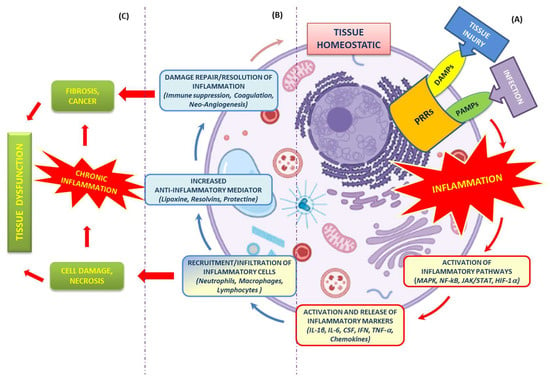
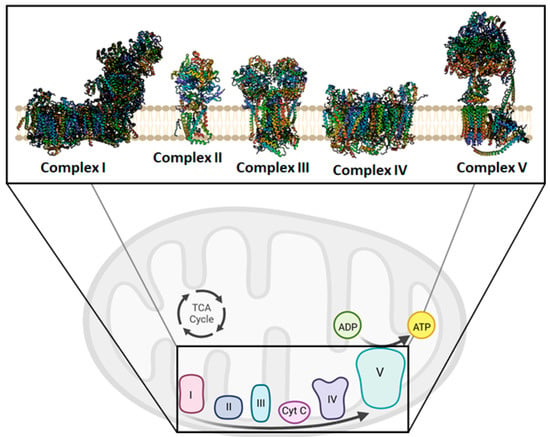
The escape of mtDNA in several devastating diseases is still an obscure molecular mechanism. The critical step in the mtDNA release process is the ROS generation, which is responsible for its oxidation. Defective OXPHOS constituents or alteration of their functionality cause oxidative stress and, consequently, ROS imbalance. Antioxidants could scavenge excess ROS and reduce inflammatory responses by suppressing the inflammation phenomenon, renewing the mitochondrial bioenergetics under pathological or drug-induced imbalance conditions [
61]. ROS-mediated oxidative modifications of biomolecules usually induces post-translational changes in proteins, i.e., phosphorylation, acetylation, ubiquitination, and SUMOylation, among others [
62], or chemical modifications, through the oxidation of DNA bases. The most common type of DNA mutation is caused by ROS modifying guanine (G), leading to the production of 8-oxoG. This lesion causes a Hoogsteen base pair, which is a type of base pairing in nucleic acids that can result in a mismatched pairing with adenine (A) in the genome, resulting in C to A (anti) substitutions [
63]. The enzymes of the base excision repair pathway are primarily responsible for the repair of 8-oxoG, achieved by exploiting their glycosylase activity. In mitochondria, 8-OxoG Glycosylase (OGG1) repairs major oxidative damage in mtDNA and, consequently, OGG1 deficiency results in increased levels of 8-oxoG in mtDNA [
64].
Ox-mtDNA is a mtDAMP and triggers the inflammatory response. Knowing the molecular process that underlies the inflammatory reactions could aid future therapeutic activity in response to microbial invasion or damage signals. Diets rich in bioactive compounds can modulate different stages of inflammation, but information related to their anti-inflammatory mechanisms is still not well understood [
66]. Ox-mtDNA presence in the cytosol triggers the NLRP3 inflammasome assembly and cGAS/STING pathway-mediated type 1 interferon production [
67]. Importantly, it was previously unknown as to how Ox-mtDNA moves into the cytoplasm to bind NLRP3 and also activate cGAS-STING signalling. The Voltage-Dependent Anion Channels (VDACs) and the PTP placed on the outer mitochondrial membrane (OMM) and the IMM, respectively, permit the escape of Ox-mtDNA from mitochondria to reach the cytosol, acting as a mtDAMP [
4,
59].
