Lipid nanoparticles (LNPs) have recently emerged as one of the most advanced technologies for the highly efficient in vivo delivery of exogenous mRNA, particularly for COVID-19 vaccine delivery. LNPs comprise four different lipids: ionizable lipids, helper or neutral lipids, cholesterol, and lipids attached to polyethylene glycol (PEG). Since its first isolation in 1961, mRNA (that encodes the protein of interest) research has taken several paths, which made us understand its diversified functions and modification-mediated potential for therapeutic applications. As a result of the COVID-19 pandemic, nucleic acid therapeutics (NATs), particularly mRNA vaccines, potentials have been enabled for emerging infectious diseases. The translation of host genetic information (DNA) into proteins by ribosomes in the cytoplasm is mediated by mRNA.
- vaccine
- mRNA
- COVID-19
- lipid nanoparticles
1. Use of mRNA as Prophylactics and Therapeutics
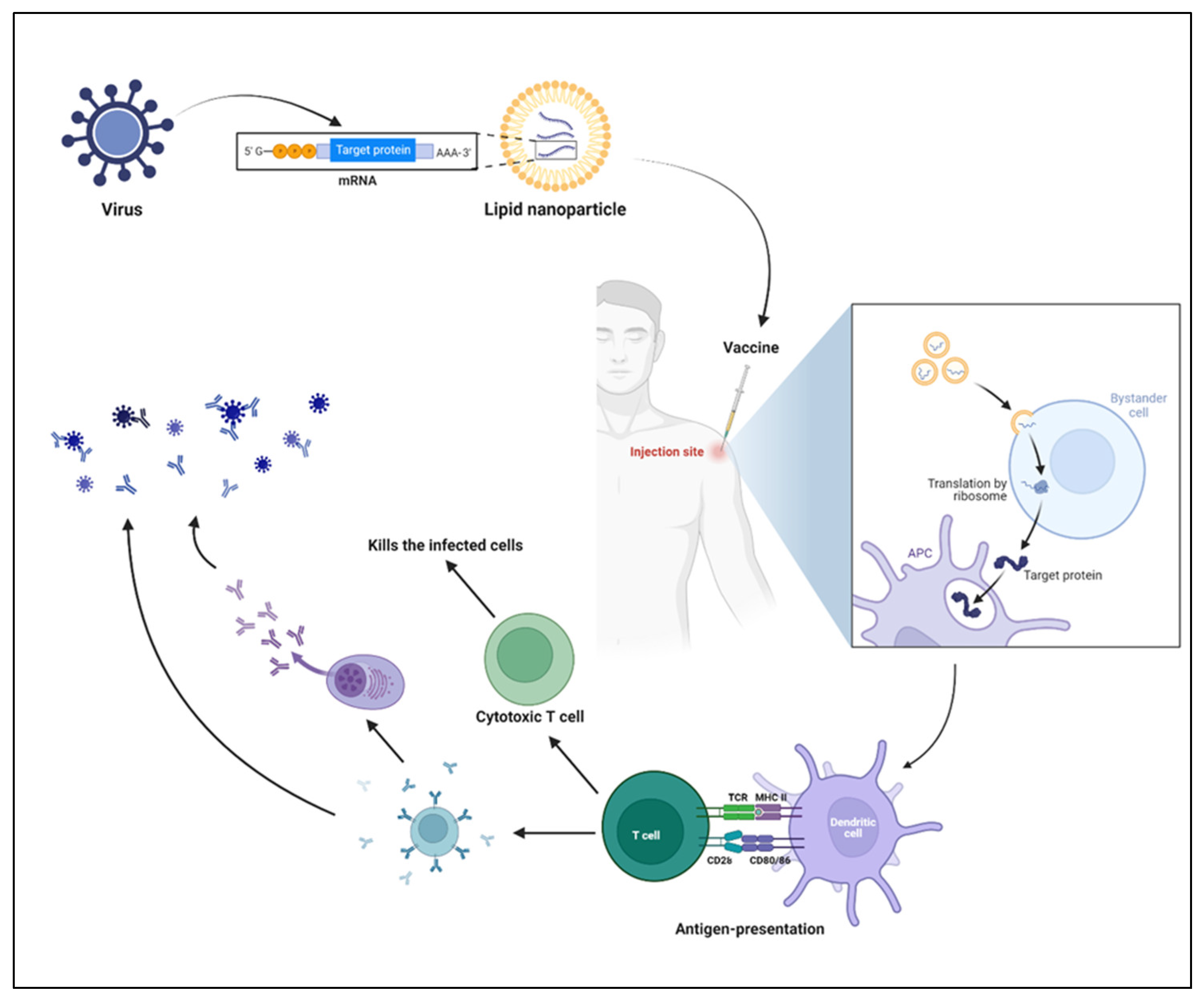
2. LNP Role in mRNA Delivery
3. Salient Features of LNP-Based Therapeutics
-
Naked mRNA is unsuitable for therapeutic purposes, as it is rapidly degraded by extracellular RNases. Several nanotechnology platforms have been set and optimized for mRNA-targeted delivery.
