Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Trophoblast cell surface antigen-2 (Trop-2) is a glycoprotein that was first described as a membrane marker of trophoblast cells and was associated with regenerative abilities. Trop-2 overexpression was also described in several tumour types. Nevertheless, the therapeutic potential of Trop-2 was widely recognized and clinical studies with drug–antibody conjugates have been initiated in various cancer types.
- Trop-2
- effect
- cancer
1. Background
1.1. Trophoblast Cell Surface Antigen-2 Biology and Functions
The transmembrane glycoprotein trophoblast cell surface antigen-2 (Trop-2) is widely expressed in various epithelial cancers as well as in specific normal tissue. Trop-2 is also known as tumour-associated calcium signal transducer 2 (TACSTD2), membrane component chromosome 1 surface marker 1 (M1S1), gastrointestinal antigen 733-1 (GA733-1), and epithelial glycoprotein-1 (EGP-1) [1].
Trop-2 was initially discovered in placental trophoblastic tissue, and the cells expressing this biomarker have the capacity to invade the uterus during placental implantation [2][3]. Lipinski et al. identified four new transmembrane glycoproteins (Trop-1, 2, 3, and 4) expressed on normal and malignant embryonal cells and, among them, only Trop-2 may similarly confer the capacity for proliferation and invasion to cancer cells [4].
Although the physiological function of Trop-2 is not fully clarified and is still under investigation, Trop-2 is implicated in several intracellular axes, including the MAPK/PI3K/AKT pathways that are implicated in proliferation, migration, and invasion of cancer cells [5][6][7]. Overexpression of Trop-2 was associated with accelerated tumour growth and a dismal prognosis in various types of cancers, including breast, gastric, and ovarian cancers [8][9][10]. Conversely, in other tumours like non-small cell lung cancer (NSCLC), TROP-2 downregulation and internalization into the cytoplasm, are related to metastasis and recurrence [11]. Trop-2 is also overexpressed in haematologic malignancies like as leukaemia, extranodal nasal type lymphoma (ENK/TL), and non-Hodgkin’s lymphoma (NHL) [12][13].
These characteristics could make Trop-2 a seductive target for cancer therapy. Currently, numerous therapeutic strategies with antibodies or antibody–drug conjugates are being developed to target Trop-2 in specific tumours.
1.2. Trophoblast Cell Surface Antigen-2 Properties, Binding Partners, and Signalling Pathways
Trop-2/TACSTD2 was first described in 1981 and its gene is located on chromosome 1p32 [2][4]. The tertiary structure of Trop-2 consists of multiple domains that extend through the cell membrane. The extracellular domain is composed of a 26-amino acid hydrophobic peptide and an N-terminal part, the largest part of the molecule consisting of 274 amino-acids, also known as the ectodomain (Trop-2EC). It is comprised of an epidermal growth factor-like repeat containing a cysteine-rich domain, a thyroglobulin type-1 domain, and a cysteine-poor domain, anchored via a single transmembrane helix (TM) followed by a short intracellular tail (Trop-2IC) [14].
The 30-amino acid cytoplasmic part shows high homology to a HIKE domain [15][16] and includes a serine residue (S303) that is phosphorylated by protein kinase C (PKC) [17] and a phosphatidyl-inositol 4,5-bisphosphate (PIP2) binding site [8].
Trop-2 is a member of a protein family (GA733 family) that includes at least two “type I” membrane proteins: GA733-1 (Trop-2) and GA733-2, also known as EpCAM (epithelial cell adhesion molecule). Trop-2 and EpCAM exhibit very high similarities in sequence and structure, with 49% homology and 65% similarity in amino acid repeats and a comparable arrangement of hydrophilic and hydrophobic parts [14][18]. Nevertheless, the promotor regions of EpCAM and Trop-2 are unrelated, resulting in different expression patterns [19] and leading to structural differences in the intracellular domain explaining the distinct intracellular signalling and functions between Trop-2 and EpCAM [20][21]. Indeed, EpCAM exhibits its role in cell differentiation, proliferation, and migration through c-myc. On the contrary, Trop-2 has been reported to interact with several proteins, such as insulin-like growth factor-1 (IGF-1) 11, claudin-1 and 7, cyclin D1, and PKC. Furthermore, due to the HIKE domain, the PIP2 binding site, and the serine phosphorylated by PKC, Trop-2 is involved in calcium signalling through which the MAPK pathway could be activated [5].
