Neuroblastoma (NB) is an extracranial tumor of the peripheral nervous system arising from neural crest cells. It is the most common malignancy in infants and the most common extracranial solid tumor in children. The treatment for high-risk NB involves chemotherapy and surgical resection followed by high-dose chemotherapy with autologous stem-cell rescue and radiation treatment. However, those with high-risk NB are susceptible to relapse and the long-term side effects of standard chemotherapy
Polyphenols, including the sub-class of flavonoids, contain more than one aromatic ring with hydroxyl groups. The literature demonstrates their utility in inducing the apoptosis of neuroblastoma cells, mostly in vitro and some in vivo. This review explores the use of various polyphenols outlined in primary studies, underlines the pathways involved in apoptotic activity, and discusses the dosage and delivery of these polyphenols.
1. Calpain-Dependent Apoptotic Pathway
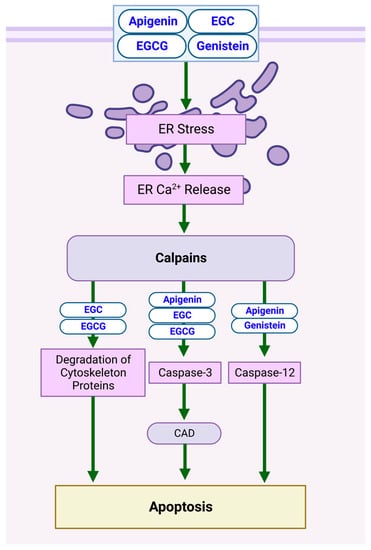
The release of calcium (Ca
2+) from the endoplasmic reticulum (ER) leads to the activation of calpains. Calpain is a Ca
2+-activated endo-protease involved in apoptotic mechanisms [
25]. Exposure to the compounds listed in
Table 1 activates proteolytic pathways involving calpain, leading to NB-cell apoptosis. Specifically, apigenin, epigallocatechin (EGC), epigallocatechin gallate (EGCG), and genistein trigger ER stress, thus increasing intracellular free Ca
2+ and causing calpain activation at the ER membrane [
26,
27], resulting in the degradation of cytoskeletal proteins and the destabilization of cellular integrity in SH-SY5Y cells. Caspase release is also stimulated, with caspase-3 activating caspase-activated DNase (CAD), contributing to DNA fragmentation [
8,
28]. Caspase-12 is also activated when apigenin and genistein are applied, reinforcing their apoptotic effects [
27]. Several studies concerning other cancer types reinforced the calpain–caspase apoptotic pathway, illustrated in
Figure 1 [
25,
26,
27,
28,
29].
Figure 1. Schematic diagram demonstrating the apoptotic effects of flavonoids on NB cell lines via a calpain-dependent pathway. The induction of ER stress induces Ca
2+ release at the ER membrane, triggering caspase and CAD release and degradation of cytoskeletal proteins. Specific biomarkers can be viewed in
Table 1. Created with
BioRender.com.
Table 1. Results of in vitro studies of polyphenols’ apoptotic effects on NB cell lines via a calpain-dependent pathway *.