-
As mRNA is thermolabile, LNPs would enhance its stability and half-life at room temperature and be useful for avoiding vaccine cold chain.
-
Modifications of the type of delivery system (carrier molecules) rule out the organ-specific mRNA delivery (for lung, spleen, liver, etc.) and its in vivo half-life.
-
Composition of LNP could decide the type of immune response induced.
-
LNPs act as adjuvants systems for mRNA vaccines.
-
The stable LNPs make the lower dose of mRNA work.
-
Composition of LNPs also decides the number of booster doses required if it is admixed with a suitable adjuvant.
-
Moreover, for chronic treatments, multiple administration via different routes of administration is possible.
4. Mechanism of mRNA Vaccines
| Name | mRNA Specific to | LNP Composition | Adverse Effects |
|---|---|---|---|
| BNT162b2 | Spike glycoprotein of SARS-CoV-2 | lipids ((4- hydroxybutyl)azanediyl) bis(hexane-6,1- diyl)bis(2- hexyldecanoate), 2 [(polyethylene glycol)- 2000]-N,Nditetradecylacetamide, 1,2-distearoyl-snglycero- 3-phosphocholine, and cholesterol), potassium chloride, monobasic potassium phosphate, sodium chloride, dibasic sodium phosphate dihydrate, and sucrose |