1.2.1. Insulin-like growth factor-1/IGF-1R
Insulin-like growth factor-1 (IGF-1), as mentioned above, binds Trop-2 leading to modulation of IGF-1 signalling and activation through PIP2 and Ca2+. Trop-2 may also bind the receptor of IGR-1 (IGF-1R), blocking IGF-1 signalling [11] and playing critical roles in cell growth, differentiation, transformation, and metastasis. This mechanism could explain the different impacts of Trop-2 overexpression in lung cancer. Indeed, in a lung cancer model, high expression of Trop-2 suppressed tumour growth by attenuating IGF-1R signalling, likely by binding IGF-1 [14].
1.2.2. Claudin
Claudin-1 and 7, two transmembrane proteins composing the tight junctions at the epithelial surface, bind to Trop-2′s ectodomain preventing claudin degradation which plays a fundamental role in epithelial barrier maintenance. Trop-2 might also indirectly affect adhesive interactions between cells by modulating the complex formation between fibronectin and P1 integrin/RACK1 (receptor for activated PKC) [14].
1.2.3. ERK1/2
Trop-2 could also initiate the ERK1/2-MAPK axis, leading to malignant transformation [11], and could dysregulate stem cell function via the Notch, Hedgehog, and Wnt pathways through the expression and activation of cyclic AMP-responsive element-binding protein (CREB1), Jun, NF-κB, Rb, STAT1, and STAT3 (Figure 1) [5]. As previously mentioned, the MAPK pathway is stimulated by increased Ca2+ and phosphorylation of MAPK, which affects cell cycle progression. Furthermore, ERK activation was observed in various tumour types characterized by Trop-2 overexpression, and this ERK1/2 activation is thought to promote tumour survival through anti-apoptotic effects [5].
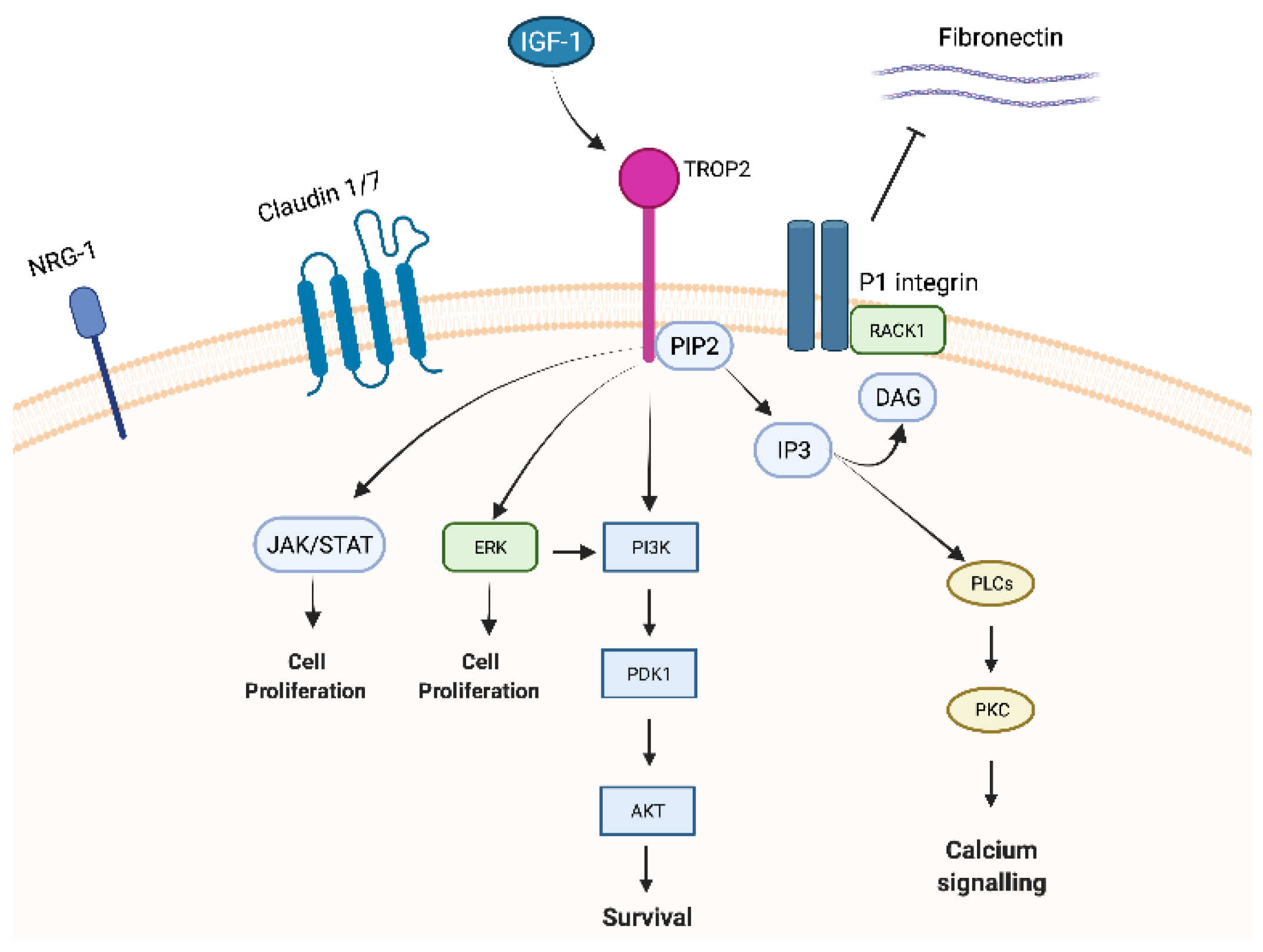
Figure 1. Trop-2 membrane-associated interacting partners and signalling pathways.
1.2.4. NRG1
Trop-2 seems to also directly affect NRG1 via the EGF-like and thyroglobulin repeat domains in the extracellular region of Trop-2. In a pre-clinical model of HNSCC cells, Zhang and colleagues knocked down Trop-2 resulting in increased membrane localization of NRG1 that binds to and increases the activation of ErbB3 [22].
1.2.5. Cyclin D1
Trop-2 could create a fusion product with cyclin D1 (bicistronic cyclin D1/Trop-2) [23] due to post transcriptional processes. This change could affect the stability of cyclin D1 increasing cell longevity and proliferation and qualifying Trop-2 as an oncogene.
