Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Environmental
Anaerobic membrane bioreactor (AnMBR) technology is emerging as an alternative to conventional anaerobic treatment due to its complete biomass retention, short start-up time, high effluent quality, and small footprint.
- anaerobic membrane bioreactors
- organics removal
- recovery of methane
- recovery of ethanol
1. Introduction
Anaerobic membrane bioreactors (AnMBRs) combine anaerobic biological processes and solid–liquid separation by membranes. Complete biomass retention can be achieved by using microfiltration (MF) or ultrafiltration (UF) modules with membranes made of polymers, ceramics, and metals. This allows solid retention time (SRT) long enough for slow-growing methanogens to develop. As a result, the start-up time of AnMBRs can be several times shorter (<2 weeks) than in conventional anaerobic reactors that retain biomass in the form of granules or biofilm [1].
In AnMBRs, submerged configuration (Figure 1A) and external (side-stream) configuration (Figure 1B) are used. In the submerged configuration, where the membrane is located inside the reactor, less energy is consumed and operating conditions, including permeate flux, are milder due to lower velocities. The external configuration with integrated membrane filtration in an external loop provides higher fluxes and easier membrane replacement; however, energy consumption is higher and biomass activity may be reduced due to the high cross-flow velocity. Ten of eleven pilot-scale AnMBRs used systems consisting of an anaerobic reactor and an external membrane tank [2]. In conventional AnMBRs, the membrane modules are accompanied by bioreactors such as a completely stirred tank reactor, an up-flow anaerobic sludge blanket reactor, an expanded granular sludge bed reactor, or an anaerobic fluidized-bed membrane bioreactor [3].
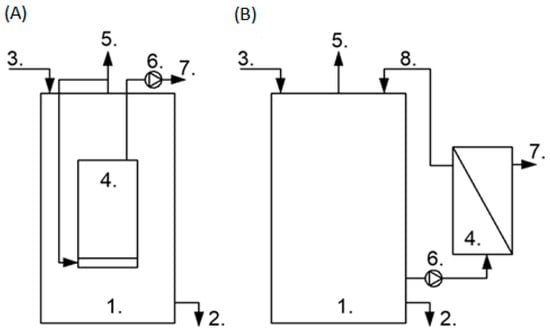
Figure 1. Submerged (A) and external (B) configuration of AnMBR; 1—anaerobic bioreactor, 2—excess sludge, 3—feed, 4—membrane module, 5—biogas, 6—pump, 7—permeate, 8—retentate.
The main factor limiting the widespread use of AnMBRs on a large scale is membrane fouling, which leads to flux decline. Due to the high viscosity, high mixed liquor suspended solids (MLSS) concentration, long SRT, and often long hydraulic retention time (HRT) required to treat complex wastewater, membrane fouling is more prevalent in AnMBRs than in aerobic MBRs. As a result, permeate flux in AnMBRs (5–12 L/(m2·h)) is significantly lower than in aerobic MBRs (20–30 L/(m2·h)) in full-scale facilities [4,5]. In general, the reasons for membrane fouling are the deposition of sludge particles and the adsorption of dissolved organic matter on the membrane surface. This decreases the permeability of the membrane, increases the frequency of membrane cleaning, and thus shortens the membrane life. Fouling in AnMBRs is affected by (1) sludge properties (MLSS, the amount of soluble microbial products (SMP) and extracellular polymers (EPS), particle size distribution, microbial species composition), which is influenced by substrate characteristics (mainly soluble/particulate COD) and bioreactor operation (SRT, organic loading rate (OLR), food to microorganism ratio (F/M), temperature); (2) membrane properties (material, pore size, hydrophobicity, surface charge, roughness); and (3) membrane operation (flux, cleaning, shear rate) [6]. Among the major energy-consuming activities in AnMBRs (operation of the biological reactor, fouling control, liquid circulation between and within reactors, operation of pumps), energy expenditure to fouling control is considered the largest energy consumer in AnMBRs [2,7]. For example, over 70% of the energy requirement is used for fouling control when biogas sparging is used [2]. Therefore, it is important to optimize the fouling reduction methods to make AnMBRs energy efficient. However, since no energy is required for aeration, the energy demand of AnMBRs in secondary wastewater treatment is still low [8].
