species [233].
Human African Trypanosomiasis & Animal Trypanosomiases. No information gathered during the study.
Chagas Disease. No information gathered during the study.
3.9 Hair/Bristles
Appendages such as hair, bristles, and nails are not referenced as target tissues for trypanosomatid survival and proliferation. Therefore, only a few studies were performed using these materials for the investigation of trypanosomatid infection.
Human and animal leishmaniases. The first series of analyses was performed on dog hair by searching for markers of infection via the analysis of volatile organic compounds. This approach is based on the hypothesis that illnesses can modify odors exhaled by individuals [234] and that canine Leishmania infection involves the liberation of some volatile compounds specific to the infection [235][236]. Therefore, with this methodology, it is not the infectious agent that is detected, nor the immunologic response nor the set of volatile compounds exhaled by the dogs. Although hair is not known as a target tissue for Leishmania, an investigation of Leishmania DNA was undertaken on CVL in a mouse experimental model of infection with major but also in the hair of wild mammals or Leporidae [235][237][99]. In mice, the DNA of L. major is detected near the inoculation site but also in hair collected in body areas far from the infection site [99]. The first evidence on the usefulness of PCR performed on hair to act as a biomarker of infectiousness of the host came from CanL cases [238]. The rationale for such an accumulation of parasite DNA into the hair of the infected animal is not entirely understood. The hypothesis of a « transdermal elimination » process has been raised. This process, observed as a secondary component of primary skin diseases, includes the elimination of endogenous substances but also exogenous infectious organisms, such as Mycobacterium tuberculosis or HIV [239]. It requires the direct incorporation of the parasite DNA among skin and hair keratinocytes at the site of inoculation. The intracellular infection of keratinocytes with Leishmania has not been detected following the infection of C57Black/6j mice [240], but the presence of Leishmania amastigotes has been observed in the hair follicles of patients with cutaneous leishmaniasis [239].
Human African Trypanosomiasis & Animal Trypanosomiases. No information gathered during the study.
Chagas Disease. No information gathered during the study.
4.Conclusions
This literature analysis reveals striking facts and gaps in the usefulness of body fluid secretions and/or appendages for the diagnosis of infection caused by Trypanosomatidae parasites. Nevertheless, diagnostic protocols that use new non-invasive biological samples should be of help to track disease in endemic areas with limited resources but they should also be of help to track diseases evolution and clinical and/or chemotherapy success.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21051684
References
- Alastair G. B. Simpson; Jamie R. Stevens; Julius Lukeš; The evolution and diversity of kinetoplastid flagellates. Trends in Parasitology 2006, 22, 168-174, 10.1016/j.pt.2006.02.006.
- NTD Modelling Consortium Discussion Group on Gambiense Human African Trypanosomiasis; Insights from quantitative and mathematical modelling on the proposed 2030 goal for gambiense human African trypanosomiasis (gHAT). Gates Open Research 2019, 3, 1553, 10.12688/gatesopenres.13070.1.
- Yulan Wang; Jürg Utzinger; Jasmina Saric; Jia Li; Jean Burckhardt; Stephan Dirnhofer; Jeremy Nicholson; Burton H. Singer; Reto Brun; Elaine Holmes; et al. Global metabolic responses of mice to Trypanosoma brucei brucei infection. Proceedings of the National Academy of Sciences 2008, 105, 6127-6132, 10.1073/pnas.0801777105.
- Plos Neglected Tropical Diseases Staff; The Plos Neglected Tropical Diseases Staff; Correction: A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies.. PLOS Neglected Tropical Diseases 2016, 10, e0004770, 10.1371/journal.pntd.0004770.
- Denis Sereno; Leishmania (Mundinia) spp.: from description to emergence as new human and animal Leishmania pathogens. New Microbes and New Infections 2019, 30, 100540, 10.1016/j.nmni.2019.100540.
- Philippe Truc; Philippe Büscher; Gérard Cuny; Mary Isabel Gonzatti; Jean Jannin; Prashant Joshi; Prayag Juyal; Zhao-Rong Lun; Raffaele Mattioli; Etienne Pays; et al. Atypical Human Infections by Animal Trypanosomes. PLOS Neglected Tropical Diseases 2013, 7, e2256, 10.1371/journal.pntd.0002256.
- Ebhodaghe, F.; Ohiolei, J.A.; Isaac, C. A systematic review and meta-analysis of small ruminant and porcine trypanosomiasis prevalence in sub-Saharan Africa (1986 to 2018). Acta Trop. 2018, 188, 118–131.
- Faith Ebhodaghe; C. Isaac; John Asekhaen Ohiolei; A meta-analysis of the prevalence of bovine trypanosomiasis in some African countries from 2000 to 2018. Preventive Veterinary Medicine 2018, 160, 35-46, 10.1016/j.prevetmed.2018.09.018.
- Kenneth Stuart; Reto Brun; Simon Croft; Alan H. Fairlamb; Ricardo E. Gürtler; Jim McKerrow; Steve Reed; Rick L. Tarleton; Kinetoplastids: related protozoan pathogens, different diseases. Journal of Clinical Investigation 2008, 118, 1301-1310, 10.1172/JCI33945.
- Fabiana L. Silva; Raquel G. Oliveira; Teane M.A. Silva; Mariana N. Xavier; Ernane F. Nascimento; Renato De Lima Santos; Venereal transmission of canine visceral leishmaniasis. Veterinary Parasitology 2009, 160, 55-59, 10.1016/j.vetpar.2008.10.079.
- Vinícius Vasconcelos Gomes De Oliveira; Leucio Câmara Alves; Valdemiro Amaro Da Silva Júnior; Transmission routes of visceral leishmaniasis in mammals. Ciência Rural 2015, 45, 1622-1628, 10.1590/0103-8478cr20141368.
- Andreia P Turchetti; Tayse D Souza; Tatiane Paixao; Renato De Lima Santos; Sexual and vertical transmission of visceral leishmaniasis.. The Journal of Infection in Developing Countries 2014, 8, 403-407, 10.3855/jidc.4108.
- Ricardo J. Bosch; Ana B. Rodrigo; Purificación Sánchez Sánchez; Maria V. De Galvez; Enrique Herrera; Presence of Leishmania organisms in specific and non-specific skin lesions in HIV-infected individuals with visceral leishmaniasis. International Journal of Dermatology 2002, 41, 670-675, 10.1046/j.1365-4362.2002.01610.x.
- Christopher Hillyer; Transmission of visceral leishmaniasis through blood transfusions from infected English Foxhounds to anemic dogs. Transfusion Medicine Reviews 2002, 16, 269, 10.1016/s0887-7963(02)80076-1.
- P.M. Nyakundi; R. Muigai; J.B.O. Were; C.N. Oster; G.S. Gachihi; G. Kirigi; Congenital visceral leishmaniasis: case report. Transactions of the Royal Society of Tropical Medicine and Hygiene 1988, 82, 564, 10.1016/0035-9203(88)90508-1.
- GeorgeC. Low; W.E. Cooke; A CONGENITAL CASE OF KALA-AZAR.. The Lancet 1926, 208, 1209-1211, 10.1016/s0140-6736(01)05214-x.
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. WHO Leishmaniasis Control Team Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671.
- Esther Von Stebut; Leishmaniasis Leishmaniasis. Der Hautarzt 2017, 68, 548-552, 10.1007/s00105-017-4001-9.
- Naouel Eddaikra; Ihcene Kherachi Djenad; Sihem Benbetka; Razika Benikhlef; Khatima Ait-Oudhia; Farida Moulti-Mati; Bruno Oury; Denis Sereno; Zoubir Harrat; Development of a Murine Infection Model withLeishmania killicki, Responsible for Cutaneous Leishmaniosis in Algeria: Application in Pharmacology. BioMed Research International 2016, 2016, 1-8, 10.1155/2016/7985104.
- Bart Ostyn; Kamlesh Gidwani; Basudha Khanal; A. Picado; Francois Chappuis; Shri Prakash Singh; Suman Rijal; Shyam Sundar; Marleen Boelaert; Incidence of Symptomatic and Asymptomatic Leishmania donovani Infections in High-Endemic Foci in India and Nepal: A Prospective Study. PLOS Neglected Tropical Diseases 2011, 5, e1284, 10.1371/journal.pntd.0001284.
- Fernando T Silveira; Ralph Lainson; José Ângelo Crescente; Adelson A.A. De Souza; Marliane B. Campos; Claudia M.C. Gomes; Márcia D. Laurenti; Carlos E.P. Corbett; A prospective study on the dynamics of the clinical and immunological evolution of human Leishmania (L.) infantum chagasi infection in the Brazilian Amazon region. Transactions of the Royal Society of Tropical Medicine and Hygiene 2010, 104, 529-535, 10.1016/j.trstmh.2010.05.002.
- Bruno L Travi; Anabela Cordeiro-Da-Silva; Filipe Dantas-Torres; Guadalupe Miró; Canine visceral leishmaniasis: Diagnosis and management of the reservoir living among us. PLOS Neglected Tropical Diseases 2018, 12, e0006082, 10.1371/journal.pntd.0006082.
- Philip D. Marsden; The survival of Trypanosoma cruzi in human saliva and urine. Transactions of the Royal Society of Tropical Medicine and Hygiene 1966, 60, 562-563, 10.1016/0035-9203(66)90286-0.
- M.Keith Howard; Mark M. Pharoah; Frank Ashall; Michael A. Miles; Human urine stimulates growth of Leishmania in vitro. Transactions of the Royal Society of Tropical Medicine and Hygiene 1991, 85, 477-479, 10.1016/0035-9203(91)90226-o.
- Y B Mebrahtu; L D Hendricks; C N Oster; P G Lawyer; P V Perkins; H Pamba; D Koech; C R Roberts; Leishmania donovani parasites in the nasal secretions, tonsillopharyngeal mucosa, and urine centrifugates of visceral leishmaniasis patients in Kenya.. The American Journal of Tropical Medicine and Hygiene 1993, 48, , .
- Manoel Sebastião Da Costa Lima; Andressa Cristina Lopes Hartkopf; Rosianne A. De Souza Tsujisaki; Elisa Teruya Oshiro; Julie Teresa Shapiro; Maria De Fatima Cepa Matos; Maria Elizabeth Dorval; Isolation and molecular characterization of Leishmania infantum in urine from patients with visceral leishmaniasis in Brazil. Acta Tropica 2018, 178, 248-251, 10.1016/j.actatropica.2017.12.011.