1.2.6. PKC
Lastly, PKC phosphorylates Trop-2′s cytoplasmic tail at S303. PIP2 has been suggested as a phosphorylation regulator of S303 by PKC through activation of the Raf and NF-κB pathways, promoting cancer cell survival [24].
2. Trophoblast Cell Surface Antigen-2 Significance in Cancer
Despite Trop-2 being first identified as a cell surface marker for trophoblast cells, a great effort has been made to elucidate the role of this marker in cancers [21].
Trop-2 was also detected in stem cells of different tissues, especially in basal cells. As an example, the expression of TROP- 2 had been associated with self-renewal, regeneration, and differentiation properties in prostate basal cells [25][26], oval cells after liver injury [27], and endometrium-regenerating cells [28]. These findings support the role of Trop-2 as a regulator of stem cell growth which is implicated in the regeneration of various tissues, perhaps playing a role in physiological events like hyperplasia.
On the other hand, as noted above with few exceptions, its overexpression has also been associated with the increase in tumour growth, proliferation, and metastasis in various epithelial cancers, i.e., head and neck, thyroid, lung, gastrointestinal tract, breast, renal, and gynaecological cancers, and glioma [29].
Beyond the prognostic value, the real weight of Trop-2 gain of function or Trop-2 loss in tumorigenesis, epithelial–mesenchymal transition (EMT), and mesenchymal trans-differentiation remains unclear [30][31].
Silencing the Trop-2 gene using small interfering (si) RNA in colon, breast, cervical, lung, and ovarian cancer cell models led to a suppression of malignant transformation inhibiting the proliferation, invasion, and the formation of colonies in vitro [30][32][33][34][35]. The knockdown of Trop-2 in gallbladder cancer inhibited vimentin and increased E-cadherin expression linked to EMT [36]. Trop-2 overexpression seems to be related to an increased risk of metastasis in patients affected by various cancer types (oral squamous, thyroid, some oesophageal, gastric, colorectal, pancreatic, ovarian, uterine, cervical, prostate, and urinary bladder). However, it is not upregulated in others (e.g., head and neck and certain lung cancers, such as lung adenosquamous and squamous cell carcinoma) [1][29][37][38][39].
