Innovative synthetic methods have been developed for the preparation of m-aryloxy phenols, which has allowed for the preparation of complex m-aryloxy phenols with functional groups, such as esters, nitriles, and halogens, that impart specific properties of these compounds.
- m-aryloxy phenols
- potential biological activities
- hydroxylation of benzenes
1. Introduction
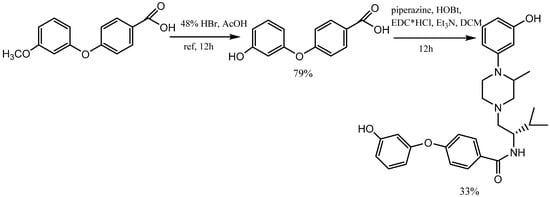
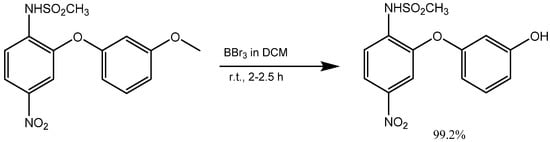
2. Synthesis of m-Aryloxy Phenols by Demethylation of m-Methoxy Phenols
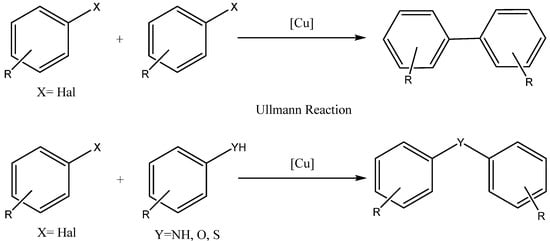
3. Synthesis of aryloxy phenols by reaction between aryl halides and resorcinol
The reaction between aryl halides and resorcinol involves a nucleophilic aromatic substitution mechanism. In this mechanism, resorcinol undergoes deprotonation under basic conditions and subsequently acts as a nucleophile on the aromatic ring of the aryl halide. This results in the formation of an intermediate, in which the nucleophile attacks the ring, leading to the substitution of the halide group. The intermediate species contains an oxide anion, which is formed due to the loss of a proton. The final product of the reaction is an aryloxy phenol, which can be obtained in good yield under appropriate reaction conditions [14].
In 2010, Vagin and colleagues published a study detailing the synthesis of 5-(3-hydroxyphenoxy)-2-nitroaniline from 5-chloro-2-nitroaniline and resorcinol. The reaction was performed using sodium hydride as a base and DMF as a solvent and was carried out under an argon atmosphere by heating the reaction mixture at 125°C for 24 hours. They then used a hydrogenation reaction with a Pd/C catalyst to obtain 4-(3-hydroxyphenoxy)-1,2-benzyldiamine (Scheme 4) [15].
4. Sonogashira Coupling: A Copper-Catalyzed Method for Biaryl Synthesis
The Sonogashira coupling reaction represents a variation of cross-coupling, wherein the interaction between aryl boronic acids and phenols is facilitated by the presence of a copper catalyst and a base. Copper (II) acetate (Cu(OAc)2) serves as a typical example of a copper catalyst for this reaction, while potassium carbonate (K2CO3) is commonly used as the base. The reaction mechanism involves the formation of a complex between the copper and phenol, which subsequently reacts with the aryl boronic acid to form an intermediate. This intermediate undergoes transmetallation to furnish the final biaryl product. Due to its high efficiency and versatility, the Sonogashira coupling reaction has gained widespread use in the synthesis of pharmaceuticals and agrochemicals, among other applications[16][17].
The report by Bryan and colleagues in 2015 summarizes research endeavors centered on forming new carbon-heteroatom bonds using organoboron reagents through copper acetate-mediated reactions under ultrasound irradiation. The methodology involves incorporating ultrasound irradiation in the Chan-Evans-Lam reaction to achieve the O-arylation of phenols, N-arylation of anilines and indoles, and S-arylation of thiols. The utilization of ultrasound irradiation was discovered to significantly reduce reaction times from 72 hours to 4 hours while increasing product yields by an average of 20% [18].