Myocarditis Pericarditis |
| mRNA- 1273 |
Spike glycoprotein of SARS-CoV-2 | LNP: Proprietary Ionic lipid SM-102, polyethylene glycol (PEG) 2000, dimyristoylglycerol (DMG), cholesterol, 1,2- distearoyl-sn-glycero-3- phosphocholine [DSPC]), tromethamine hydrochloride, acetic acid, sodium acetate, and sucrose |
Myocarditis Pericarditis |
| LUNARCOv- 19 (ARCT- 021) |
Self-replicating mRNA specific to Spike glycoprotein of SARS-CoV-2 |
Arcturus Therapeutics proprietary ionizable lipid, DSPC, cholesterol, and PEG2000-DMG dissolved in ethanol |
NA |
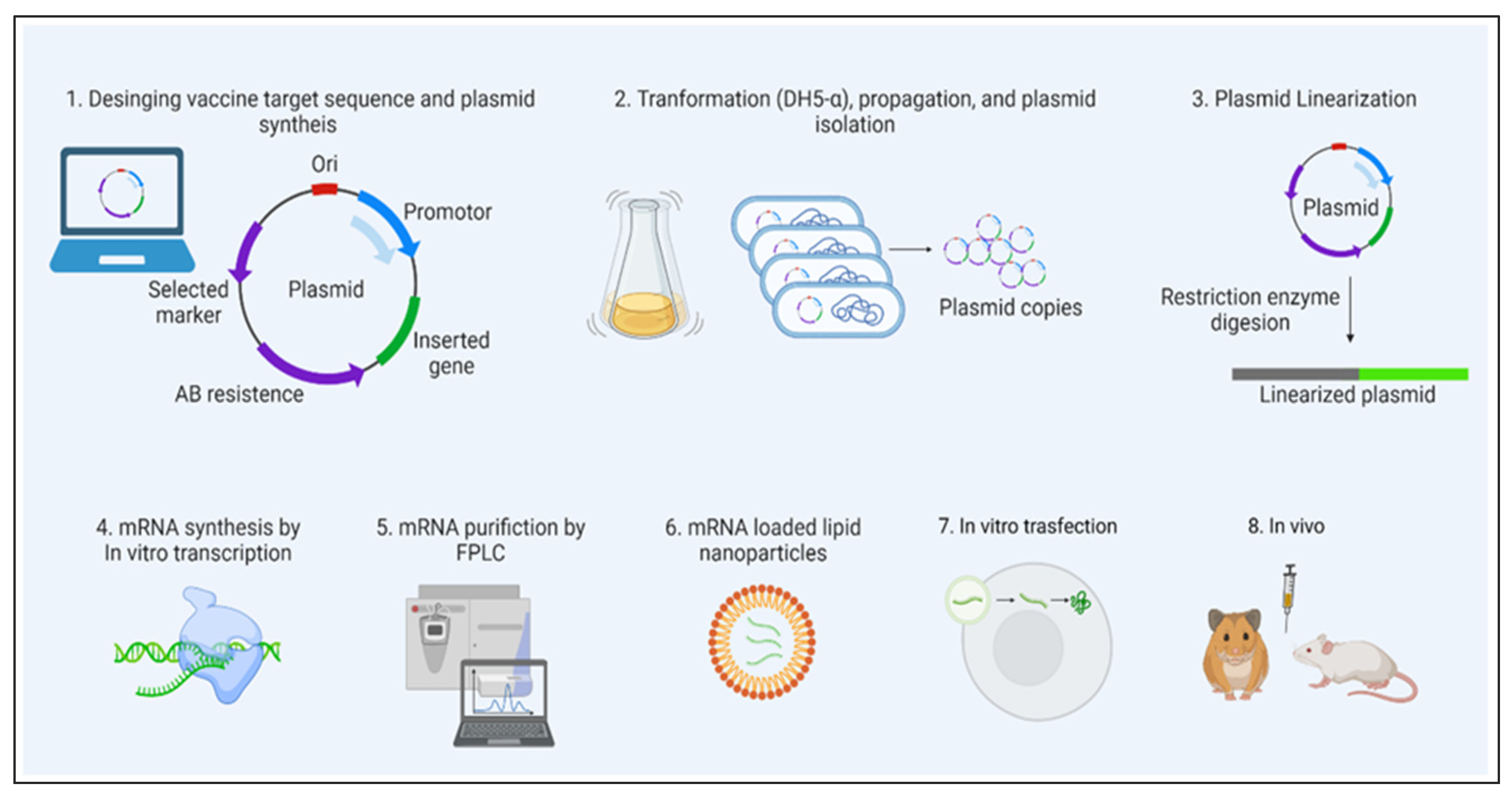
This entry is adapted from the peer-reviewed paper 10.3390/vaccines11030658
References
- Oude Munnink, B.B.; Nieuwenhuijse, D.F.; Stein, M.; O’Toole, Á.; Haverkate, M.; Mollers, M.; Kamga, S.K.; Schapendonk, C.; Pronk, M.; Lexmond, P.; et al. The Dutch-Covid-19 response, t., Rapid SARS-CoV-2 whole-genome sequencing and analysis for informed public health decision-making in the Netherlands. Nat. Med. 2020, 26, 1405–1410.
- Verbeke, R.; Hogan, M.J.; Loré, K.; Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 2022, 55, 1993–2005.