In gastric cancer, the poor prognostic value seems to be related to the co-expression of Trop-2 and amphiregulin [40], while tumour necrosis factor-α (TNF-α) axes could regulate this effect in colorectal cancer. Indeed, a low concentration of this cytokine correlates with an increase in Trop-2 protein expression, while higher concentrations of TNF-α reduce migration and cell invasiveness [41]. These authors linked these activities to the crosstalk between TNF-α and the ERK1/2 pathway, showing that an ERK1/2 inhibitor can suppress the cytokine’s upregulation of Trop-2 [41]. The ERK1/2 pathway was also involved in pancreatic cancer, gynaecological cancers, and HNSCC, where Trop-2 expression increases the phosphorylation of ERK1/2 leading to the activation of the ERK/MAPK pathway, increasing the levels of cyclin D1 and cyclin E that resulted in a cell cycle dysregulation [5][32][42]. High cyclin D1 expression seems to also be the result of the activation of Trop-2 via in breast cancer [32][43].
In a prostatic model, Goldstein et al. showed that a malignant transformation could arise from the Trop-2+ basal cells in immunodeficient mice [26]. Thus, basal cells expressing Trop-2 and CD44 can develop luminal phenotype tumours [25][26][44][45]. This is consistent with studies implicating Trop-2 as a critical regulator of β1 integrin activities and promoting prostate cancer cell motility [3][46]. Interestingly, Trop-2+ exosomes purified from prostate cancer promote migration of Trop-2-negative prostate cancer cells on fibronectin, suggesting that Trop-2 could induce cells lacking Trop-2 to gain Trop-2 regulatory properties affecting migration [47].
The role of Trop-2 in haematological disease is still unclear, since it is expressed in Hodgkin’s lymphoma and chronic lymphocytic leukaemia1, but not in anaplastic large cell lymphoma [13].
To summarize, the Trop-2 gene is related to several transcription factors leading to a dysregulation of the numerous pathways connected with this glycoprotein. Although not yet fully elucidated, it activates CREB1, Jun, NF-κB, Rb, STAT1, and STAT3 via induction of the cyclin D1 and ERK/MEK pathways affecting malignant transformation and metastasis [8][33][48][49].
This entry is adapted from the peer-reviewed paper 10.3390/cancers15061744
References
- Shvartsur, A.; Bonavida, B. Trop2 and its overexpression in cancers: Regulation and clinical/therapeutic implications. Genes Cancer 2015, 6, 84–105.
- Lipinski, M.; Parks, D.R.; Rouse, R.V.; Herzenberg, L.A. Human trophoblast cell-surface antigens defined by monoclonal antibodies. Proc. Natl. Acad. Sci. USA 1981, 78, 5147–5150.
- Trerotola, M.; Jernigan, D.L.; Liu, Q.; Siddiqui, J.; Fatatis, A.; Languino, L.R. Trop-2 promotes prostate cancer metastasis by modulating βintegrin functions. Cancer Res. 2013, 73, 3155–3167.
- Bignotti, E.; Zanotti, L.; Calza, S.; Falchetti, M.; Lonardi, S.; Ravaggi, A.; Romani, C.; Todeschini, P.; Bandiera, E.; Tassi, R.A.; et al. Trop-2 protein overexpression is an independent marker for predicting disease recurrence in endometrioid endometrial carcinoma. BMC Clin. Pathol. 2012, 12, 22.
- Cubas, R.; Zhang, S.; Li, M.; Chen, C.; Yao, Q. Trop2 expression contributes to tumor pathogenesis by activating the ERK MAPK pathway. Mol. Cancer 2010, 9, 253.
- Guan, H.; Guo, Z.; Liang, W.; Li, H.; Wei, G.; Xu, L.; Xiao, H.; Li, Y. Trop2 enhances invasion of thyroid cancer by inducing MMP2 through ERK and JNK pathways. BMC Cancer 2017, 17, 486.
- Guerra, E.; Trerotola, M.; Tripaldi, R.; Aloisi, A.L.; Simeone, P.; Sacchetti, A.; Relli, V.; D’Amore, A.; La Sorda, R.; Lattanzio, R.; et al. Trop-2 Induces Tumor Growth Through AKT and Determines Sensitivity to AKT Inhibitors. Clin. Cancer Res. 2016, 22, 4197–4205.