| Compound |
Cell Line |
Incubation Period |
Concentration(s) |
Biomarker Changes |
Reference |
| Flavonoids |
|
|
|
|
|
| Apigenin |
SH-SY5Y |
24 h |
50 µM |
↑ Intracellular free [Ca2+]
↑ Calpain activation
↑ Caspase-12, -3
↑ CAD |
[27] |
| EGC |
SH-SY5Y |
24 h |
50 µM |
↑ Intracellular free [Ca2+]
↑ Calpain activation
↑ Cytoskeletal protein degradation
↑ Caspase-3
↑ CAD |
[27] |
| EGCG |
SH-SY5Y |
24 h |
50 µM |
↑ Intracellular free [Ca2+]
↑ Calpain activation
↑ Cytoskeletal protein degradation
↑ Caspase-3
↑ CAD |
[27] |
| Genistein |
SH-SY5Y |
24 h |
100 µM |
↑ Intracellular free [Ca2+]
↑ Calpain activation
↑ Caspase-12 |
[27] |
2. Anti-Proliferative Pathways
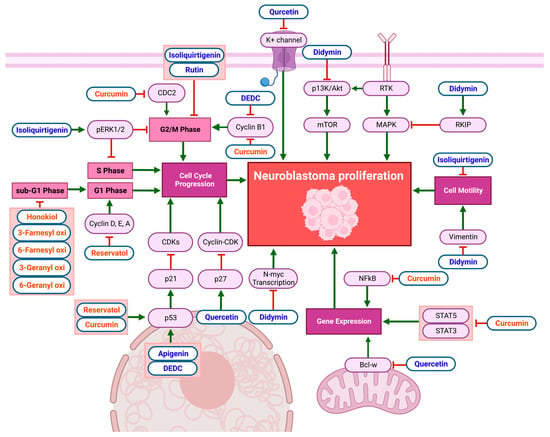
In vitro studies on the anti-cancer effects of flavonoids and non-flavonoid polyphenols in NB cell lines reveal remarkable anti-proliferative effects, as demonstrated in
Figure 2 and
Table 2. Quercetin exhibits anti-proliferative effects by increasing p27 mRNA expression, inhibiting the formation and activity of the cyclin–cyclin-dependent kinase (cyclin/CDK) complex, disrupting the cell cycle in NB. Quercetin also reduces B-cell lymphoma-w (Bcl-w) mRNA expression, which decreases tumor-gene expression and induces apoptosis in NB cells [
30]. Quercetin can further inhibit NB-cell growth by blocking voltage-gated potassium (K
+) channel activity [
31]. Other flavonoids, such as apigenin and 2-(cis-1,2-dihydroxy-4-oxo-cyclohex-5-enyl)-5,7-dihydroxy-chromone (DEDC), increase p53 and p21 mRNA expression while decreasing cyclin-B1 expression in NB [
32,
33]. Didymin decreases the proliferation of NB cells via the downregulation of the phosphoinositide 3-kinase (PI3K) and Akt pathways, accompanied by reduced vimentin levels, indicating a decrease in cell motility. Furthermore, proto-oncogene N-Myc transcription was inhibited by didymin. Increased Raf-1 kinase inhibitor protein (RKIP) levels inhibit the mitogen-activated protein kinase (MAPK) pathway, also decreasing proliferation [
34]. Isoliquiritigenin inhibits cell motility and increases the activation of extracellular regulated kinase 1/2 (pERK1/2), which inhibits NB-cell migration and proliferation while arresting the cell cycle in the S phase [
35]. Isoliquiritigenin and rutin both enhance G2/M-phase arrest in NB [
35,
36].
Figure 2. Schematic diagram demonstrating the anti-proliferative effects of flavonoids (blue) and non-flavonoid polyphenols (orange) on NB cell lines. The inhibition of cell-cycle progression, cell motility, and gene expression limits NB-cell proliferation. Compounds caused cell-cycle arrest in the S phase, sub-G1 phase, G1 phase, and G2/M phase. Specific biomarkers can be viewed in
Table 2. Created with
BioRender.com.
Non-flavonoid polyphenols affect multiple cell lines through multiple pathways. Curcumin decreases CDC2 and cyclin-B1, resulting in NB-cell-cycle arrest in the G2/M phase [
37]. Furthermore, it reduces NF-κB activator protein (AP-1) and STAT3 and STAT5 activation, suppressing gene transcription [
38]. Honokiol inhibits NB-cell-cycle progression at the sub-G1 phase [
39]. Resveratrol reduced Cyclin D1 levels in NB cells, causing cell-cycle arrest in the S phase [
40]. Similarly, the treatment of NB cells with resveratrol resulted in a significant drop in pAkt, Cyclin D, E, A, and CDK2 levels and increased p53 and NF-κB, resulting in cell-cycle arrest in the S phase [
41]. Resveratrol causes p21 levels to rise, which inhibits CDK levels and causes cell-cycle arrest in the G1, G2/M, and S phases in NB cell lines [
42]. Prenyl hydroxy coumarin derivatives also have notable anti-proliferative effects on NB cell lines, inducing cell-cycle arrest in the sub-G1 phase, with no effect on normal lymphocytic cells [
43].