Due to high effluent quality, energy production, low sludge production, low impact of influent fluctuations on reactor performance, and small footprint, AnMBRs have been used to treat various industrial wastewater. Municipal wastewater is not considered suitable for anaerobic treatment due to its low organic load. Although there are still some challenges to overcome to make AnMBR technically and economically feasible, including low methane yield, high methane loss in wastewater, and high capital requirements [9], the application of AnMBR for municipal wastewater treatment is close to full-scale implementation [10]. Especially since AnMBRs allow wastewater to be treated at psychrophilic temperatures where increased methane solubility results in energy losses [11]. Other reasons that anaerobic treatment was rarely used for municipal wastewater were the difficulties in retaining the slow-growing methanogenic microorganisms when treating low-strength wastewater at short HRT and in meeting the effluent standards in terms of nitrogen. The introduction of a membrane section leading to the size exclusion of solids and their complete retention allows independent control of SRT and HRT, resulting in methanogens being retained in the biomass at short HRT [12]. This property allows expanding the application of AnMBRs in the treatment of municipal wastewater. In anaerobic processes, nutrients are partially used for biomass growth and converted to soluble forms (ammonia and phosphate), resulting in high concentrations in the effluent. Subsequently, recovery of the nutrients (e.g., for fertilizer production) or downstream treatment is required if the effluent is to be reused. Since most organic matter is converted to methane, conventional nitrification/denitrification is not effective for AnMBR effluents. Anaerobic ammonia oxidation (Anammox), ion exchange, or use as fertilizer can be employed [13]. Phosphorus can be used as fertilizer after chemical precipitation. In addition, AnMBRs have a high potential for water reuse because they remove a wide range of trace organic pollutants [14,15].
2. Anaerobic Membrane Bioreactors—Wastewater Treatment and Biogas Production
AnMBRs can achieve excellent COD removal (>99%) and biogas production at OLR up to 40 kg COD/(m3·d), making their potential comparable to that of high-rate up-flow anaerobic sludge blanket reactors [16].
2.1. Industrial Wastewater
AnMBRs have been used to treat various industrial wastewater with high organic load, for example, from food processing [17,18,19,20,21], the paper industry [22], textile production [23], or pharmaceutical wastewater [24].
The data presented indicate the possibility of energy-positive treatment of food wastewater with AnMBRs. In the treatment of meat-processing wastewater, the permeate flux was stable at an OLR between 0.4 and 1.3 kg COD/(m3·d); increasing the OLR to 3.2 kg COD/(m3·d) resulted in unrecoverable fouling. Methane yields were 33–38% of influent COD and the system produced a net energy benefit of 0.16–1.82 kWh/m3 [19]. From the leachate of food waste stabilization at 21–22 °C, 86 ± 3% of COD was removed at an HRT of 13 d and an SRT of 75 d; biogas recovery was 850 kWh/t [25].
The high efficiency of food processing wastewater treatment in AnMBR is due to the fact that this system easily adapts to lipids by stimulating biomass growth and activity. Increasing the oleate-Na from 0 to 2 g/L decreased the total organic carbon (TOC) removal for a few days and then recovered to over 90% [29]. These fluctuations were consistent with biogas and methane production (which were maintained at 0.61 and 0.41 L/g COD, respectively). Increasing oleate-Na to 4 g/L reduced TOC removal to 62% and biogas production to 0.083 L/g COD due to accumulation of volatile fatty acids (VFAs) and inhibition of Methanosarcina.
Although numerous case studies indicate the excellent performance of AnMBRs and the ease of adaptation of the microorganisms to environmental conditions, it should be considered that some toxic compounds may cause deflocculation of the sludge, leading to deterioration of the membrane flux. In this case, pretreatment may be necessary [26].
2.2. Municipal Wastewater
Despite low organic strength, heavy membrane fouling, and inefficient energy recovery, AnMBRs have emerged as a potential treatment technology for municipal wastewater because they completely retain anaerobic microorganisms, producing a high-quality effluent and generating energy that partially compensates for its consumption. Another advantage is the low biosolid production; regardless of temperature (between 8 and 30 °C), it was 0.051 g VSS (volatile suspended solids)/g CODremoved [30] or 0.04–0.09 g VSS/g COD [8].