- El Sewedy, T.; Fornaro, M.; Alberti, S. Cloning of the murine TROP2 gene: Conservation of a PIP2-binding sequence in the cytoplasmic domain of TROP-2. Int. J. Cancer 1998, 75, 324–330.
- Miotti, S.; Canevari, S.; Ménard, S.; Mezzanzanica, D.; Porro, G.; Pupa, S.M.; Regazzoni, M.; Tagliabue, E.; Colnaghi, M.I. Characterization of human ovarian carcinoma-associated antigens defined by novel monoclonal antibodies with tumor-restricted specificity. Int. J. Cancer 1987, 39, 297–303.
- Mühlmann, G.; Spizzo, G.; Gostner, J.; Zitt, M.; Maier, H.; Moser, P.; Gastl, G.; Müller, H.M.; Margreiter, R.; Ofner, D.; et al. TROP2 expression as prognostic marker for gastric carcinoma. J. Clin. Pathol. 2009, 62, 152–158.
- Lin, J.C.; Wu, Y.Y.; Wu, J.Y.; Lin, T.C.; Wu, C.T.; Chang, Y.L.; Jou, Y.S.; Hong, T.M.; Yang, P.C. TROP2 is epigenetically inactivated and modulates IGF-1R signalling in lung adenocarcinoma. EMBO Mol. Med. 2012, 4, 472–485.
- Govindan, S.V.; Goldenberg, D.M. New antibody conjugates in cancer therapy. Sci. World J. 2010, 10, 2070–2089.
- Bobos, M.; Kotoula, V.; Kaloutsi, V.; Karayannopoulou, G.; Papadimitriou, C.S.; Kostopoulos, I. Aberrant CCND1 copies and cyclin D1 mRNA expression do not result in the production of functional cyclin D1 protein in anaplastic large cell lymphoma. Histol. Histopathol. 2009, 24, 1035–1048.
- Vidmar, T.; Pavšič, M.; Lenarčič, B. Biochemical and preliminary X-ray characterization of the tumor-associated calcium signal transducer 2 (Trop2) ectodomain. Protein. Expr. Purif. 2013, 91, 69–76.
- Alberti, S. HIKE, a candidate protein binding site for PH domains, is a major regulatory region of Gbeta proteins. Proteins 1999, 35, 360–363.
- Ciccarelli, F.D.; Acciarito, A.; Alberti, S. Large and diverse numbers of human diseases with HIKE mutations. Hum. Mol. Genet. 2000, 9, 1001–1007.
- Basu, A.; Goldenberg, D.M.; Stein, R. The epithelial/carcinoma antigen EGP-1, recognized by monoclonal antibody RS7-3G11, is phosphorylated on serine 303. Int. J. Cancer 1995, 62, 472–479.
- Szala, S.; Kasai, Y.; Steplewski, Z.; Rodeck, U.; Koprowski, H.; Linnenbach, A.J. Molecular cloning of cDNA for the human tumor-associated antigen CO-029 and identification of related transmembrane antigens. Proc. Natl. Acad. Sci. USA 1990, 87, 6833–6837.
- Villablanca, E.J.; Renucci, A.; Sapède, D.; Lec, V.; Soubiran, F.; Sandoval, P.C.; Dambly-Chaudière, C.; Ghysen, A.; Allende, M.L. Control of cell migration in the zebrafish lateral line: Implication of the gene “tumour-associated calcium signal transducer,” tacstd. Dev. Dyn. 2006, 235, 1578–1588.
- Linnenbach, A.J.; Seng, B.A.; Wu, S.; Robbins, S.; Scollon, M.; Pyrc, J.J.; Druck, T.; Huebner, K. Retroposition in a family of carcinoma-associated antigen genes. Mol. Cell Biol. 1993, 13, 1507–1515.
- McDougall, A.R.; Tolcos, M.; Hooper, S.B.; Cole, T.J.; Wallace, M.J. Trop2: From development to disease. Dev. Dyn. 2015, 244, 99–109.