In addition to isolated flavonoids and non-flavonoid polyphenolic compounds, recent research supports the potent anti-cancer effects of whole plants or plant extracts characterized by numerous phytochemicals acting synergistically or additively [
44,
45,
46,
47]. For example, a recent study by Morandi et al. (2021) demonstrated the capacity of olive-leaf extract (rich in phenolic compounds) to inhibit the proliferation of NB cells through cell arrest in the G0/G1 phase and the accumulation of cells in the sub-G0 phase, accompanied by the induction of apoptosis [
48]. Numerous other recent studies highlight the anti-cancer potential of plant extracts; for example, the fruit extract of
Kigelia Africana, a plant rich in flavonoids that are used in traditional African medicine, inhibited proliferation and other mechanisms associated with carcinogenesis in NB cells [
49]. Interestingly, research results obtained by Roomi et al. (2013) suggested the therapeutic potential of a nutrient mixture of lysine, proline, ascorbic acid, and green-tea extracts for NB management through the inhibition of tumor growth and proliferation and the induction of apoptosis in neuroblastoma models in vitro and in vivo [
50]. Indeed, green tea is rich in numerous phytochemicals, mainly catechins. Green-tea catechins, including ECG, EGCG, and EGC, are phytochemicals with strong anti-cancer effects [
51,
52].
Table 2. Results of in vitro studies of polyphenols’ anti-proliferative effects on NB cell lines *.
| Compound |
Cell Line |
Incubation Period |
Concentration(s) |
Biomarker Changes and Effects |
Reference |
| Flavonoids |
|
|
|
|
|
| Apigenin |
NUB-7 and LAN-5 |
24 h |
10, 50, 100, 150, 200 µM
IC50: 35 μM in NUB-7
IC50: 22 μM in LAN-5 |
↑ p53
↑ p21WAF−1/CIP−1 |
↓ Proliferation |
[33] |
| DEDC |
SH-SY5Y |
24 h |
7.5 µg/mL |
↑ p53 mRNA
↑ p21 mRNA
↓ Cyclin-B1 |
[32] |
| Didymin |
CHLA-90 and SK-N-BE2 (p53-mutant) l + SMS-KCNR and LAN-5 (p53 wild-type) |
24 h |
50 μmol/L |
↓ P13K
↓ Akt |
↓ Proliferation |
[34] |
| ↓ Vimentin |
↓ Motility of tumor cells |
| ↓ N-Myc transcription |
| ↑ RKIP |
↓ MAPK pathway
↓ Proliferation |
| Isoliquiritigenin |
SH-SY5Y |
24 h |
10–100 µM
IC50: 25.4 µM |
↑ pERK1/2 |
↓ Cell migration
↓ Proliferation
↑ S + G2/M-phase arrest |
[35] |
| Rutin |
LAN-5 |
24 h |
0, 25, 50, 100 μM |
|
↑ G2/M-phase arrest |
[36] |
| Quercetin |
Neuro2a (mouse cell line) |
24 h |
10, 20, 40, 80, 120 μM
IC50: 40 µM |
↑ p27 |
↓ Cyclin–CDK complex binding |
[30] |
| ↓ Bcl-w |
↓ Tumor-cell-gene expression |
| Quercetin |
Neuroblastoma X glioma NG 108-15 cells (mouse cell line) |
48 h |
10 µM, 20 µM
IC50: 10 µM |
↓ K+-channel activity |
↓ Cell growth |
[31] |
| Non-Flavonoid Polyphenols |
|
|
|
|
| Curcumin |
SK-N-SH |
24 h |
8, 16, 32 µM |
↓ CDC2
↓ Cyclin B1 |
↑ G2/M-phase arrest |
[37] |