In general, AnMBRs are able to remove more than 85% COD and 99% of total suspended solids (TSS), while the removal of total nitrogen (TN) or total phosphorus (TP) is usually negligible, and therefore downstream treatment is required if the effluent is to be reused [1]. Hydrolysis of particulate organic matter accumulated in the membrane and cell decay can increase TN and TP levels [31]. In terms of COD, TSS, and the number of pathogens, the effluents meet the more stringent discharge standards than conventional anaerobic treatment [32], while the low removal of TN and TP could be beneficial if the effluents are to be used for agriculture or irrigation. To increase the performance of AnMBR, it was equipped with a forward osmosis membrane, which removed >96% of organic carbon, almost 100% of TP, and 62% of ammonia nitrogen; methane production was 0.21 L CH4/g CODremoved [33].
The use of AnMBRs in domestic wastewater treatment is very promising at low and ambient temperatures. Efficient COD removal (>90%) and consumption of 70% of the influent COD for methane production were achieved even at temperatures of 9–11 °C [2]. Similarly, at psychrophilic conditions (18 °C), 90% COD removal was achieved without the need for membrane cleaning during three years of continuous operation [34].
Although the performance of both AnMBR configurations used for municipal wastewater treatment is similar in terms of COD removal efficiency and biogas productivity, their fouling potentials differ significantly. Despite over 91% COD removal and methane yield of 160 L CH4/kg CODremoved in external and submerged AnMBRs, VFA accumulation was observed in the submerged AnMBR [37]. This was because more SMP and EPS were obtained in the mixed liquor and cake layer when the membrane was submerged in the fermenter, which increased the resistance of the cake layer and the fouling rate. Fouling was mainly caused by the deposition of low molecular weight biopolymers. In conclusion, external AnMBRs have better biomass quality and lower fouling tendency, making them a better solution for municipal wastewater treatment.
Another challenge related to the energetic effectiveness of AnMBRs arises from the low COD/sulfate ratio of the wastewater. The sulfate present in the influent reduces the available COD for methane production as the sulfate-reducing bacteria outcompete the methane-producing Archaea for the substrate. At a sulfate concentration >99 mg/L, methane yield (0.08–0.15 L CH4/g COD) was lower than at low-sulfate concentrations, where it reached 0.22 L CH4/g COD [2]. The importance of the COD/sulfate ratio is greatest at low COD (<300 mg/L) [8]. According to Lei et al. [8], the use of AnMBRs for the treatment of low-sulfate wastewater or municipal wastewater other than sulfate-rich provides cost savings of up to 28%.
AnMBRs show high efficiency in treating streams with high solids content. Their use has been studied for the treatment of sewage sludge under mesophilic (35 °C) and thermophilic (55 °C) conditions [38]. A reduction of digester volume was possible, compared to conventional anaerobic systems due to efficient treatment at OLRs of 6.4 and 4.6 kg COD/(m3·d) at 55 and 35 °C, respectively. Temperature differences distributed the inert COD to the soluble and particulate fractions. At 55 °C, a higher permeate flux was achieved due to lower viscosity. However, higher SRT increased irreversible fouling.
3. Wastewater Treatment with AnMBRs—Recovery of Energy
3.1. Methane Production and Recovery
Biogas produced in AnMBRs typically contains 70–88% methane, 3–15% carbon dioxide, and 0–15% nitrogen; however, 80–90% methane has also been observed [26]. Biogas production ranges from 0 to 220 L/d in pilot-scale plants, while it can be as high as 800 Nm3/d in full-scale facilities [39]. It has been demonstrated that for energy-efficient AnMBR performance and for energy recovery, the OLR should be 0.43–0.90 kg COD/(kg VSS·d) and the SRT should be 50 d to infinity [40]. To achieve energy-neutral operation, the fluxes should be between 8.3 and 9.5 L/(m2·h) at 35 °C and between 6.0 and 6.7 L/(m2·h) at 25 °C.
The AnMBRs, where HRT and SRT are decoupled, physically retaining the solids prevents the loss of methane bound to the biomass particles [41]. This results in greater transfer of dissolved methane to the reactor headspace and concentrations of dissolved methane approaching thermodynamic equilibrium. Even at this equilibrium, large amounts of dissolved methane can be lost due to the large volume of water flowing through the system. For example, the dissolved methane concentrations that were 1.009 times the thermodynamic equilibrium concentration resulted in a loss of 43% of the total methane produced as dissolved methane in the effluent [42]. Methane losses via permeate can reach 24–58% [43], 45% [44], 63% [11], or 67% [45] of the total methane produced.