- Zhang, K.; Jones, L.; Lim, S.; Maher, C.A.; Adkins, D.; Lewis, J.; Kimple, R.J.; Fertig, E.J.; Chung, C.H.; Van Tine, B.A.; et al. Loss of Trop2 causes ErbB3 activation through a neuregulin-1-dependent mechanism in the mesenchymal subtype of HNSCC. Oncotarget 2014, 5, 9281–9294.
- Huang, H.; Groth, J.; Sossey-Alaoui, K.; Hawthorn, L.; Beall, S.; Geradts, J. Aberrant expression of novel and previously described cell membrane markers in human breast cancer cell lines and tumors. Clin. Cancer Res. 2005, 11, 4357–4364.
- Cubas, R.; Li, M.; Chen, C.; Yao, Q. Trop2: A possible therapeutic target for late stage epithelial carcinomas. Biochim. Biophys. Acta 2009, 1796, 309–314.
- Goldstein, A.S.; Lawson, D.A.; Cheng, D.; Sun, W.; Garraway, I.P.; Witte, O.N. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc. Natl. Acad. Sci. USA 2008, 105, 20882–20887.
- Goldstein, A.S.; Huang, J.; Guo, C.; Garraway, I.P.; Witte, O.N. Identification of a cell of origin for human prostate cancer. Science 2010, 329, 568–571.
- Okabe, M.; Tsukahara, Y.; Tanaka, M.; Suzuki, K.; Saito, S.; Kamiya, Y.; Tsujimura, T.; Nakamura, K.; Miyajima, A. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development 2009, 136, 1951–1960.
- Memarzadeh, S.; Zong, Y.; Janzen, D.M.; Goldstein, A.S.; Cheng, D.; Kurita, T.; Schafenacker, A.M.; Huang, J.; Witte, O.N. Cell-autonomous activation of the PI3-kinase pathway initiates endometrial cancer from adult uterine epithelium. Proc. Natl. Acad. Sci. USA 2010, 107, 17298–17303.
- Zeng, P.; Chen, M.B.; Zhou, L.N.; Tang, M.; Liu, C.Y.; Lu, P.H. Impact of TROP2 expression on prognosis in solid tumors: A Systematic Review and Meta-analysis. Sci. Rep. 2016, 6, 33658.
- Wang, J.; Day, R.; Dong, Y.; Weintraub, S.J.; Michel, L. Identification of Trop-2 as an oncogene and an attractive therapeutic target in colon cancers. Mol. Cancer Ther. 2008, 7, 280–285.
- Wang, J.; Zhang, K.; Grabowska, D.; Li, A.; Dong, Y.; Day, R.; Humphrey, P.; Lewis, J.; Kladney, R.D.; Arbeit, J.M.; et al. Loss of Trop2 promotes carcinogenesis and features of epithelial to mesenchymal transition in squamous cell carcinoma. Mol. Cancer Res 2011, 9, 1686–1695.
- Liu, T.; Liu, Y.; Bao, X.; Tian, J.; Yang, X. Overexpression of TROP2 predicts poor prognosis of patients with cervical cancer and promotes the proliferation and invasion of cervical cancer cells by regulating ERK signaling pathway. PLoS ONE 2013, 8, e75864.
- Trerotola, M.; Cantanelli, P.; Guerra, E.; Tripaldi, R.; Aloisi, A.L.; Bonasera, V.; Lattanzio, R.; de Lange, R.; Weidle, U.H.; Piantelli, M.; et al. Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene 2013, 32, 222–233.
- Gao, X.Y.; Zhu, Y.H.; Zhang, L.X.; Lu, H.Y.; Jiang, A.G. siRNA targeting of Trop2 suppresses the proliferation and invasion of lung adenocarcinoma H460 cells. Exp. Ther. Med. 2015, 10, 429–434.
- Wu, B.; Yu, C.; Zhou, B.; Huang, T.; Gao, L.; Liu, T.; Yang, X. Overexpression of TROP2 promotes proliferation and invasion of ovarian cancer cells. Exp. Ther. Med. 2017, 14, 1947–1952.
- Li, X.; Teng, S.; Zhang, Y.; Zhang, W.; Zhang, X.; Xu, K.; Yao, H.; Yao, J.; Wang, H.; Liang, X.; et al. TROP2 promotes proliferation, migration and metastasis of gallbladder cancer cells by regulating PI3K/AKT pathway and inducing EMT. Oncotarget 2017, 8, 47052–47063.