- Roser Fisa; Israel Molina; M. Gállego; Cristina Riera; Montserrat Portús; Vicenç Falcó; Paulo López-Chejade; Esteban Ribera; Leishmania infantum DNA detection in urine from patients with visceral leishmaniasis and after treatment control.. The American Journal of Tropical Medicine and Hygiene 2008, 78, 741-744, 10.4269/ajtmh.2008.78.741.
- Cristina Riera; Josep Enric Valladares; Cristina Riera; Viable Leishmania infantum in urine and semen in experimentally infected dogs. Parasitology Today 1996, 12, 412, 10.1016/0169-4758(96)90062-9.
- Martin Balzan; Frederick Fenech; Acute renal failure in visceral leishmaniasis treated with sodium stibogluconate. Transactions of the Royal Society of Tropical Medicine and Hygiene 1992, 86, 515-516, 10.1016/0035-9203(92)90091-p.
- Cid Carlos Soares De Alcântara; Laís Regina Lacerda Santana; Priscila Dourado Evangelista; André Costa Teixeira; Geraldo Bezerra Da Silva; Elizabeth De Francesco Daher; Renal dysfunction in Leishmaniasis and Chagas disease coinfection: a case report. Revista do Instituto de Medicina Tropical de São Paulo 2018, 60, , 10.1590/S1678-9946201860073.
- Koutinas, A.F.; Koutinas, C.K. Pathologic mechanisms underlying the clinical findings in canine leishmaniasis due to Leishmania infantum/chagasi. Vet. Pathol. 2014, 51, 527–538.
- Maria-Grazia Pennisi; Luís Cardoso; Gad Baneth; Patrick Bourdeau; A F Koutinas; Guadalupe Miró; Gaetano Oliva; Laia Solano-Gallego; LeishVet update and recommendations on feline leishmaniosis.. Parasites & Vectors 2015, 8, 302, 10.1186/s13071-015-0909-z.
- Anna Clementi; Giorgio Battaglia; Matteo Floris; Pietro Castellino; Claudio Ronco; Dinna N. Cruz; Renal involvement in leishmaniasis: a review of the literature.. NDT Plus 2011, 4, 147-52, 10.1093/ndtplus/sfr008.
- Gilberto Silva Nunes Bezerra; Walter Lins Barbosa; Elis Dionísio Da Silva; Nilma Cintra Leal; Zulma Maria De Medeiros; Urine as a promising sample for Leishmania DNA extraction in the diagnosis of visceral leishmaniasis - a review.. The Brazilian Journal of Infectious Diseases 2019, 23, 111-120, 10.1016/j.bjid.2019.04.001.
- Tribhuvan M. Mohapatra; Dharmendra P. Singh; Rahul K. Goyal; Rahul K. Singh; S. Sundar; In Search of an Ideal Test for Diagnosis and Prognosis of Kala-azar. Journal of Health, Population and Nutrition 2010, 28, 281-285, 10.3329/jhpn.v28i3.5557.
- Asad Mirzaei; Fereshteh Ahmadipour; Arnaud Cannet; Pierre Marty; Pascal Delaunay; Pascale Perrin; Franck Dorkeld; Denis Sereno; Mohammad Akhoundi; Immunodetection and molecular determination of visceral and cutaneous Leishmania infection using patients' urine. Infection, Genetics and Evolution 2018, 63, 257-268, 10.1016/j.meegid.2018.05.021.
- Ben-Abid, M.; Galaï, Y.; Habboul, Z.; Ben-Abdelaziz, R.; Ben-Sghaier, I.; Aoun, K.; Bouratbine, A. Diagnosis of Mediterranean visceral leishmaniasis by detection of Leishmania -related antigen in urine and oral fluid samples. Acta Trop. 2017, 167, 71–72.
- Nicolas Veland; Diego A. Espinosa; Braulio Mark Valencia; Ana Pilar Ramos; Flor Calderon; Jorge Arevalo; Nald E. Low; Alejandro Llanos-Cuentas; Andrea K. Boggild; Polymerase Chain Reaction Detection of Leishmania kDNA from the Urine of Peruvian Patients with Cutaneous and Mucocutaneous Leishmaniasis. The American Journal of Tropical Medicine and Hygiene 2011, 84, 556-561, 10.4269/ajtmh.2011.10-0556.
- Laia Solano-Gallego; Alhelí Rodríguez-Cortés; Michele Trotta; Claudia Zampieron; Luis Razia; Tommaso Furlanello; Marco Caldin; Xavier Roura; J. Alberola; Detection of Leishmania infantum DNA by fret-based real-time PCR in urine from dogs with natural clinical leishmaniosis. Veterinary Parasitology 2007, 147, 315-319, 10.1016/j.vetpar.2007.04.013.
- Laura Manna; Stefano Reale; Esther Picillo; Fabrizio Vitale; Angelo Elio Gravino; Urine sampling for real-time polymerase chain reaction based diagnosis of canine leishmaniasis.. Journal of Veterinary Diagnostic Investigation 2008, 20, 64-67, 10.1177/104063870802000112.
- Atchara Phumee; Vich Tampanya; Asda Vibhagool; Kanyarat Kraivichian; Padet Siriyasatien; Nopadon Noppakun; Vivornpun Sanprasert; Henry Wilde; Sarunyou Chusri; Detection of Leishmania siamensis DNA in Saliva by Polymerase Chain Reaction. The American Journal of Tropical Medicine and Hygiene 2013, 89, 899-905, 10.4269/ajtmh.12-0612.
- A. Franceschi; V. Merildi; G. Guidi; F. Mancianti; Occurrence of Leishmania DNA In Urines of Dogs Naturally Infected with Leishmaniasis. Veterinary Research Communications 2006, 31, 335-341, 10.1007/s11259-006-3477-z.
- Gordon W. Gribble; Charles F. Nutaitis; [1.1.1.1.1]paracyclophane and [1.1.1.1.1.1]paracyclophane. Tetrahedron Letters 1985, 26, 6023-6026, 10.1016/s0040-4039(00)95115-3.
- K Arai; T Koshi; Y Ehara; T Edano; M Ohkuchi; M Hirata; T Okabe; Characterization of immunoreactive endothelin in human urine.. Biochemical and Biophysical Research Communications 1992, 182, , .
- J Kohanteb; S M Ardehali; H R Rezai; Detection of Leishmania donovani soluble antigen and antibody in the urine of visceral leishmaniasis patients.. Transactions of the Royal Society of Tropical Medicine and Hygiene 1987, 81, , .
- Mohammad Zahidul Islam; Makoto Itoh; S. M. Shamsuzzaman; Rusella Mirza; Farzana Matin; Iftikhar Ahmed; A. K. M. Shamsuzzaman Choudhury; M. Akram Hossain; Xu-Guang Qiu; Nilufar Begam; et al. Diagnosis of Visceral Leishmaniasis by Enzyme-Linked Immunosorbent Assay Using Urine Samples. Clinical and diagnostic laboratory immunology 2002, 9, 789-794, 10.1128/CDLI.9.4.789-794.2002.
- Mohammad Zahidul Islam; Iftikhar Ahmed; Abdul Halim Sarder; Rusella Mirza; Eisaku Kimura; Yoshihisa Hashiguchi; Makoto Itoh; Saifuddin Ekram; S. M. Shamsuzzaman; DIRECT AGGLUTINATION TEST WITH URINE SAMPLES FOR THE DIAGNOSIS OF VISCERAL LEISHMANIASIS. The American Journal of Tropical Medicine and Hygiene 2004, 70, 78-82, 10.4269/ajtmh.2004.70.78.
- Mohammad Zahidul Islam; Atsuhide Takesue; Eisaku Kimura; Makoto Itoh; Ajijur Rahman; Anwar Ul Islam; Saifuddin Ekram; Yoshihisa Hashiguchi; Hidekazu Takagi; Enzyme-linked immunosorbent assay to detect urinary antibody against recombinant rKRP42 antigen made from Leishmania donovani for the diagnosis of visceral leishmaniasis.. The American Journal of Tropical Medicine and Hygiene 2008, 79, 599-604, 10.4269/ajtmh.2008.79.599.
- Gulam Musawwir Khan; Mohammad Shafiul Alam; Milka Patracia Podder; Makoto Itoh; Kazi M Jamil; Mamun Kabir; Yukiko Wagatsuma; Evaluation of rK-39 strip test using urine for diagnosis of visceral leishmaniasis in an endemic area in Bangladesh. Parasites & Vectors 2010, 3, 114-114, 10.1186/1756-3305-3-114.
- Dharmendra Singh; Krishna Pandey; Vidya Nand Rabi Das; Sushmita Das; Neena Verma; Alok Ranjan; Sekhar Chandra Lal; Kamal Roshan Topno; Shubhankar Kumar Singh; Rakesh Bihari Verma; et al. Evaluation of rK-39 Strip Test Using Urine for Diagnosis of Visceral Leishmaniasis in an Endemic Region of India. The American Journal of Tropical Medicine and Hygiene 2013, 88, 222-226, 10.4269/ajtmh.2012.12-0489.
- Jaya Chakravarty; Subodh Kumar; Rajiv Kumar; Shalini Gautam; Madhukar Rai; Shyam Sundar; Evaluation of rk39 immunochromatographic test with urine for diagnosis of visceral leishmaniasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 2011, 105, 537-9, 10.1016/j.trstmh.2011.05.008.
- Rudra Goswami; Sukhen Das; Y Ray; M Rahman; Goswami R P; Das S; Ray Y; Rahman M; Testing urine samples with rK39 strip as the simplest non-invasive field diagnosis for visceral leishmaniasis: An early report from eastern India. Journal of Postgraduate Medicine 2012, 58, 180, 10.4103/0022-3859.101378.
- Sarfaraz Ahmad Ejazi; Pradyot Bhattacharya; Asjad Karim Bakhteyar; Aquil Ahmad Mumtaz; Krishna Pandey; Vidya Nand Ravi Das; Pradeep Das; Mehebubar Rahaman; Rama Prosad Goswami; Nahid Ali; et al. Noninvasive Diagnosis of Visceral Leishmaniasis: Development and Evaluation of Two Urine-Based Immunoassays for Detection of Leishmania donovani Infection in India. PLOS Neglected Tropical Diseases 2016, 10, e0005035, 10.1371/journal.pntd.0005035.