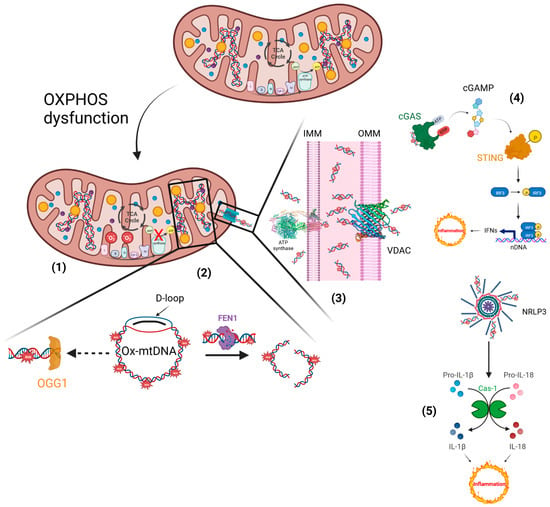
Conversely, mitochondrial dysfunction supports Ox-mtDNA generation, in which the mtDNA misses the opportunity for OGG1 repair, and the mutated DNA is cleaved by the Flap Endonuclease-1 (FEN1). Ox-mtDNA and ROS production are not related to FEN1 activity, but instead work to respond to the creation of a bio-signal using mitochondrial Damage-Associated Molecular Patterns (mtDAMPs). Additionally, 500–650 bp Ox-mtDNA fragments are the products of mtDNA cleavage by FEN1 that exit through the pores of the mitochondrial membranes, acting as mtDAMPs (
Figure 3). In the fragments of Ox-mtDNA, it was noticed that the sequences corresponding to a region within the mitochondrial genome, the D-loop, were overrepresented and released in the cytosol [
65].
Figure 3. Inflammatory responses elicited by impaired mitochondria. (1) Defective electron transfer in OXPHOS produces ROS, responsible for the oxidation of mtDNA. (2) Ox-mtDNA might be repaired by OGG1 or cleaved by FEN1 to fragments that are expelled from mitochondria. (3) PTP and VDAC are located on the IMM and OMM, respectively, and translocate the Ox-mtDNA into the cytosol to activate the inflammatory reactions. (4) OX-mtDNA acts as mtDAMPs and promotes cGAS/STING signalling, responsible for type I IFN production. (5) In addition, OX-mtDNA binds NRLP3 to trigger inflammasome assembly and caspase 1 (Cas-1) activation, and consequently promotes proteolytic maturation of the biologically inactive precursors of interleukin-1β (IL-1β) and interleukin-18 (IL-18), thus generating potent proinflammatory and pyrogenic activities. This figure was created using BioRender (7 March 2023;
https://biorender.com/).
5. Phytosome and Pathologies
5.1. Curcumin Phytosome
It is known that human exposure to Aluminum Chloride (AlCl3) causes hepatotoxicity, which can be counteracted by the Curcumin Phytosome (CP). Curcumin is a polyphenolic compound extracted from Curcuma longa. In studies conducted in rats, Al-Kahtani et al. demonstrated that treatment with AlCl3 increases the concentrations of Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), Alkaline Phosphatase (ALP), Lactate dehydrogenase (LDH), total bilirubin, and Lipid Peroxidation (LPO), reducing, in addition, the stores of albumin, Reduced Glutathione (GSH), Superoxide Dismutase (SOD) and Glutathione Peroxidase (GPx). Histological lesions have also been reported. All of this results in an increased expression of caspase-3 and a decreased expression of Bcl-2.
On the contrary, in the presence of CP, the endogenous antioxidant status is favored; therefore, caspase-3 expression is decreased and Bcl-2 expression increases, with an improvement in liver dysfunction [
108]. The antioxidant and anti-inflammatory properties of curcumin and its nano-phytosome were also tested in mice that exhibited acute inflammation following the administration of carrageenan. In particular, the mice were treated, for 7 days, with an oral dose equal to 15 mg/Kg of indomethacin, curcumin and its nano-phytosome. After 7 days of treatment, the mice were administered carrageenan (1%) at the level of the subplantar region of the left paw, to induce the inflammatory process. A serum antioxidant enzyme assay found that carrageenan reduced the antioxidant activities of SOD, catalase (CAT), GPx, and glutathione reductase (GRx). Conversely, these activities increased in the presence of the curcumin nano-phytosome, both separately and in combination with indomethacin, demonstrating that curcumin nano-phytosome could enhance inflammatory-related antioxidant responses [
109].
5.2. Silybin Phytosome
Among the research related to the application of phytosomes, a very interesting study was conducted to improve the bioavailability of silybin, a natural compound known for its anticancer, antioxidant and hepatoprotective properties. Silybin is one of the main polyphenols found in silymarin, which is a complex of seven flavonolignans and polyphenols extracted from milk thistle (Silybum marianum). In particular, Chi et al. tested the phytosome-nanosuspensions formulation for silybin, defined as SPCs-NPs, in a mouse model of oxyhepatitis, induced by treatment with Carbon Tetrachloride (CCl4). CCl4 causes liver damage, following lipid peroxidation, a lower activity of antioxidant enzymes and increased generation of free radicals and ROS. The choice of silybin was dictated by the known properties of this natural compound, which is capable of protecting the liver from oxidative stress and inflammation, linked to ROS and secondary cytokine production. The CCl4-induced liver injury resulted in an increased presence of ALT, AST and ALP in the bloodstream, inducing centrilobular necrosis, ballooning degeneration and cellular infiltration. Only in the group of mice treated with SPCs-NPs was a significant reduction in ALT, AST and ALP levels recorded, while no significant changes in these parameters were reported in mice treated with silybin alone compared to the positive control.