- Stepan, L.P.; Trueblood, E.S.; Hale, K.; Babcook, J.; Borges, L.; Sutherland, C.L. Expression of Trop2 cell surface glycoprotein in normal and tumor tissues: Potential implications as a cancer therapeutic target. J. Histochem. Cytochem. 2011, 59, 701–710.
- Xu, P.; Zhao, Y.; Liu, K.; Lin, S.; Liu, X.; Wang, M.; Yang, P.; Tian, T.; Zhu, Y.Y.; Dai, Z. Prognostic role and clinical significance of trophoblast cell surface antigen 2 in various carcinomas. Cancer Manag. Res. 2017, 9, 821–837.
- Chou, J.; Trepka, K.; Sjöström, M.; Egusa, E.A.; Chu, C.E.; Zhu, J.; Chan, E.; Gibb, E.A.; Badura, M.L.; Contreras-Sanz, A.; et al. TROP2 Expression Across Molecular Subtypes of Urothelial Carcinoma and Enfortumab Vedotin-resistant Cells. Eur. Urol. Oncol. 2022, 5, 714–718.
- Zhao, W.; Ding, G.; Wen, J.; Tang, Q.; Yong, H.; Zhu, H.; Zhang, S.; Qiu, Z.; Feng, Z.; Zhu, J. Correlation between Trop2 and amphiregulin coexpression and overall survival in gastric cancer. Cancer Med. 2017, 6, 994–1001.
- Zhao, P.; Zhang, Z. TNF-α promotes colon cancer cell migration and invasion by upregulating TROP-2. Oncol. Lett. 2018, 15, 3820–3827.
- Nakanishi, H.; Taccioli, C.; Palatini, J.; Fernandez-Cymering, C.; Cui, R.; Kim, T.; Volinia, S.; Croce, C.M. Loss of miR-125b-1 contributes to head and neck cancer development by dysregulating TACSTD2 and MAPK pathway. Oncogene 2014, 33, 702–712.
- Lin, H.; Huang, J.F.; Qiu, J.R.; Zhang, H.L.; Tang, X.J.; Li, H.; Wang, C.J.; Wang, Z.C.; Feng, Z.Q.; Zhu, J. Significantly upregulated TACSTD2 and Cyclin D1 correlate with poor prognosis of invasive ductal breast cancer. Exp. Mol. Pathol. 2013, 94, 73–78.
- Park, J.W.; Lee, J.K.; Phillips, J.W.; Huang, P.; Cheng, D.; Huang, J.; Witte, O.N. Prostate epithelial cell of origin determines cancer differentiation state in an organoid transformation assay. Proc. Natl. Acad. Sci. USA 2016, 113, 4482–4487.
- Stoyanova, T.; Cooper, A.R.; Drake, J.M.; Liu, X.; Armstrong, A.J.; Pienta, K.J.; Zhang, H.; Kohn, D.B.; Huang, J.; Witte, O.N.; et al. Prostate cancer originating in basal cells progresses to adenocarcinoma propagated by luminal-like cells. Proc. Natl. Acad. Sci. USA 2013, 110, 20111–20116.
- Trerotola, M.; Li, J.; Alberti, S.; Languino, L.R. Trop-2 inhibits prostate cancer cell adhesion to fibronectin through the β1 integrin-RACK1 axis. J. Cell Physiol. 2012, 227, 3670–3677.
- Trerotola, M.; Ganguly, K.K.; Fazli, L.; Fedele, C.; Lu, H.; Dutta, A.; Liu, Q.; De Angelis, T.; Riddell, L.W.; Riobo, N.A.; et al. Trop-2 is up-regulated in invasive prostate cancer and displaces FAK from focal contacts. Oncotarget 2015, 6, 14318–14328.
- Guerra, E.; Trerotola, M.; Aloisi, A.L.; Tripaldi, R.; Vacca, G.; La Sorda, R.; Lattanzio, R.; Piantelli, M.; Alberti, S. The Trop-2 signalling network in cancer growth. Oncogene 2013, 32, 1594–1600.
- Ripani, E.; Sacchetti, A.; Corda, D.; Alberti, S. Human Trop-2 is a tumor-associated calcium signal transducer. Int. J. Cancer 1998, 76, 671–676.
This entry is offline, you can click here to edit this entry!