- Ermias Diro; Yoseph Techane; Tedros Tefera; Yibeltal Assefa; Tadesse Kebede; Abebe Genetu; Yenew Kebede; Abiye Tesfaye; Bahiru Ergicho; Asfawesen Gebre-Yohannes; et al. Field evaluation of FD-DAT, rK39 dipstick and KATEX (urine latex agglutination) for diagnosis of visceral leishmaniasis in northwest Ethiopia. Transactions of the Royal Society of Tropical Medicine and Hygiene 2007, 101, 908-914, 10.1016/j.trstmh.2007.05.002.
- D. Singh; K. Pandey; V. N. R. Das; S. Das; S. Kumar; R. K. Topno; P. Das; Novel Noninvasive Method for Diagnosis of Visceral Leishmaniasis by rK39 Testing of Sputum Samples ▿. Journal of Clinical Microbiology 2009, 47, 2684-2685, 10.1128/JCM.00988-09.
- Sarita Mohapatra; Jyotish C Samantaray; Arnab Ghosh; A Comparative Study of Serum, Urine and Saliva Using rk39 Strip for the Diagnosis of Visceral Leishmaniasis. Journal of Arthropod-Borne Diseases 2015, 10, 87-91, .
- Mohammad Zahidul Islam; Makoto Itoh; Anwar Ul Islam; Saifuddin Ekram; Ajijur Rahman; Hidekazu Takagi; Atsuhide Takesue; Yoshihisa Hashiguchi; Eisaku Kimura; ELISA with Recombinant rKRP42 Antigen Using Urine Samples: A Tool for Predicting Clinical Visceral Leishmaniasis Cases and Its Outbreak. The American Journal of Tropical Medicine and Hygiene 2012, 87, 658-662, 10.4269/ajtmh.2012.12-0168.
- Rafael Barrera; Francisco Centeno; Jose A. Tapia; C Zaragoza; Esther Durán; Marta González; M. Cinta Mañé; SDS-PAGE and Western blot of urinary proteins in dogs with leishmaniasis. Veterinary Research 2003, 34, 137-151, 10.1051/vetres:2002061.
- Laia Solano-Gallego; A. Rodríguez; L. Iniesta; Margarita Arboix; M. Portús; J. Alberola; Detection of Anti-Leishmania Immunoglobulin G Antibodies in Urine Specimens of Dogs with Leishmaniasis. Clinical and diagnostic laboratory immunology 2003, 10, 849-855, 10.1128/CDLI.10.5.849-855.2003.
- Felicitat Todolí; Laia Solano-Gallego; Ana Ojeda; Josefina Quintana; Albert Lloret; Xavier Roura; J. Alberola; Alhelí Rodríguez-Cortés; Anti-Leishmania IgA in urine samples from dogs with clinical leishmaniasis. Veterinary Parasitology 2009, 159, 17-23, 10.1016/j.vetpar.2008.10.010.
- Maria Colmenares; M. Portus; C. Riera; M. Gállego; M. J. Aisa; S. Torras; Carme Muñoz; Short Report: Detection of 72–75-kD and 123-kD Fractions of Leishmania Antigen in Urine of Patients with Visceral Leishmaniasis. The American Journal of Tropical Medicine and Hygiene 1995, 52, 427-428, 10.4269/ajtmh.1995.52.427.
- A. A. Azazy; M. L. Chance; Eileen Devaney; A time-course study of circulating antigen and parasite-specific antibody in cotton rats infected withLeishmania donovani. Annals of Tropical Medicine & Parasitology 1997, 91, 153-162, 10.1080/00034983.1997.11813125.
- Suely S. Kashino; Claudia Abeijon; Lizeng Qin; Kelly Aparecida Kanunfre; Flávia Kubrusly; Fernando O. Silva; Dorcas L. Costa; Dioclécio Campos; Carlos H.N. Costa; Isaias Raw; et al. Identification of Leishmania infantum chagasi proteins in urine of patients with visceral leishmaniasis: a promising antigen discovery approach of vaccine candidates.. Parasite Immunology 2012, 34, 360-71, 10.1111/j.1365-3024.2012.01365.x.
- Claudia Abeijon; Suely S. Kashino; Fernando O. Silva; Dorcas L. Costa; Ricardo Fujiwara; Carlos H. N. Costa; Antonio Campos-Neto; Identification and Diagnostic Utility of Leishmania infantum Proteins Found in Urine Samples from Patients with Visceral Leishmaniasis. Clinical and Vaccine Immunology 2012, 19, 935-943, 10.1128/CVI.00125-12.
- Ferlizza, E. Urine Proteome in Animals of Veterinary Interest: Species Comparison and New Biomarkers of Nephropathy. Master’s Thesis, Università di Bologna, Bologna, Italy, 2015.
- Claudia Abeijon; Fabiana Alves; Severine Monnerat; Monique Wasunna; Jane Mbui; Agostinho G. Viana; Lilian L. Bueno; Williane F. Siqueira; Silvio G. Carvalho; Neha Agrawal; et al. Development of a Multiplexed Assay for Detection of Leishmania donovani and Leishmania infantum Protein Biomarkers in Urine Samples of Patients with Visceral Leishmaniasis. Journal of Clinical Microbiology 2019, 57, , 10.1128/jcm.02076-18.
- Zamil J Attar; M. L. Chance; Sayda El-Safi; James Carney; Ahmed Azazy; Maha El-Hadi; Cibele Dourado; Marcel Hommel; Latex agglutination test for the detection of urinary antigens in visceral leishmaniasis. Acta Tropica 2001, 78, 11-16, 10.1016/s0001-706x(00)00155-8.
- Bahador Sarkari; Michael Chance; Marcel Hommel; Antigenuria in visceral leishmaniasis: detection and partial characterisation of a carbohydrate antigen. Acta Tropica 2002, 82, 339-348, 10.1016/s0001-706x(02)00043-8.
- Cristina Vilaplana; S. Blanco; Jose Domínguez; M. Giménez; Vicente Ausina; Cristina Tural; Carme Muñoz; Noninvasive Method for Diagnosis of Visceral Leishmaniasis by a Latex Agglutination Test for Detection of Antigens in Urine Samples. Journal of Clinical Microbiology 2004, 42, 1853-1854, 10.1128/JCM.42.4.1853-1854.2004.
- S H El-Safi; A Abdel-Haleem; A Hammad; I El-Basha; A Omer; H G Kareem; M Boelaert; M Chance; Marcel Hommel; Field evaluation of latex agglutination test for detecting urinary antigens in visceral leishmaniasis in Sudan.. Eastern Mediterranean Health Journal 2003, 9, , .
- Motahareh Motazedian; Mehdi Fakhar; Mohammad Hossein Motazedian; Gholamreza Hatam; Fattaneh Mikaeili; A urine-based polymerase chain reaction method for the diagnosis of visceral leishmaniasis in immunocompetent patients. Diagnostic Microbiology and Infectious Disease 2008, 60, 151-154, 10.1016/j.diagmicrobio.2007.09.001.
- Cristina Riera; Roser Fisa; P. Lopez; Esteban Ribera; I. Molina; J. Carrió; Vicenç Falcó; M. Gállego; M. Portús; Evaluation of a latex agglutination test (KAtex) for detection of Leishmania antigen in urine of patients with HIV-Leishmania coinfection: value in diagnosis and post-treatment follow-up. European Journal of Clinical Microbiology and Infectious Diseases 2004, 23, , 10.1007/s10096-004-1249-7.
- Marleen Boelaert; Kristien Verdonck; Joris Menten; Temmy Sunyoto; Johan Van Griensven; Francois Chappuis; Suman Rijal; Rapid tests for the diagnosis of visceral leishmaniasis in patients with suspected disease. Cochrane Database of Systematic Reviews 2014, , 1-119, 10.1002/14651858.cd009135.pub2.
- Maina Ngotho; John Maina Kagira; Beatrice Muthoni Gachie; Simon Muturi Karanja; Maxwell Wambua Waema; Dawn Nyawira Maranga; Naomi Maina; Loop Mediated Isothermal Amplification for Detection of Trypanosoma brucei gambiense in Urine and Saliva Samples in Nonhuman Primate Model. BioMed Research International 2015, 2015, 1-7, 10.1155/2015/867846.
- Marleen Boelaert; Kristien Verdonck; Joris Menten; Temmy Sunyoto; Johan Van Griensven; Francois Chappuis; Suman Rijal; Rapid tests for the diagnosis of visceral leishmaniasis in patients with suspected disease. Cochrane Database of Systematic Reviews 2014, , 1-119, 10.1002/14651858.cd009135.pub2.
- Maina Ngotho; John Maina Kagira; Beatrice Muthoni Gachie; Simon Muturi Karanja; Maxwell Wambua Waema; Dawn Nyawira Maranga; Naomi Maina; Loop Mediated Isothermal Amplification for Detection of Trypanosoma brucei gambiense in Urine and Saliva Samples in Nonhuman Primate Model. BioMed Research International 2015, 2015, 1-7, 10.1155/2015/867846.
- P F Boreham; C A Facer; Fibrinogen and fibrinogen/fibrin degradation products in the urine of rabbits infected with Trypanosoma (trypanozoon) brucei.. Parasitology Research 1977, 52, , .
- O.K.A. Itazi; J.C. Enyaru; The nature of proteins excreted in the urine of rabbits infected with T. brucei subgroup organisms. Transactions of the Royal Society of Tropical Medicine and Hygiene 1973, 67, 263, 10.1016/0035-9203(73)90168-5.
- John R. Seed; James Edwin Hall; John Sechelski; Phenylalanine metabolism in Microtus montanus chronically infected with Trypanosoma brucei gambiense. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 1982, 71, 209-215, 10.1016/0305-0491(82)90242-5.
- J E Hall; J R Seed; J B Sechelski; Multiple alpha-keto aciduria in Microtus montanus chronically infected with Trypanosoma brucei gambiense.. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 1985, 82, , .
- Hall, J.E.; Seed, J.R. Quantification of aromatic amino acid catabolites in urine of mice acutely infected with Trypanosoma brucei gambiense. Comp. Biochem. Physiol 1981, 69B, 791-796.
- James Edwin Hall; John R. Seed; Increased urinary excretion of aromatic amino acid catabolites by Microtus montanus chronically infected with Trypanosoma brucei gambiense. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 1984, 77, 755-760, 10.1016/0305-0491(84)90309-2.