5.3. Quercetin Phytosome
An interesting flavanol for human health is quercetin, which cannot be produced by man, but is present in abundance in foods such as fruit, especially citrus fruits, green leafy vegetables, broccoli, olive oil, cherries, and blueberries. Similar to the other flavanols, however, quercetin has a low bioavailability, due to its poor solubility in water, even if it is quite soluble in alcohol and lipids. Therefore, the quercetin-loaded phytosome nanoparticles (QP) have made it possible to overcome this difficulty, increasing the bioavailability for humans of orally administered quercetin in this formulation by about 20 times. This turns out to be very interesting, given the known antiviral, anti-atopic, pro-metabolic, and anti-inflammatory properties of quercetin. Moreover, quercetin has a protective effect on mPTP opening [
114].
5.4. Berberine Phytosome
The efficacy of another natural product, such as berberine, was tested in women affected by Polycystic Ovary Syndrome (PCOS), an endocrine pathology characterized by hormonal imbalances, dysmetabolism and inflammation. In particular, berberine is an alkaloid used to fight infections, Type 2 diabetes and cancer, but also dyslipidemia in subjects intolerant to statins, and has been shown to enhance the expression of antioxidant enzyme activity. In the study, patients were treated with two daily oral doses of the Berberine Phytosome (BBR-PP) and evaluations were performed at baseline and after 60 days of treatment. The recorded data report a reduction in insulin resistance and acne, and an improvement in lipid metabolism and body composition, but also indicate a reduction in inflammation, with lower levels of CRP and TNF-α.
5.5. Mulberry and Ginger Phytosome
Phytosomes have also been used in the treatment of Metabolic Syndrome (MetS), characterized by visceral adiposity, insulin resistance, hypertension, high triglyceride levels, and low High-Density Lipoprotein Cholesterol (HDL-C) levels. Patients with MetS have an increased risk of developing Type 2 Diabetes Mellitus (T2DM) and Atherosclerotic Cardiovascular Disease (ASCVD). Both genetic and acquired factors generate oxidative stress, cellular dysfunction and the systemic inflammation process responsible for the pathogenesis of MetS [
120]. In particular, the phytosome containing the combined extracts of mulberry (
Morus alba Linn. Var. Chiangmai) and ginger (
Zingiber officinale Roscoe) (PMG) was tested in an animal model of MetS. Specifically, male Wistar rats were fed a high-carbohydrate, high-fat diet for 16 weeks, to induce MetS. In the following 21 days, rats with MetS signs were subjected to daily oral treatment with three different doses of PMG, equal to 50, 100 and 200 mg/Kg. Data analysis demonstrated that PMG has a positive effect on body weight gain, and lipid and glucose values, as well as improving Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) and Angiotensin-Converting Enzyme (ACE) levels. Even the parameters relating to the density and size of the adipocytes, to the weight of the adipose tissue, have undergone an improvement after this treatment. At the adipose tissue level, PMG also reduced inflammatory cytokines such as IL-6 and TNF-α, as well as reducing oxidative stress and Histone Deacetylase 3 (HDAC3) expression, while simultaneously increasing PPAR-γ expression.
5.5. Eufortyn® Colesterolo Plus
Recently, the ANEMONE study was conducted, involving 60 healthy subjects with moderate polygenic hypercholesterolemia, treated with Eufortyn® Colesterolo Plus. This nutraceutical consists of the standardized bergamot polyphenolic fraction phytosome (Vazguard®), as well as artichoke extract (Pycrinil®), artichoke dry extract (Cynara scolymus L.), zinc and CoQ10 phytosome (Ubiqosome®).
5.7. Naringenin Phytosome
Furthermore, a few years ago, Yu et al. studied the effect of Naringenin (NG) on acute lung injury. This is a respiratory pathology in which the lung undergoes an important inflammatory process. NG is a plant bioflavonoid found in citrus fruits such as bergamot, grapefruit, tangerine, known for its antioxidant, anti-inflammatory, antiproliferative and anticancer properties. In the study, rats with acute lung injury were treated with Dipalmitoylphosphatidylcholine (DPPC) phytosomes NG-loaded for dry powder inhalation (NPDPIs); in particular, NPDPIs, consisting of mannitol/DPPC/NG in a 4:2:1, w/w/w ratio, were found to be effective.