The loss of methane due to its solubility in wastewater is particularly important in the treatment of low-strength municipal wastewater due to the high methane solubility in the effluent and process limitations due to inhibiting substances [5]. The presence of dissolved methane in the permeate contributes to global warming, as the greenhouse effect of methane is about 25 times higher than that of carbon dioxide [46], and reduces the energy efficiency of the process, thus reducing the advantage of AnMBRs as energy-efficient systems. Compared to high-rate activated sludge with anaerobic digestion, conventional activated sludge with anaerobic digestion, and an aerobic MBR with anaerobic digestion for domestic wastewater treatment at 15 °C, AnMBRs had the highest impact on global warming [47].
Methane loss is related to OLR. Dissolved methane was only 3.4–11% of the input COD [19]. Its lowest accumulation was observed at the highest OLR of 3.2 kg COD/(m3·d), where the biogas production rate was the highest. Dissolved methane in AnMBR effluent accounted for 25–67% of the total methane content at temperatures of 15–25 °C [8]. According to Henry’s law, the solubility of methane increases with decreasing temperature, which makes psychrophilic treatment more difficult. The dissolved methane was 1.5 times higher at 15 °C than at 35 °C [1].
Large differences in biogas production in different municipal wastewater treatment reactors (from 0.128 to 0.90 L/(Lreactor·d)) result from the fact that dissolved methane in the permeate was not considered in some studies [8]. To achieve energy-neutral or energy-positive wastewater treatment and reduce greenhouse gas emissions through the use of AnMBRs, the dissolved methane must be captured with minimal energy input. Without these treatments, methane dissolution in the permeate is still a critical limiting factor for the use of AnMBRs for low-strength wastewater.
Methods for removing or recovering dissolved methane from anaerobic permeates include biological oxidation, aeration, air stripping, and membrane-based recovery.
Biological oxidation uses methane-oxidizing bacteria. They were used in a down-flow hanging sponge (DHS) reactor inoculated with activated sludge [48]. To provide oxygen, the aeration rate was 3.8–10 m3air/(m3·d). With the lowest aeration and an HRT of 2 h, 95% methane removal was achieved at a rate of 0.2 kg CH4/(m3·d). At the highest aeration and an HRT of 0.5 h, the efficiency decreased to 60%, but the removal rate increased to 0.55 kg CH4/(m3·d). Methane oxidation occurred preferentially over ammonium oxidation. Aeration is usually provided by vacuum-packed towers, bubble columns, or forced draft aerators [49].
Membrane-based recovery of dissolved methane from anaerobic permeates is one of the solutions. It is based on the use of a dense membrane that separates the dissolved gas from the liquid. Although this recovery has been shown to be effective, the operating costs are higher for commercial processes than for conventional gas-stripping systems [8]. Membrane contractors (MC) are used, which achieve efficiencies close to 99% for short-term operation. For long-term operation, mass-transfer resistances must be reduced [50]. Hollow-fiber MC resulted in removal of more than 98.9% of dissolved methane [49]. For methane recovery, non-porous and microporous membranes were compared. Since AnMBR permeates have a very low residual organic content due to their higher COD removal efficiency, the use of microporous membranes is more effective as they can achieve recovery of up to 98.9% dissolved methane [11]. Among the proposed solutions for dissolved methane recovery, vacuum MCs are considered the most energy-efficient process. They require 0.009 kWh/m3 of energy [11].
3.2. Ethanol Production
Apart from methane, ethanol serves as a renewable fuel that can be used as an alternative to traditional fossil fuels. In the ethanol production system, a permselective membrane aims to separate the cells from the medium, thus increasing the concentration of biomass in the bioreactor, improving the removal of the inhibitor, or recovering the product. Mainly MF and UF membranes are used. The ethanol yield (92.7% of theoretical yield with a productivity of 20 g/(L·h)) from glucose was higher in AnMBRs than in conventional systems [51]. High concentration of the product (ethanol) may inhibit the process; it was removed in the membrane system. Tapioca hydrolysate, wood hydrolysate, lactose, and thin stillage wastewater have also been used effectively [51,52].