- Ahmed El Sawalhy; John R. Seed; James Edwin Hall; Hassam El Attar; Increased excretion of aromatic amino acid catabolites in animals infected with Trypanosoma brucei evansi.. Journal of Parasitology 1998, 84, 469, 10.2307/3284707.
- C Nowicki; Juan J. Cazzulo; Aromatic amino acid catabolism in trypanosomatids. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 2008, 151, 381-390, 10.1016/j.cbpa.2007.03.010.
- Julien Bonnet; Camille Garcia; Thibaut Leger; Marie-Pauline Couquet; Philippe Vignoles; Gedeao Vatunga; Joseph Mathu Ndung’U; Clotilde Boudot; Sylvie Bisser; Bertrand Courtioux; et al. Proteome characterization in various biological fluids of Trypanosoma brucei gambiense-infected subjects. Journal of Proteomics 2019, 196, 150-161, 10.1016/j.jprot.2018.11.005.
- Simaren, J.; Ogunnaike, M. Urinary biochemical changes, histopathologic effect of kidney damage observed in rats infected with Trypanosoma b. brucei. Tropical Medicine and Parasitology 1989, 11, 35-46.
- P. Dukes; W.C. Gibson; J.K. Gashumba; K.M. Hudson; T.J. Bromidge; A. Kaukus; T. Asonganyi; E. Magnus; Absence of the LiTat 1.3 (CATT antigen) gene in Trypanosoma brucei gambiense stocks from Cameroon. Acta Tropica 1992, 51, 123-134, 10.1016/0001-706x(92)90054-2.
- Monroy, F.P.; Dusanic, D.G. The kidney form of Trypanosoma musculi: A distinct stage in the life cycle? Parasitology today (Personal ed.) 2000, 16, 107-110.
- L.F. Arias; E. Duque; C. Ocampo; J. Henao; G. Zuluaga; G. Varela; J. Carvajal; J. Duque; M. Robledo-Villegas; M. Arbeláez; et al. Detection of Amastigotes of Trypanosoma Cruzi in a Kidney Graft With Acute Dysfunction. Transplantation Proceedings 2006, 38, 885-887, 10.1016/j.transproceed.2006.02.033.
- G González; Dan Sunnemark; A Orn; K O Grönvik; Detection of cruzipain, the major cysteine proteinase from Trypanosoma cruzi and its C-terminal extension in biological fluids during experimental infection in mice.. Scandinavian Journal of Immunology 1996, 44, , .
- Verónica Yauri; Manuela Renee Verástegui; Fernando Recuenco; Edith Malaga; Cesar Miguel Gavidia; Robert H. Gilman; Noelia Angulo; Ines Cabello; Caryn Bern; Yagahira E. Castro-Sesquen; et al. Domestic Pig (Sus scrofa) as an Animal Model for Experimental Trypanosoma cruzi Infection. The American Journal of Tropical Medicine and Hygiene 2016, 94, 1020-1027, 10.4269/ajtmh.15-0233.
- Yagahira E. Castro-Sesquen; Robert H. Gilman; Verónica Yauri; Jaime Cok; Noelia Angulo; Hermes Escalante; Caryn Bern; Detection of Soluble Antigen and DNA of Trypanosoma cruzi in Urine Is Independent of Renal Injury in the Guinea Pig Model. PLOS ONE 2013, 8, e58480, 10.1371/journal.pone.0058480.
- A.M. Katzin; A. Marcipar; H. Freilij; R. Corral; J.F. Yanovsky; Rapid determination of Trypanosoma cruzi urinary antigens in human chronic chagas disease by agglutination test. Experimental Parasitology 1989, 68, 208-215, 10.1016/0014-4894(89)90099-4.
- H L Freilij; R S Corral; A M Katzin; S Grinstein; Antigenuria in infants with acute and congenital Chagas' disease.. Journal of Clinical Microbiology 1987, 25, 133-137, 10.1128/jcm.25.1.133-137.1987.
- R.S. Corral; J. Altcheh; H.L. Freilij; Presence of IgM antibodies to Trypanosoma cruzi urinary antigen in sera from patients with acute Chagas' disease. International Journal for Parasitology 1998, 28, 589-594, 10.1016/s0020-7519(98)00017-4.
- R S Corral; A Orn; H L Freilij; T Bergman; S Grinstein; Purification and characterization of an 80-kilodalton Trypanosoma cruzi urinary antigen.. Journal of Clinical Microbiology 1989, 27, 145-151, 10.1128/jcm.27.1.145-151.1989.
- R S Corral; G M Bertot; Patricia B. Petray; J. Altcheh; M Singh; A Orn; M F Rapoport; S Grinstein; An iron-binding Trypanosoma cruzi urinary antigen.. Parasite 1995, 2, , .
- Eufrosina S. Umezawa; M A Shikanai-Yasuda; J F Da Silveira; P C Cotrim; G Paranhos; A M Katzin; Trypanosoma cruzi: detection of a circulating antigen in urine of chagasic patients sharing common epitopes with an immunodominant repetitive antigen.. Experimental Parasitology 1993, 76, , .
- Gowdhaman Et Al. Gowdhaman Et Al.; Tjprc; Sourabh Jain Et Al. Sourabh Jain Et Al.; Test 1. International Journal of Agricultural Science and Research 2018, 8, 1-15, 10.24247/ijdrdjun20186.
- Temple A. Douglas; Davide Tamburro; Claudia Fredolini; Benjamin H. Espina; Benjamin S. Lepene; Leopold L. Ilag; Virginia Espina; Emanuel F. Petricoin; Lance A. Liotta; Alessandra Luchini; et al. The use of hydrogel microparticles to sequester and concentrate bacterial antigens in a urine test for Lyme disease. Biomaterials 2010, 32, 1157-66, 10.1016/j.biomaterials.2010.10.004.
- Mackie, F.P. Note on some bodies of unknown nature found in faeces of kala-azar patients. Indian. J. Med. Res 1914, 510-515.
- Shortt, H.E.; Smith, R.O.A.; D'Silva, M.A.H.; Swaminath, C.S. Leishmania donovani in human faeces in indian kala-azar. Indian. J. Med. Res 1923, 644-646.
- G. Nery, I.D.S.M.I.T.D.F.L.S.M.B.-M. Ocorrência de Leishmania infantum em fezes de cão. Arq. Bras. Med. Vet. Zootec 2015, 67, 1249-1253.
- Ibrahim Hamad; Claire-Lise Forestier; Martine Peeters; Eric Delaporte; Didier Raoult; Fadi Bittar; Wild Gorillas as a Potential Reservoir ofLeishmania major. The Journal of Infectious Diseases 2014, 211, 267-273, 10.1093/infdis/jiu380.
- Ibrahim Hamad; Claire-Lise Forestier; Gilbert Greub; Katia Jaton; Didier Raoult; Fadi Bittar; Reply to Bastien et al.. The Journal of Infectious Diseases 2015, 212, 506-8, 10.1093/infdis/jiv130.
- Jan Votýpka; Barbora Pafčo; David Modrý; Donald Mbohli; Nikki Tagg; Klára J. Petrželková; An unexpected diversity of trypanosomatids in fecal samples of great apes. International Journal for Parasitology: Parasites and Wildlife 2018, 7, 322-325, 10.1016/j.ijppaw.2018.09.003.
- Denis Sereno; F. Dorkeld; Mohammad Akhoundi; Pascale Perrin; Pathogen Species Identification from Metagenomes in Ancient Remains: the Challenge of Identifying Human Pathogenic Species of Trypanosomatidae Via Bioinformatic Tools. 2018, , , 10.20944/preprints201807.0124.v1.
- Tasha M. Santiago-Rodriguez; Gino Fornaciari; Stefania Luciani; Scot E. Dowd; Gary A. Toranzos; Isolina Marota; Raul J. Cano; Taxonomic and predicted metabolic profiles of the human gut microbiome in pre-Columbian mummies. FEMS Microbiology Ecology 2016, 92, , 10.1093/femsec/fiw182.
- Antônio R. L. Teixeira; Florêncio Figueiredo; Joffre Rezende Filho; Vanize Macêdo; Chagas' Disease: a Clinical, Parasitological, Immunological, and Pathological Study in Rabbits *. The American Journal of Tropical Medicine and Hygiene 1983, 32, 258-272, 10.4269/ajtmh.1983.32.258.
- Alexandre Fernandes; Alena Mayo Iñiguez; Valdirene S Lima; Sheila M F Mendonça De Souza; Luiz Fernando Ferreira; Ana Carolina P Vicente; Ana M Jansen; Pre-Columbian Chagas disease in Brazil: Trypanosoma cruzi I in the archaeological remains of a human in Peruaçu Valley, Minas Gerais, Brazil.. Memórias do Instituto Oswaldo Cruz 2008, 103, 514-516, 10.1590/s0074-02762008000500021.
- A Araújo; Ana Maria Jansen; Karl Reinhard; Luiz Fernando Ferreira; Paleoparasitology of Chagas disease: a review. Memórias do Instituto Oswaldo Cruz 2009, 104, 9-16, 10.1590/s0074-02762009000900004.
- Deila J. Franco; Annamaria R. Vago; Egler Chiari; Fidel C.A. Meira; Lucia M.C. Galvão; Conceição R.S. Machado; Trypanosoma cruzi: mixture of two populations can modify virulence and tissue tropism in rat.. Experimental Parasitology 2003, 104, 54-61, 10.1016/s0014-4894(03)00119-x.
- Luciana O. Andrade; Conceição R.S. Machado; Egler Chiari; Sérgio D.J. Pena; A M Macedo; Differential tissue distribution of diverse clones of Trypanosoma cruzi in infected mice.. Molecular and Biochemical Parasitology 1999, 100, 163-172, 10.1016/s0166-6851(99)90035-x.
- Michael Lewis; Amanda Fortes Francisco; Martin C. Taylor; Hollie Burrell-Saward; Alex P. McLatchie; Michael A. Miles; John M. Kelly; Bioluminescence imaging of chronicTrypanosoma cruziinfections reveals tissue-specific parasite dynamics and heart disease in the absence of locally persistent infection. Cellular Microbiology 2014, 16, 1285-1300, 10.1111/cmi.12297.
- Laura-Isobel McCall; Anupriya Tripathi; Fernando Vargas; Rob Knight; Pieter C. Dorrestein; Jair L. Siqueira-Neto; Experimental Chagas disease-induced perturbations of the fecal microbiome and metabolome. PLOS Neglected Tropical Diseases 2018, 12, e0006344, 10.1371/journal.pntd.0006344.