| Curcumin |
GI-L-IN, HTLA-230, SH-SY5Y, LAN5, SK-NBE2c, and IMR-32 |
18–72 h |
0.1–25 µM |
↓ NFκβ activator protein (AP-1)
↓ STAT3, STAT5 activation |
↓ Cell growth |
[38] |
| Curcumin |
NUB-7, LAN-5, IMR-32 and SK-N-BE(2) |
2–8 days |
0–100 µM *
* Significantly inhibited proliferation in the range of 5–10 µM |
↑ p53 translocation from cytoplasm to nucleus
↑ p21WAF−1/CIP−1 |
↑ G1-, G2/M-, and S-phase arrest |
[42] |
| Honokiol |
Neuro-2a (mouse cell line) and NB41A3 |
72 h |
2.5, 5, 10, 20,
30, 40, 50, 60,
80, 100 µM
LC50: 63.3 µM |
|
↑ Sub-G1-phase arrest |
[39] |
| Prenyl hydroxy-coumarins |
Neuro-2a (mouse cell line) |
24, 48, 72 h |
6.25–200 µg/mL |
|
↑ Sub-G1-phase arrest |
[43] |
| Resveratrol |
B103 (rat cell line) |
48 h |
5–20 µM
IC50: 17.86 µM |
↓ Cyclin D1 |
↑ G1-phase arrest |
[40] |
| Resveratrol |
B65 (rat dopaminergic cell line) |
24 h |
25, 50, 100 µM |
↓ pAkt
↓ Cyclin D, E, A
↓ CDK2
↑ p53
↑ NFκβ |
↑ S-phase arrest |
[41] |
| Resveratrol |
NUB-7, LAN-5, IMR-32 and SK-N-BE(2) |
2–8 days |
25–160 µM |
↑ p53 translocation from cytoplasm to nucleus
↑ p21WAF−1/CIP−1 |
↑ G1-, G2/M-, and S-phase arrest |
[53] |
3. Mitochondrial and ER-Stress-Related Apoptotic Pathways
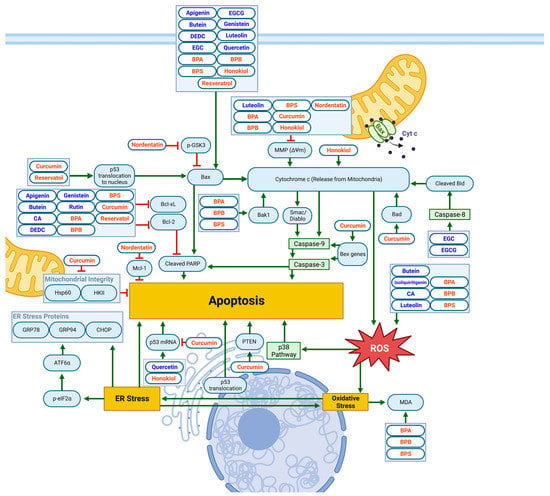
Results of several studies have also identified Mitochondrial and ER-Stress-Related Apoptotic Pathways, as demonstrated in Figure 4 and Table 3.
Figure 4. Schematic diagram demonstrating the apoptotic effects of flavonoids (blue) and non-flavonoid polyphenols (orange) on NB cell lines via mitochondrial or ER/oxidative-stress-related pathways. Elevated Bax/Bcl-2 ratio, increased PARP cleavage, loss of MMP and cytochrome C release, and ROS generation all contribute to apoptotic cell death. Specific biomarkers can be viewed in
Table 3. Created with
BioRender.com.
Table 3. Results of in vitro studies of polyphenols’ apoptotic effects on NB cell lines via receptor-mediated pathways *.
| Compound |
Cell Line |
Incubation Period |
Concentration(s) |
Biomarker Changes |
Reference |
| Flavonoids |
|
|
|
|
| DEDC |
SH-SY5Y |
24 h |
7.5 µg/mL |
↓ Phosphor-STAT3 expression (ROS mediated) |
[27] |
| Genistein |
SK-N-DZ |
24 h |
10 µM |
↑ TNF-α
↑ FasL
↑ TRADD
↑ FADD |
[27] |
| EGC |
SH-SY5Y |
24 h |
50 µM |
↑ Caspase-8 activation
↑ Proteolytic cleavage of Bid to tBid
↑ Bax oligomerization |
[27] |
| EGCG |
SH-SY5Y |
24 h |
100 µM |
↑ Caspase-8 activation
↑ Proteolytic cleavage of Bid to tBid
↑ Bax oligomerization |
[27] |
| Rutin |
LAN-5 |
24 h |
25, 50, 100 μM |
↑ TNF-α secretion |
[36] |
| Non-Flavonoid Polyphenols |
| Curcumin |
LAN-5 |
3, 5, 24 h |
5, 10, 15, 20 µM |
↑ Bad
↑ PTEN
↑ ROS |
[77] |
| Honokiol |
Neuro-2a (mouse cell line) |
30, 60, 120 µM |
24, 48, 72 h |
↑ RIP3
↑ ROS |
[80] |
This entry is adapted from the peer-reviewed paper 10.3390/biom13030563