The possibility of in situ integration of pervaporation into the fermentor by using an ethanol-selective membrane on the wall surface of the bioreactor was investigated [53]. In this system, ethanol is selectively removed from the bioreactor through the membrane wall surface, which minimizes water consumption. Different membranes were tested. For example, a silicalite membrane was used to obtain an 85% (v/v) ethanol solution. Among the membranes used, the ethanol–water separation factors were in the following order: polydimethylsiloxane (PDMS) < poly(1-trimethylsilyl-1-propyne) (PTMSP) < composite membranes < zeolite membranes. Zeolite membranes are more expensive than polymer membranes (PDMS, PTMSP), but have higher separation efficiency and flow rate.
3.3. Hydrogen Production
Maintaining high cell density and decoupling SRT from HRT in AnMBRs help to improve hydrogen production. Hydrogen production can be achieved by inhibiting the methanogenesis phase, e.g., by manipulating hydrogen partial pressure, pH control, chemical inhibition, and promoting ferric-reducing conditions [20]. The hydrogen content in biogas can be as high as 62.6% [54].
The addition of iron and sulfur significantly affects dark fermentation for hydrogen production. Under mesophilic conditions in AnMBR, hydrogen production of 41.6 L/d was 1.59 times higher at 10.9 mg FeSO4/L than at 2.7 mg FeSO4/L [54].
The effect of OLR on hydrogen production was studied in an OLR range of 4–30 kg COD/(m3·d) [55]. Hydrogen yield was the highest at 22 kg COD/(m3·d). Hydrogen production in the AnMBR was 50% higher than in the CSTR, which was found to be overloaded. The results suggest that the AnMBR is preferable when operated near the OLR, which causes overloading in terms of substrate utilization. When brewery wastewater was treated in the submerged AnMBR, increasing the OLR from 30 to 60 kg COD/(m3·d) decreased the hydrogen production rate and yield due to the shift of metabolism to solventogenesis [56].
Regarding the effect of SRT on hydrogen production, it increased with increasing SRT; however, at an SRT of 90 d, it began to decrease [57]. This decrease was attributed to the low VSS/TSS ratio, a shift in metabolism to lactate, and a negative effect of EPS accumulation on microbial growth. It may also be caused by the formation of inhibitory by-products such as short-chain VFAs, alcohols, and the development of hydrogen-consuming microorganisms [58].
The instability of the process and the low hydrogen yield due to the incomplete conversion of the substrates hinder the introduction of biohydrogen production plants on an industrial scale. In this context, other membrane-based technologies besides AnMBRs are suitable to achieve high-rate biohydrogen production. These include microbial electrolysis cells (MECs) and downstream membrane-based technologies, such as electrodialysis (ED) [59].
3.4. Bioelectrochemical Processes Using Microbial Fuel Cells
Bioelectrochemical systems are represented by microbial fuel cells (MFCs). MFCs use electrochemically active microorganisms that donate electrons directly to an anode to oxide substrates, thereby generating electricity from energy contained in wastewater. Although MFCs have been extensively studied for municipal wastewater treatment, they were found to be insufficient to meet stringent wastewater quality requirements [60]. Therefore, MFCs have been combined with MBRs to generate electricity and treat wastewater. MBRs improve pollutant removal, while the electricity generated by MFCs reduces energy consumption during MBR operation. This integration leverages the advantages of both technologies to obtain better effluent, reduce membrane fouling, and create an energy-neutral system [61,62]. The maximum power density varies widely, reaching about 0.38 W/m2 anode area [62].
The MFC can serve as a pretreatment or post-treatment of the MBR (external configuration), or the MFC can be directly immersed in the MBR or vice versa. External configurations represent two-stage systems where wastewater is processed from one stage to another. In the internal system, the anode chamber of the MFC is immersed in the MBR, while the cathode chamber consists of an aeration tank of the MBR [63]. For example, the MFC was combined with a fluidized-bed AnMBR (external configuration), where > 94% COD and 80% ammonia were removed from municipal wastewater. To achieve a more compact design, better treatment efficiency, and less membrane fouling, the internal configuration is a more common solution. MBR modules were inserted into the anode or cathode chamber of the MFC to filter the water treated by the MFC [62]. For example, in two dual-chamber MFCs immersed in the MBR, simultaneous nitrification and denitrification occurred in the bio-cathode, achieving 84.3% of nitrogen removal [64].
This entry is adapted from the peer-reviewed paper 10.3390/en16062829
This entry is offline, you can click here to edit this entry!