- S Urdaneta-Morales; I Nironi; Trypanosoma cruzi in the anal glands of urban opossums: I- isolation and experimental infections. Memórias do Instituto Oswaldo Cruz 1996, 91, 399-403, 10.1590/s0074-02761996000400002.
- Claude E. Forkner; Lily S. Zia; VIABLE LEISHMANIA DONOVANI IN NASAL AND ORAL SECRETIONS OF PATIENTS WITH KALA-AZAR AND THE BEARING OF THIS FINDING ON THE TRANSMISSION OF THE DISEASE. Journal of Experimental Medicine 1934, 59, 491-499, 10.1084/jem.59.4.491.
- Maria E.F. Brito; Ericka Lima Almeida; Angela Cristina Rapela Medeiros; Roberto Pereira Werkhauser; Joanna Lucia De Almeida Alexandre; Bruna Santos Lima Figueiredo Sá; Eduardo Henrique Gomes Rodrigues; Sinval Pinto Brandão-Filho; Leishmania (Viannia) braziliensis isolated from the saliva of patients in a cutaneous leishmaniasis-endemic area of northeastern Brazil. Memórias do Instituto Oswaldo Cruz 2018, 113, , 10.1590/0074-02760170250.
- Sarunyou Chusri; Thanaporn Hortiwakul; Khachornsakdi Silpapojakul; Padet Siriyasatien; Consecutive Cutaneous and Visceral Leishmaniasis Manifestations Involving a Novel Leishmania Species in Two HIV Patients in Thailand. The American Journal of Tropical Medicine and Hygiene 2012, 87, 76-80, 10.4269/ajtmh.2012.11-0749.
- Padet Siriyasatien; Sarunyou Chusri; Kanyarat Kraivichian; Narissara Jariyapan; Thanaporn Hortiwakul; Khachornsakdi Silpapojakul; Adam M. Pym; Atchara Phumee; Early detection of novel Leishmania species DNA in the saliva of two HIV-infected patients.. BMC Infectious Diseases 2016, 16, 89, 10.1186/s12879-016-1433-2.
- Chaichontat Sriworarat; Atchara Phumee; Mathirut Mungthin; Saovanee Leelayoova; Padet Siriyasatien; Development of loop-mediated isothermal amplification (LAMP) for simple detection of Leishmania infection.. Parasites & Vectors 2015, 8, 591, 10.1186/s13071-015-1202-x.
- Manisha Vaish; Sanjana Mehrotra; Jaya Chakravarty; Shyam Sundar; Noninvasive Molecular Diagnosis of Human Visceral Leishmaniasis▿. Journal of Clinical Microbiology 2011, 49, 2003-2005, 10.1128/JCM.00130-11.
- Sushmita Das; Avishek Halder; Vidya Nand Rabidas; Abhishek Mandal; Pradeep Das; Specific Noninvasive Detection of Leishmania donovani in Desquamated Buccal Cell Swab Samples from Human Visceral Leishmaniasis-HIV Coinfected Patients. Journal of Clinical Microbiology 2014, 52, 1238-1241, 10.1128/jcm.02819-13.
- Gabriella Lombardo; Maria-Grazia Pennisi; Tiziana Lupo; Antonella Migliazzo; Alessandra Caprì; Laia Solano-Gallego; Detection of Leishmania infantum DNA by real-time PCR in canine oral and conjunctival swabs and comparison with other diagnostic techniques. Veterinary Parasitology 2012, 184, 10-17, 10.1016/j.vetpar.2011.08.010.
- Sidney De Almeida Ferreira; Gregório Almeida; Soraia De Oliveira Silva; Gabriela Peixoto Vogas; Ricardo Fujiwara; Antero Silva Ribeiro De Andrade; Maria Norma Melo; Nasal, Oral and Ear Swabs for Canine Visceral Leishmaniasis Diagnosis: New Practical Approaches for Detection of Leishmania infantum DNA. PLOS Neglected Tropical Diseases 2013, 7, e2150, 10.1371/journal.pntd.0002150.
- M.A Masum; D.A Evans; Agglutinating anti-leishmanial antibodies in the saliva of kala-azar patients. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994, 88, 660, 10.1016/0035-9203(94)90216-X.
- Ana Cantos-Barreda; Damián Escribano; Luis J. Bernal; José Joaquín Cerón; Silvia Martínez-Subiela; Quantification of anti- Leishmania antibodies in saliva of dogs. Veterinary Parasitology 2017, 242, 54-58, 10.1016/j.vetpar.2017.05.017.
- Ben-Abid, M.; Galaï, Y.; Habboul, Z.; Ben-Abdelaziz, R.; Ben-Sghaier, I.; Aoun, K.; Bouratbine, A. Diagnosis of Mediterranean visceral leishmaniasis by detection of Leishmania -related antigen in urine and oral fluid samples. Acta Tropica 2017, 167, 71-72, doi:10.1016/j.actatropica.2016.12.026.
- Lejon, V.; Kwete, J.; Büscher, P. Towards saliva-based screening for sleeping sickness? Tropical medicine & international health : TM & IH 2003, 8, 585-588.
- P.D. Marsden; Jack W.C. Hagstrom; Trypanosoma cruzi in the saliva of beagle puppies. Transactions of the Royal Society of Tropical Medicine and Hygiene 1966, 60, 189-191, 10.1016/0035-9203(66)90026-5.
- P.D. Marsden; J.W.C. Hagstrom; Experimental Trypanosoma cruzi infection in beagle puppies. The effect of variations in the dose and source of infecting trypanosomes and the route of inoculation on the course of the infection. Transactions of the Royal Society of Tropical Medicine and Hygiene 1968, 62, 816-824, 10.1016/0035-9203(68)90010-2.
- R.T. Pinho; R.C. Pedrosa; P. Costa-Martins; L.R.R Castello-Branco; Saliva ELISA: a method for the diagnosis of chronic Chagas disease in endemic areas. Acta Tropica 1999, 72, 31-38, 10.1016/s0001-706x(98)00075-8.
- Nuria Cortes-Serra; María-Jesus Pinazo; Leonardo De La Torre; Melina Galizzi; Joaquim Gascon; Juan Bustamante; Diagnosis of Trypanosoma cruzi Infection Status using Saliva of Infected Subjects. The American Journal of Tropical Medicine and Hygiene 2018, 98, 464-467, 10.4269/ajtmh.17-0141.
- João Lucas M. Lourenço; Thaís T.C. Minuzzi-Souza; Larissa R. Silva; Amanda C. Oliveira; Vagner J. Mendonça; Nadjar Nitz; Ludmilla M.S. Aguiar; Rodrigo Gurgel-Gonçalves; High frequency of trypanosomatids in gallery forest bats of a Neotropical savanna. Acta Tropica 2018, 177, 200-206, 10.1016/j.actatropica.2017.10.012.
- A.M. El Hassan; Eltahir Khalil; E.A. El Sheikh; E E Zijlstra; Amna Osman; M.E. Ibrahim; Post kala-azar ocular leishmaniasis.. Transactions of the Royal Society of Tropical Medicine and Hygiene 1998, 92, 177-179, 10.1016/s0035-9203(98)90736-2.
- Dongmei Yuan; Hanxiao Qin; Jianguo Zhang; Lin Liao; Qiwei Chen; Da-Li Chen; Jian-Ping Chen; Phylogenetic analysis of HSP70 and cyt b gene sequences for Chinese Leishmania isolates and ultrastructural characteristics of Chinese Leishmania sp.. Parasitology Research 2016, 116, 693-702, 10.1007/s00436-016-5335-4.
- P Reinecke; H Gabbert; W Strunk; C Losche; Ocular scleromalacia caused by leishmaniasis: a rare cause of scleral perforation. British Journal of Ophthalmology 2001, 85, 238-238, 10.1136/bjo.85.2.238c.
- Masoud Doroodgar; Moein Doroodgar; Abbas Doroodgar; Unusual Presentation of Cutaneous Leishmaniasis: Ocular Leishmaniasis. Case Reports in Infectious Diseases 2017, 2017, 1-4, 10.1155/2017/3198547.
- M Abrishami; M Soheilian; A Farahi; Y Dowlati; Successful treatment of ocular leishmaniasis.. European Journal of Dermatology 2002, 12, , .
- Bita Kiafar; Malihe Nikandish; Vahid Mashayekhi Goyonlo; Ahmad Reza Taheri; Ocular leishmaniasis treated by intralesional amphotericin B. Middle East African Journal of Ophthalmology 2016, 23, 153-155, 10.4103/0974-9233.171801.
- Enrique Mencía-Gutiérrez; Esperanza Gutiérrez-Díaz; José L Rodríguez-Peralto; Juan Monsalve-Córdova; Old World eyelid cutaneous leishmaniasis: a case report.. Dermatology online journal 2005, 11, , .
- Iraj Mohammadpour; Mohammad Hossein Motazedian; Farhad Handjani; Gholamreza Hatam; Cutaneous Leishmaniasis of the Eyelids: A Case Series with Molecular Identification and Literature Review. The Korean Journal of Parasitology 2016, 54, 787-792, 10.3347/kjp.2016.54.6.787.
- Dejean; Viallefont; Boudet; Boulad; [A case of cutaneous leishmaniasis of the lower eyelid].. Bulletin des societes d'ophtalmologie de France 1958, 2, , .
- Reza Razeghinejad; Ahmad Monabati; Mohammad Rahim Kadivar; Abdolvahab Alborzi; Conjunctival leishmaniasis in a case of disseminated cutaneous leishmaniasis. Tropical Doctor 2016, 47, 53-55, 10.1177/0049475516631881.
- Perrin-Terrin, A.; Auriol, S.; Mahieu, L.; Debard, A.; Eden, A.; Cassagne, M.; Pagot-Mathis, V.; Malecaze, F.; Soler, V. [Recurrent bilateral anterior uveitis due to Leishmania infantum in a patient with immune deficiency related to HIV infection: a case report and literature review]. Journal francais d'ophtalmologie 2014, 37, 514-519, doi:10.1016/j.jfo.2014.02.006.
- A Nandy; M Addy; A B Chowdhury; Leishmanial blepharo-conjunctivitis.. Tropical and geographical medicine 1991, 43, , .