5.8. Centella Asiatica Phytosome
In a mouse model of phthalic anhydride-induced Atopic Dermatitis (AD), the anti-inflammatory effect of the
Centella Asiatica phytosome (CA phytosome) was investigated;it is a medicinal herb used in Ayurvedic, traditional African and Chinese medicine for the treatment of venous insufficiency of legs and diabetes wounds. Following the onset of AD, lesions on the dorsal skin and ear of mice were treated by applying the CA phytosome three times a week for 4 weeks. As a result, inhibition of hyperkeratosis, mast cell proliferation and inflammatory cell infiltration has been reported. The data obtained demonstrated that the CA phytosome inhibits the NF-κB signaling pathway, the release of TNF-α, IL-1β, and IgE, as well as inhibiting the expression of iNOS and COX-2 and the production of NO, resulting in it being a good treatment for AD [
125].
5.9. Leucoselect Phytosome
A few years ago, an open-label phase I lung cancer chemoprevention study was conducted on smokers and ex-smokers to test the chemoprotective effects of the leucoselect phytosome. This phytosome consisted of Grape Seed procyanidin Extract (GSE) complexed with soy phospholipids. At the end of the first month of treatment with leucoselect phytosome, an increase in the levels of omega-3 Polyunsaturated Fatty Acids (n-3 PUFAs) was reported, in particular of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), known for their anticancer properties. Three months after the start of the administration of the leucoselect phytosome, an increase in the serum levels of prostaglandin E3 (PGE3) was also recorded. The latter represents a metabolite of EPA, which, in addition to having antineoplastic properties, is also an anti-inflammatory molecule. Thus, the leucoselect phytosome represents a good chemopreventive agent for lung cancer [
126].
5.10. CoQ10 Phytosome
Moreover, in humans, the effect of CoQ
10 deficiency is known. CoQ
10 is a natural compound known for its antioxidant properties, its ability to prevent damage caused by free radicals, as well as its ability to inhibit inflammation signaling pathways. The CoQ
10 deficiency can be of a primary or secondary form, and determines the onset of serious diseases, such as encephalomyopathy, cerebellar ataxia and cardiovascular disease. Therefore, maintaining correct concentrations of CoQ
10 is important from a therapeutic point of view, even if it is very difficult to achieve this result given its high molecular weight, high lipophilicity, light sensitivity and thermolability [
128]. To solve these problems, over the years, studies have been conducted on various formulations of CoQ
10 that could increase its oral bioavailability. One such study is by Rizzardi et al., published in 2021. In this in vitro study, the authors evaluated the effects of the CoQ
10 phytosomal formulation (UBIQSOME, UBQ) on the bioenergetic and antioxidant status of human intestinal epithelial cells (I407) and rat cardiomyoblasts (H9c2). In this formulation, CoQ
10 was administered in association with lecithin.
5.11. Phytosomes and Cytotoxicity
Much evidence points to the efficacy of phytosomal formulations in increasing the cytotoxicity of certain natural compounds. Specifically, Alhakamy et al. conducted a study on treating ovarian cancer cells, OVCAR-3, with the phytosome Icariin (ICA). ICA is a flavonol glycoside found in Epimedium grandiflorum. It is best known for its efficacy in treating atherosclerosis and neurodegenerative disorders. It also possesses antioxidant, anti-inflammatory, cardioprotective and hepatoprotective activities. In addition, the antitumor activity of ICA has been demonstrated through its cytotoxicity, apoptotic activity, and regulation of cell cycle protein expression against different cell types. ICA also has anti-angiogenic, anti-metastatic, and immunomodulatory effects, explicitly enhancing the chemosensitivity of ovarian cancer cells. The limitation of ICA treatments is related to its pharmacokinetic properties; it has poor bioavailability due to its chemical structure and a short half-life when administered orally (3.15 h) and intravenously (0.56 h). Therefore, the phytosomal formulation seems to have many advantages for the cytotoxic efficacy of ICAs, particularly for ovarian cancer. This pathology represents a very serious and widespread gynecologic neoplasm worldwide, being fatal in most cases, as it has no early symptoms or adequate treatment. In the study by Alhakamy et al. an increase in the cytotoxic activity of the ICA phytosome on OVCAR-3 cells was demonstrated. Specifically, there was an increase in ICA phytosome-treated cells in the G2/M and pre-G1 phases of the cell cycle. Furthermore, Annexin V staining demonstrated an increase in early, late, and total apoptotic cells.