- Arevalo, C.P.; Bolton, M.J.; Le Sage, V.; Ye, N.; Furey, C.; Muramatsu, H.; Alameh, M.-G.; Pardi, N.; Drapeau, E.M.; Parkhouse, K.; et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 2022, 378, 899–904.
- Bonam, S.R.; Partidos, C.D.; Halmuthur, S.K.M.; Muller, S. An Overview of Novel Adjuvants Designed for Improving Vaccine Efficacy. Trends Pharmacol. Sci. 2017, 38, 771–793.
- Riley, R.S.; Kashyap, M.V.; Billingsley, M.M.; White, B.; Alameh, M.-G.; Bose, S.K.; Zoltick, P.W.; Li, H.; Zhang, R.; Cheng, A.Y.; et al. Ionizable lipid nanoparticles for in utero mRNA delivery. Sci. Adv. 2021, 7, eaba1028.
- Vaca, G.B.; Meyer, M.; Cadete, A.; Hsiao, C.J.; Golding, A.; Jeon, A.; Jacquinet, E.; Azcue, E.; Guan, C.M.; Sanchez-Felix, X.; et al. Intranasal mRNA-LNP vaccination protects hamsters from SARS-CoV-2 infection. bioRxiv 2023, 11, 523616.
- Jansen, E.M.; Frijlink, H.W.; Hinrichs, W.L.J.; Ruigrok, M.J.R. Are inhaled mRNA vaccines safe and effective? A review of preclinical studies. Expert Opin. Drug Deliv. 2022, 19, 1471–1485.
- Phua, K.K.L.; Leong, K.W.; Nair, S.K. Transfection efficiency and transgene expression kinetics of mRNA delivered in naked and nanoparticle format. J. Control. Release 2013, 166, 227–233.
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E.; Lee, A.; Woods, A.; Yuzhakov, O.; Himansu, S.; Deterling, J.; Geilich, B.M.; Ketova, T.; et al. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Mol. Ther. Nucleic Acids 2019, 15, 1–11.
- Ols, S.; Yang, L.; Thompson, E.A.; Pushparaj, P.; Tran, K.; Liang, F.; Lin, A.; Eriksson, B.; Karlsson Hedestam, G.B.; Wyatt, R.T.; et al. Route of Vaccine Administration Alters Antigen Trafficking but Not Innate or Adaptive Immunity. Cell Rep. 2020, 30, 3964–3971.e7.
- Davies, N.; Hovdal, D.; Edmunds, N.; Nordberg, P.; Dahlén, A.; Dabkowska, A.; Arteta, M.Y.; Radulescu, A.; Kjellman, T.; Höijer, A.; et al. Functionalized lipid nanoparticles for subcutaneous administration of mRNA to achieve systemic exposures of a therapeutic protein. Mol. Ther. —Nucleic Acids 2021, 24, 369–384.
- Alameh, M.-G.; Tombácz, I.; Bettini, E.; Lederer, K.; Ndeupen, S.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892.e7.
- Chen, J.; Chen, J.; Xu, Q. Current Developments and Challenges of mRNA Vaccines. Annu. Rev. Biomed. Eng. 2022, 24, 85–109.
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022, 40, 840–854.
- Naveed, Z.; Li, J.; Wilton, J.; Spencer, M.; Naus, M.; Velásquez García, H.A.; Kwong, J.C.; Rose, C.; Otterstatter, M.; Janjua, N.Z. Comparative Risk of Myocarditis/Pericarditis Following Second Doses of BNT162b2 and mRNA-1273 Coronavirus Vaccines. J. Am. Coll. Cardiol. 2022, 80, 1900–1908.
- Wagenhäuser, I.; Reusch, J.; Gabel, A.; Krone, L.B.; Kurzai, O.; Petri, N.; Krone, M. Bivalent BNT162b2mRNA original/Omicron BA.4-5 booster vaccination: Adverse reactions and inability to work compared to the monovalent COVID-19 booster. medRxiv 2022, 2022, 22281982.