- Simona Di Pietro; Valentina Rita Francesca Bosco; Chiara Crinò; Francesco Francaviglia; Giudice Elisabetta; Prevalence, type, and prognosis of ocular lesions in shelter and owned-client dogs naturally infected by Leishmania infantum. Veterinary World 2016, 9, 633-637, 10.14202/vetworld.2016.633-637.
- E E McConnell; E F Chaffee; I G Cashell; F M Garner; Visceral leishmaniasis with ocular involvement in a dog.. Journal of the American Veterinary Medical Association 1970, 156, , .
- Richter; Schaarschmidt, K.; Krudewig. Ocular signs, diagnosis and long-term treatment with allopurinol in a cat with leishmaniasis. Schweizer Archiv fur Tierheilkunde 2014, 156, 289-294, doi:10.1024/0036-7281/a000593.
- M Garcia-Alonso; C G Nieto; A Blanco; José M Requena; C Alonso; I Navarrete; Presence of antibodies in the aqueous humour and cerebrospinal fluid during Leishmania infections in dogs. Pathological features at the central nervous system.. Parasite Immunology 1996, 18, , .
- Mercedes García Alonso; A. Blanco; D. Reina; F.J. Serrano; C. Alonso; C.G. Nieto; Immunopathology of the uveitis in canine leishmaniasis. Parasite Immunology 1996, 18, 617-623, 10.1046/j.1365-3024.1996.d01-39.x.
- Virgínia Bodelão Richini‐Pereira; Pâmela Merlo Marson; Enio Yoshinori Hayasaka; Cassiano Victoria; Rodrigo Silva; H. Langoni; Molecular detection of Leishmania spp. in road-killed wild mammals in the Central Western area of the State of São Paulo, Brazil. Journal of Venomous Animals and Toxins including Tropical Diseases 2014, 20, 27-27, 10.1186/1678-9199-20-27.
- Carolina Naranjo; Dolors Fondevila; Marta Leiva; Xavier Roura; Teresa Peña; Characterization of lacrimal gland lesions and possible pathogenic mechanisms of keratoconjunctivitis sicca in dogs with leishmaniosis. Veterinary Parasitology 2005, 133, 37-47, 10.1016/j.vetpar.2005.05.017.
- V.T. Barbosa; M.A.G. Silva; M.G. Sousa; A.P. Gering; H.D. Santos; J.L. Laus; Detecção de formas amastigotas em exame parasitológico de esfregaço obtido a partir de suabe conjuntival de cães com leishmaniose visceral. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 2012, 64, 1465-1470, 10.1590/s0102-09352012000600009.
- Brett P. Bielory; Hamed B. Lari; Neena Mirani; Rajendra Kapila; Valerie A. Fitzhugh; Roger E. Turbin; Conjunctival Squamous Cell Carcinoma Harboring Leishmania Amastigotes in a Human Immunodeficiency Virus–Positive Patient. Archives of Ophthalmology 2011, 129, 1230, 10.1001/archophthalmol.2011.252.
- Carolina Naranjo; Dolors Fondevila; Laura Altet; Olga Francino; J. Ríos; Xavier Roura; Teresa Peña; Evaluation of the presence of Leishmania spp. by real-time PCR in the lacrimal glands of dogs with leishmaniosis. The Veterinary Journal 2012, 193, 168-173, 10.1016/j.tvjl.2011.10.001.
- Dalit Strauss-Ayali; Charles L. Jaffe; Ofer Burshtain; Liat Gonen; Gad Baneth; Polymerase Chain Reaction Using Noninvasively Obtained Samples, for the Detection of Leishmania infantum DNA in Dogs. The Journal of Infectious Diseases 2004, 189, 1729-1733, 10.1086/383281.
- Vanessa Figueredo Pereira; Julia Cristina Benassi; Wilma Starke-Buzetti; Diogo Tiago Silva; Helena L. Ferreira; Lara Borges Keid; Rodrigo Martins Soares; Vera Letticie De Azevedo Ruiz; Trícia Maria Ferreira De Sousa Oliveira; Detection of canine visceral leishmaniasis by conjunctival swab PCR. Revista da Sociedade Brasileira de Medicina Tropical 2016, 49, 104-106, 10.1590/0037-8682-0191-2015.
- Sidney De Almeida Ferreira; Leonardo Trindade Ituassu; Maria Norma De Melo; Antero Silva Ribeiro De Andrade; Evaluation of the conjunctival swab for canine visceral leishmaniasis diagnosis by PCR–hybridization in Minas Gerais State, Brazil. Veterinary Parasitology 2008, 152, 257-263, 10.1016/j.vetpar.2007.12.022.
- Rodrigo Souza Leite; Sidney De Almeida Ferreira; Leonardo Trindade Ituassu; Maria Norma De Melo; Antero Silva Ribeiro De Andrade; PCR diagnosis of visceral leishmaniasis in asymptomatic dogs using conjunctival swab samples. Veterinary Parasitology 2010, 170, 201-206, 10.1016/j.vetpar.2010.02.020.
- Trícia Maria Ferreira De Sousa Oliveira; Vanessa Figueredo Pereira; Graziella Ulbricht Benvenga; Maria Fernanda Alves Martin; Julia Cristina Benassi; Diogo Tiago Da Silva; Wilma Starke-Buzetti; Conjunctival swab PCR to detect Leishmania spp. in cats. Revista Brasileira de Parasitologia Veterinária 2015, 24, 220-222, 10.1590/s1984-29612015016.
- Julia Cristina Benassi; Graziella U. Benvenga; Helena L. Ferreira; Vanessa F. Pereira; Lara B. Keid; Rodrigo Soares; Trícia Maria Ferreira De Sousa Oliveira; Detection of Leishmania infantum DNA in conjunctival swabs of cats by quantitative real-time PCR. Experimental Parasitology 2017, 177, 93-97, 10.1016/j.exppara.2017.04.004.
- Mehmet Karakus; Suha Kenan Arserim; Özge Erişöz Kasap; Metin Pekağırbaş; Duygu Aküzüm; Bülent Alten; Seray Töz; Yusuf Özbel; Vector and reservoir surveillance study in a canine and human leishmaniasis endemic area in most western part of Turkey, Karaburun. Acta Tropica 2019, 190, 177-182, 10.1016/j.actatropica.2018.11.020.
- Angela Monica Ionică; Georgiana Deak; Zsuzsa Kalmar; Calin Gherman; Andrei Daniel Mihalca; Mirabela O Dumitrache; Molecular Survey on Leishmania Infantum Infection in Red Foxes (Vulpes Vulpes) From Romania. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Veterinary Medicine 2017, 74, 230, 10.15835/buasvmcn-vm:0054.
- Ln Nsiangani; D Kaimbo Wa Kaimbo; Ml Kazumba; Anterior uveitis as the first sign of human African trypanosomiasis: a case report. Médecine et Santé Tropicales 2016, 26, 334-336, 10.1684/mst.2016.0587.
- W. I. Morrison; M. Murray; P. D. Sayer; J. M. Preston; The pathogenesis of experimentally induced Trypanosoma brucei infection in the dog. I. Tissue and organ damage.. The American Journal of Pathology 1981, 102, 168-181, .
- Panigrahi, P.N.; Mahendran, K.; Jena, S.C.; Behera, P.; Mahajan, S.; Arjun, K.; Dey, S. Trypanosoma evansi infection in a German shepherd dog — Apparent successful treatment using serial low dose of diminazene aceturate. Veterinary Parasitology: Regional Studies and Reports 2015, 1-2, 70-74, doi:10.1016/j.vprsr.2016.03.003.
- J Mortelmans; A Neetens; Ocular lesions in experimental Trypanosoma brucei infection in cats.. Acta zoologica et pathologica Antverpiensia 1975, , , .
- Aleksandro S. Da Silva; F. Pierezan; P. Wolkmer; M.M. Costa; C.B. Oliveiro; A.A. Tonin; J.M. Santurio; S.T.A. Lopes; S.G. Monteiro; Pathological Findings Associated with Experimental Infection by Trypanosoma evansi in Cats. Journal of Comparative Pathology 2010, 142, 170-176, 10.1016/j.jcpa.2009.10.020.
- Inmaculada Morales; Mónica De León; Manuel Morales; Francesca Dalla; Carlos Gutiérrez; Ocular lesions associated with Trypanosoma evansi in experimentally infected goats. Veterinary Parasitology 2006, 141, 325-329, 10.1016/j.vetpar.2006.06.007.
- A Prata; Evolution of the clinical and epidemiological knowledge about Chagas disease 90 years after its discovery.. Memórias do Instituto Oswaldo Cruz 1999, 94, 81-88, 10.1590/s0074-02761999000700008.
- P. D. Marsden; Trypanosoma cruziinfections in CFI mice. Annals of Tropical Medicine & Parasitology 1967, 61, 62-67, 10.1080/00034983.1967.11686459.
- O. K. Giddings; Chris Eickhoff; T. J. Smith; L. A. Bryant; D. F. Hoft; Anatomical Route of Invasion and Protective Mucosal Immunity in Trypanosoma cruzi Conjunctival Infection. Infection and Immunity 2006, 74, 5549-5560, 10.1128/IAI.00319-06.
- Maria Terezinha Bahia; Washington Luiz Tafuri; Marcelo V. Caliari; Vanja Maria Veloso; Claudia M. Carneiro; George Luiz Lins Machado-Coelho; Marta Lana; Comparison of Trypanosoma cruzi infection in dogs inoculated with blood or metacyclic trypomastigotes of Berenice-62 and Berenice-78 strains via intraperitoneal and conjunctival routes.. Revista da Sociedade Brasileira de Medicina Tropical 2002, 35, , .
- Christopher D Conrady; Kimberly E Hanson; Sonia Mehra; Adrienne Carey; Marissa LaRochelle; Akbar Shakoor; The First Case of Trypanosoma cruzi–Associated Retinitis in an Immunocompromised Host Diagnosed With Pan-Organism Polymerase Chain Reaction. Clinical Infectious Diseases 2018, 67, 141-143, 10.1093/cid/ciy058.
- Leidi Herrera; Clara Martinez; Hernan Jose Carrasco; Ana Maria Jansen; Servio Urdaneta-Morales; Cornea as a tissue reservoir of Trypanosoma cruzi. Parasitology Research 2006, 100, 1395-1399, 10.1007/s00436-006-0403-9.
- Malihe Nourollahpour Shiadeh; Maryam Niyyati; Shirzad Fallahi; Ali Rostami; Human parasitic protozoan infection to infertility: a systematic review. Parasitology Research 2015, 115, 469-477, 10.1007/s00436-015-4827-y.
- Teichmann, C.E.; Da Silva, A.S.; Monteiro, S.G.; Barbosa, C.F.; Barcelos, R. Evidence of Venereal and Transplacental Transmission of Canine Visceral Leishmaniasis in Southern Brazil. ACTA SCIENTIAE VETERINARIAE 2011, 39.
- Naucke, T.J.; Lorentz, S. Non-sandfly transmission of canine leishmaniasis. TIERAERZTLICHE UMSCHAU 2013, 68, 121-125.
- Veera Karkamo; Anu Kaistinen; Anu Näreaho; Kati Dillard; Katri Vainio-Siukola; Gabriele Vidgren; Niina Tuoresmäki; Marjukka Anttila; The first report of autochthonous non-vector-borne transmission of canine leishmaniosis in the Nordic countries.. Acta Veterinaria Scandinavica 2014, 56, 84, 10.1186/s13028-014-0084-9.
- Mahdi Mosayebi; Mehdi Mohebali; Aliasghar Farazi; Mohammad Reza Shirzadi; Davood Akhlaghi; Reza Hajhossein; Samira Elikaee; Leishmaniasis Caused by Leishmania major on the Glans Penis: A Case Report.. 2019, 14, 472-476, .
- Ismery Cabello; Alejandro Caraballo; Yaneth Millan; Leishmaniasis in the genital area.. Revista do Instituto de Medicina Tropical de São Paulo 2002, 44, 105-107, 10.1590/s0036-46652002000200009.
- Carin Cain; Mary Stone; Mark Thieberg; Mary E. Wilson; Nonhealing Genital Ulcers. Archives of Dermatology 1994, 130, 1316, 10.1001/archderm.1994.01690100103020.
- Z A Andrade; S G Andrade; [Some new aspects of the kala-azar pathology. (Morphologic study of 13 autopsy cases)].. Revista do Instituto de Medicina Tropical de São Paulo 1966, 8, , .
- Francisco Martinez-Garcia; Javier Regadera; Rodolfo Mayer; Susan Sanchez; Manuel Nistal; Protozoan Infections in the Male Genital Tract. Journal of Urology 1996, 156, 340-349, 10.1016/s0022-5347(01)65846-4.
- Soraia De Araújo Diniz; M. S. Melo; A. M. Borges; R. Bueno; B. P. Reis; W. L. Tafuri; E. F. Nascimento; Renato De Lima Santos; Genital Lesions Associated with Visceral Leishmaniasis and Shedding of Leishmania sp. in the Semen of Naturally Infected Dogs. Veterinary Pathology 2005, 42, 650-658, 10.1354/vp.42-5-650.
- F. Mir; E. Fontaine; Edouard Reyes-Gomez; M. Carlus; A. Fontbonne; Subclinical leishmaniasis associated with infertility and chronic prostatitis in a dog. Journal of Small Animal Practice 2012, 53, 419-422, 10.1111/j.1748-5827.2012.01224.x.
- Viviane Cardoso Boechat; Artur Augusto Velho Mendes Júnior; Maria De Fátima Madeira; Luiz Claudio Ferreira; Fabiano Borges Figueiredo; Francisco Das Chagas De Carvalho Rodrigues; Valéria Da Costa Oliveira; Raquel De Vasconcellos Carvalhaes De Oliveira; Rodrigo Caldas Menezes; Occurrence of Leishmania infantum and associated histological alterations in the genital tract and mammary glands of naturally infected dogs. Parasitology Research 2016, 115, 2371-2379, 10.1007/s00436-016-4987-4.
- L.C. Silva; V.P. Assis; V.M. Ribeiro; W L Tafuri; J.C. Toledo Júnior; S.O. Silva; M.N. Melo; Milene Alvarenga Rachid; Guilherme Ribeiro Valle; Detection of Leishmania infantum in the smegma of infected dogs. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 2014, 66, 731-736, 10.1590/1678-41626610.
- Leticia Hernández; Ana Montoya; Rocío Checa; Diana Dado; Rosa Gálvez; Domenico Otranto; Maria Stefania Latrofa; Gad Baneth; Guadalupe Miró; Course of experimental infection of canine leishmaniosis: follow-up and utility of noninvasive diagnostic techniques.. Veterinary Parasitology 2015, 207, 149-155, 10.1016/j.vetpar.2014.10.035.
- Fabiana L. Silva; Antonio A.M. Rodrigues; Isabela O.P. Rego; Raquel L.H. Santos; Raquel G. Oliveira; Teane M.A. Silva; Mariana N. Xavier; Ernane F. Nascimento; Renato De Lima Santos; Mariana X. Byndloss; et al. Genital lesions and distribution of amastigotes in bitches naturally infected with Leishmania chagasi. Veterinary Parasitology 2008, 151, 86-90, 10.1016/j.vetpar.2007.09.032.
- S. G. Rhind; P. N. Shek; Cytokines in the pathogenesis of human African trypanosomiasis: antagonistic roles of TNF-α and IL-10. Progress in Human African Trypanosomiasis, Sleeping Sickness 1999, , 119-135, 10.1007/978-2-8178-0857-4_7.
- Apted, F.I.C. Clinical manifestations and diagnosis of sleep- ing sickness. Mulligan, H.W., Pott, W.H., Eds. Allen & Unwin Ltd.: 1970; pp. 661-683.
- G Rocha; A Martins; G Gama; F Brandao; Jorge Atouguia; Possible cases of sexual and congenital transmission of sleeping sickness.. The Lancet 2004, 363, 247, 10.1016/S0140-6736(03)15345-7.
- Keisuke Suganuma; Sandagdorj Narantsatsral; Banzragch Battur; Shino Yamasaki; Davaajav Otgonsuren; Simon Peter Musinguzi; Batdorj Davaasuren; Badgar Battsetseg; Noboru Inoue; Isolation, cultivation and molecular characterization of a new Trypanosoma equiperdum strain in Mongolia.. Parasites & Vectors 2016, 9, 481, 10.1186/s13071-016-1755-3.
- T. Guimarães; Graça Lopes; Marlene Cavaleiro Pinto; Eliane Silva; Carla Miranda; M.J. Correia; L. Damasio; Gertrude Thompson; A. Rocha; Colloid centrifugation of fresh stallion semen before cryopreservation decreased microorganism load of frozen-thawed semen without affecting seminal kinetics. Theriogenology 2015, 83, 186-191, 10.1016/j.theriogenology.2014.09.003.
- Ahmed, Y.; Hagos, A.; Merga, B.; Van Soom, A.; Duchateau, L.; Goddeeris, B.M.; Govaere, J. Trypanosoma equiperdum in the horse - a neglected threat? VLAAMS DIERGENEESKUNDIG TIJDSCHRIFT 2018, 87, 66-75.
- Al-Busadah, K.A.; El-Bahr, S.M.; Khalafalla, A.I. Serum biochemical profile and molecular detection of pathogens in semen of infertile male dromedary camels ( Camelus dromedarius ). Animal Reproduction Science 2017, 180, 58-65, doi:10.1016/j.anireprosci.2017.03.003.
- Francis Abidemi Ogundele; Oluyinka Oluseyi Okubanjo; Olagunju Joseph. Ajanusi; Samuel Tanko Fadason; Semen characteristics and reaction time of Yankasa rams experimentally infected with Trypanosoma evansi infection. Theriogenology 2016, 86, 667-673, 10.1016/j.theriogenology.2016.02.018.
- Nicholas Morais Bezerra; Gabriela Hemylin Ferreira Moura; Hélio Noberto De Araújo; Francisco Bezerra; Kaliane Alessandra Rodrigues De Paiva; Kizzy Millenn De Freitas Mendonça Costa; Wirton Peixoto Costa; Dayse Ariane Soares Medeiros; Jael Soares Batista; Detection of Trypanosoma vivax DNA in semen from experimentally infected goats. Veterinary Research Communications 2018, 42, 131-135, 10.1007/s11259-018-9715-3.
- B.O. Ikede; Genital lesions in experimental chronic Trypanosoma brucei infection in rams. Research in Veterinary Science 1979, 26, 145-151, 10.1016/s0034-5288(18)32907-2.
- V.O. Anosa; T.T. Isoun; Further observations on the testicular pathology in Trypanosoma vivax infection of sheep and goats. Research in Veterinary Science 1980, 28, 151-160, 10.1016/s0034-5288(18)32738-3.
- T T Isoun; V O Anosa; Lesions in the reproductive organs of sheep and goats experimentally infected with Trypanosoma vivax.. Tropenmedizin und Parasitologie 1974, 25, , .
- G P Kaaya; D Oduor-Okelo; The effects of Trypanosoma congolense infection on the testis and epididymis of the goat.. Bulletin of Animal Health and Production in Africa 1980, 28, , .
- S. Adamu; M.Y. Fatihu; N.M. Useh; M. Mamman; V.O. Sekoni; K.A.N. Esievo; Sequential testicular and epididymal damage in Zebu bulls experimentally infected with Trypanosoma vivax. Veterinary Parasitology 2007, 143, 29-34, 10.1016/j.vetpar.2006.07.022.
- V. O. Anosa; T. T. Isoun; Pathology of Experimental Trypanosoma vivax Infection in Sheep and Goats. Zentralblatt für Veterinärmedizin Reihe B 2010, 30, 685-700, 10.1111/j.1439-0450.1983.tb01894.x.
- F. Claes; Suman K. Vodnala; Nick Van Reet; Nathalie Boucher; Hilda Lundén-Miguel; Théo Baltz; Bruno Goddeeris; Philippe Büscher; Martin Rottenberg; Bioluminescent Imaging of Trypanosoma brucei Shows Preferential Testis Dissemination Which May Hamper Drug Efficacy in Sleeping Sickness. PLOS Neglected Tropical Diseases 2009, 3, e486, 10.1371/journal.pntd.0000486.
- Tânia Carvalho; Sandra Trindade; Sílvia Pimenta; Ana B. Santos; Filipa Rijo-Ferreira; Luisa M. Figueiredo; Trypanosoma brucei triggers a marked immune response in male reproductive organs. PLOS Neglected Tropical Diseases 2018, 12, e0006690, 10.1371/journal.pntd.0006690.
- A L Bittencourt; [Chagasic placentitis and congenital transmission of Chagas' disease].. Revista do Instituto de Medicina Tropical de São Paulo 1963, 5, , .
- Contribuição para o estudo da anatomia patolojica da . , , , .
- Amilcar Martins; Valdemar Versiani; Antonio Tupínambá; Estudos sobre a Tripanosomiase americana em Minas Gerais, Brasil. Memórias do Instituto Oswaldo Cruz 1940, 35, , 10.1590/s0074-02761940000200001.
- Lamano Carvalho, T.L.; Ferreira, A.L.; Sahao, M.A. Alteracoes do testIculo humano na molestia de Chagas. II- Estudo morfometrico do tecido intersticial. Rev. Inst. Med. Trop. Sao Paulo 1982, 214-214.
- H L Lenzi; Morgana T.L Castelo-Branco; Marcelo Pelajo-Machado; Denise N Oliveira; Cerli R Gattass; Trypanosoma cruzi: compromise of reproductive system in acute murine infection.. Acta Tropica 1998, 71, 117-129, 10.1016/s0001-706x(98)00058-8.
- Luiz Otávio Pereira Carvalho; Ana Lucia Abreu-Silva; Daiana De Jesús Hardoim; Roberto Carlos Tedesco; Verônica Gonçalves Mendes; Sylvio Celso Gonçalves Da Costa; Kátia Da Silva Calabrese; Trypanosoma cruzi and myoid cells from seminiferous tubules: interaction and relation with fibrous components of extracellular matrix in experimental Chagas' disease. International Journal of Experimental Pathology 2009, 90, 52-57, 10.1111/j.1365-2613.2008.00592.x.
- Clara Crespillo-Andújar; Marta Díaz-Menéndez; Marta Mora-Rillo; Evidence for Previously Unidentified Sexual Transmission of Protozoan Parasites. Emerging Infectious Diseases 2018, 24, 602-603, 10.3201/eid2403.171838.
- Breniere, S.-F.; Waleckx, E.; Aznar, C. American Trypanosomiasis (Chagas Disease), One hundred Years of Research, 2nd edition. Telleria, J., Tibayrenc, M., Eds. Elservier INC: 2017; pp. 561-578.
- Anderson, C. Nouveaux essais de culture de Leishmania donovani. Archive de l'institut Pasteur de Tunis 1935, 24, 131-131.
- A. Henry; J. Guilhon; Le rôle du lait dans la transmission de quelques protozooses. Le Lait 1944, 24, 23-35, 10.1051/lait:1944231-2332.
- Nathan-Larrier, L. Sur le passage des trypanosomes dans le lait. Revue de Pathologie Comparée 1913, 282-282.
- M L Calvo-Méndez; B Nogueda-Torres; R Alejandre-Aguilar; M Cortés-Jiménez; [Experimental Trypanosoma cruzi infection via contaminated water and food].. Revista latinoamericana de microbiologia 1994, 36, , .
- R Disko; H E Krampitz; [Occurrence of Trypanosoma cruzi in the milk of infected mice].. Zeitschrift fur Tropenmedizin und Parasitologie 1971, 22, , .
- Miles, M.A. Trypanosoma cruzi – milk transmission of infection and immunity from mother to young. Parasitology 1972, 65, 1-1, doi:10.1017/S0031182000044188.
- Maria Das Dores Medina-Lopes; Vanize Macêdo; Trypanosoma cruzi no colostro humano. Revista da Sociedade Brasileira de Medicina Tropical 1983, 16, 170-170, 10.1590/s0037-86821983000300010.
- M E Jörg; [The transmission of Trypanosoma cruzi via human milk].. Revista da Sociedade Brasileira de Medicina Tropical 1992, 25, , .
- V Amato Neto; L Matsubara; R Campos; A A Moreira; P L Pinto; R Faccioli; M Zugaib; [Trypanosoma cruzi in the milk of women with chronic Chagas disease].. Revista do Hospital das Clínicas 1992, 47, , .
- R Campos; P L Pinto; A A Moreira; V Amato Neto; M I Duarte; E J De Sant'ana; G G Tiago; [Experimental study on the transmission of Chagas' disease by milk].. Revista do Hospital das Clínicas 1988, 43, , .
- Francesca F Norman; Rogelio Lopez-Velez; Chagas Disease and Breast-feeding. Emerging Infectious Diseases 2013, 19, 1561-1566, 10.3201/eid1910.130203.
- R. D. Ribeiro; R. A. Lopes; T. A. R. Garcia; A. Campos; Histopathological study of the mammary gland in Trypanosoma cruzi-infected mice. Parasitology Research 1988, 74, 290-292, 10.1007/bf00539580.
- Cláudio Santos Ferreira; Prazeres Conceição Martinho; Vicente Amato Neto; Roseana Rodrigues Bressane Cruz; Pasteurization of human milk to prevent transmission of Chagas disease.. Revista do Instituto de Medicina Tropical de São Paulo 2001, 43, 161-162, 10.1590/s0036-46652001000300008.
- Cláudio Santos Ferreira; V Amato Neto; Erika Gakiyai; Rita Cristina Bezerra; Ruth Semira Rodríguez Alarcón; Microwave treatment of human milk to prevent transmission of Chagas disease. Revista do Instituto de Medicina Tropical de São Paulo 2003, 45, 41-42, 10.1590/s0036-46652003000100008.
- Nicole Harrison; Julia Walochnik; Reinhard Ramsebner; Luzia Veletzky; Heimo Lagler; Michael Ramharter; Progressive Perforation of the Nasal Septum Due to Leishmania major: A Case of Mucosal Leishmaniasis in a Traveler. The American Journal of Tropical Medicine and Hygiene 2017, 96, 653-655, 10.4269/ajtmh.16-0809.
- B L Travi; C Ferro; H Cadena; C J Tabares; Y Osorio; Canine visceral leishmaniasis in Colombia: relationship between clinical and parasitologic status and infectivity for sand flies.. The American Journal of Tropical Medicine and Hygiene 2001, 64, 119-124, 10.4269/ajtmh.2001.64.119.
- Lt Eric S. Goodrich; Lt Stephen C. Sears; Capt Timothy Sorrells; Capt James K. Radike; Lcdr Anis Miladi; Cdr Jonathan S. Glass; A Case of Cutaneous Leishmaniasis guyanensis Mimicking Otitis Externa. Military Medicine 2017, 182, , 10.7205/milmed-d-17-00054.
- Dustin Penn; Chemical signals and parasite-mediated sexual selection. Trends in Ecology & Evolution 1998, 13, 391-396, 10.1016/s0169-5347(98)01473-6.
- Jairo Torres Magalhães-Junior; Paulo Roberto Ribeiro Mesquita; Wyllian Franz Dos Santos Oliveira; Fábio Santos Oliveira; Carlos Roberto Franke; Frederico De Medeiros Rodrigues; Jailson B. De Andrade; Stella Maria Barrouin-Melo; Identification of biomarkers in the hair of dogs: new diagnostic possibilities in the study and control of visceral leishmaniasis. Analytical and Bioanalytical Chemistry 2014, 406, 6691-6700, 10.1007/s00216-014-8103-2.
- Lidia S. De Oliveira; Frederico De M. Rodrigues; F DeOliveira; Paulo R. R. Mesquita; Danielle C. Leal; Adriano Costa De Alcântara; Barbara M. Souza; Carlos Franke; Pedro. A. De P. Pereira; Jailson B. De Andrade; et al. Headspace solid phase microextraction/gas chromatography–mass spectrometry combined to chemometric analysis for volatile organic compounds determination in canine hair: A new tool to detect dog contamination by visceral leishmaniasis. Journal of Chromatography B 2008, 875, 392-398, 10.1016/j.jchromb.2008.09.028.
- Rubén Muñoz-Madrid; Silvia Belinchón-Lorenzo; Virginia Iniesta; Javier Fernández-Cotrina; Juan Carlos Parejo; Francisco Javier Serrano; Isabel Monroy; VictorA Baz; Adela Gómez-Luque; Luis Carlos Gómez-Nieto; et al. First detection of Leishmania infantum kinetoplast DNA in hair of wild mammals: Application of qPCR method to determine potential parasite reservoirs. Acta Tropica 2013, 128, 706-709, 10.1016/j.actatropica.2013.08.009.
- Rafaela De Sousa Gonçalves; Carlos Franke; Jairo T. Magalhães-Junior; Bárbara M.P.S. Souza; Manuela Da Silva Solcà; Daniela F. Larangeira; Stella Maria Barrouin-Melo; Association between Leishmania infantum DNA in the hair of dogs and their infectiousness to Lutzomyia longipalpis. Veterinary Parasitology 2016, 232, 43-47, 10.1016/j.vetpar.2016.11.010.
- Sarah Karram; Asif Loya; Hadi Hamam; Robert H. Habib; Ibrahim Khalifeh; Transepidermal elimination in cutaneous leishmaniasis: a multiregional study. Journal of Cutaneous Pathology 2012, 39, 406-412, 10.1111/j.1600-0560.2012.01890.x.
- Cidia Vasconcellos; Mirian Nacagami Sotto; Experimental cutaneous leishmaniasis: transmission electron microscopy of the inoculation site. International Journal of Experimental Pathology 1997, 78, 81-89, 10.1046/j.1365-2613.1997.d01-243.x.
- Rafaela De Sousa Gonçalves; Carlos Franke; Jairo T. Magalhães-Junior; Bárbara M.P.S. Souza; Manuela Da Silva Solcà; Daniela F. Larangeira; Stella Maria Barrouin-Melo; Association between Leishmania infantum DNA in the hair of dogs and their infectiousness to Lutzomyia longipalpis. Veterinary Parasitology 2016, 232, 43-47, 10.1016/j.vetpar.2016.11.010.
- Sarah Karram; Asif Loya; Hadi Hamam; Robert H. Habib; Ibrahim Khalifeh; Transepidermal elimination in cutaneous leishmaniasis: a multiregional study. Journal of Cutaneous Pathology 2012, 39, 406-412, 10.1111/j.1600-0560.2012.01890.x.
- Cidia Vasconcellos; Mirian Nacagami Sotto; Experimental cutaneous leishmaniasis: transmission electron microscopy of the inoculation site. International Journal of Experimental Pathology 1997, 78, 81-89, 10.1046/j.1365-2613.1997.d01-243.x.