5. Synthesis of m-Aryloxy phenols using Grignard Reagents
The utilization of Grignard reagents, a type of organometallic compound, in the synthesis of aryloxy phenols via reaction with aldehydes or ketones has received widespread attention in the field of organic chemistry. Through interaction with a carbonyl compound, a complex intermediate is formed, which upon treatment with acidic conditions, gives rise to the corresponding alcohol. The alcohol can then be oxidized through the use of oxidation reagents such as hydrogen peroxide (H2O2) or sodium hypochlorite (NaClO) to produce the desired aryloxy phenol.
It is important to note that the reaction conditions, such as the choice of solvent and the presence of catalysts or bases, have a significant impact on the reactivity, selectivity, yield, and stereochemistry of the Grignard reaction and the final product [19].
In 2012, researchers led by Pidathala used 4-(3-methoxyphenoxy)benzaldehyde in a Grignard reaction to produce an intermediate alcohol.
6. Hydrolysis of Diazonium Salts Using a Two-Phase System
In 2015, Taniguchi et al. published findings on the synthesis of 3-phenoxyphenol and 3-(4-nitrophenoxy)phenol. The method involved the hydrolysis of intermediate diazonium salts derived from anilines in a two-phase system of cyclopentyl methyl ether (CPME) and water, resulting in high yields of the desired compounds. These products were found to be key components for the production of raw materials for functional plastics, specifically polyimide resin [20].
7. Synthesis of 3-aryloxyphenols from 3-chlorocyclohex-2-en-1-one
In 2023, a chemical reaction was reported by Clive's group wherein 3-chlorocyclohex-2-en-1-one was treated with phenols in the presence of K2CO3 resulting in the formation of 3-(aryloxy)cyclohex-2-en-1-ones . The synthesized products were then subjected to bromination at the C(2) position using NBS in DMF, ultimately forming the brominated products. Subsequently, the brominated compounds were aromatized to form 3-(aryloxy)phenols through treatment with DBU in PhMe or MeCN. This multi-step reaction sequence necessitated the use of several reagents and conditions to generate the final compounds. The method presently utilized operates at ambient temperature, and does not employ any heavy metals or ligands. Moreover, it circumvents the procedural steps required to overcome the o,p-directing effect of oxygen, owing to the readily available 1,3-functional group relationship inherent in the starting material, namely cyclohexane-1,3-dione [21].
8. Conclusion
In summary, the past decade has witnessed remarkable advances in the synthesis and utilization of m-aryloxy phenols. The implementation of various synthetic methodologies has resulted in the preparation of structurally diverse m-aryloxy phenols, including biologically active compounds with promising pharmacological applications. Moreover, m-aryloxy phenols have demonstrated versatility in the fields of materials science and organic electronics. These achievements have propelled further research towards the synthesis, characterization, and application of m-aryloxy phenols, and it is anticipated that this research area will continue to expand in the foreseeable future. The growing interest in m-aryloxy phenols underscores their potential to emerge as key compounds in the field of synthetic organic chemistry and their relevance to a diverse range of technological applications.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28062657
References
- Latha, G.; Devarajan, N.; Karthik, M.; Suresh, P. Nickel-Catalyzed Oxidative Hydroxylation of Arylboronic Acid: Ni(HBTC)BPY MOF as an Efficient and Ligand-Free Catalyst to Access Phenolic Motifs. Catal. Commun. 2020, 136, 105911.
- Wang, S.K.; Chen, M.T.; Zhao, D.Y.; You, X.; Luo, Q.L. Iodine-Catalyzed Oxidative Aromatization: A Metal-Free Concise Approach to meta-Substituted Phenols from Cyclohex-2-enones. Adv. Synth. Catal. 2016, 358, 4093–4099.
- Ullmann, F. Ueber eine neue Bildungsweise von Diphenylaminderivaten. Ber. Der Dtsch. Chem. Ges. 1903, 36, 2382–2384.
- Ullmann, F. Ueber eine neue Darstellungsweise von Phenyläthersalicylsäure. Ber. Der Dtsch. Chem. Ges. 1904, 37, 853–854.
- Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl-aryl Bond Formation One Century after the Discovery of the Ullmann Reaction. Chem. Rev. 2002, 102, 1359–1470.
- Zuo, L.; Yao, S.; Wang, W.; Duan, W. An Efficient Method for Demethylation of Aryl Methyl Ethers. Tetrahedron Lett. 2008, 49, 4054–4056.
- Bradsher, C.K.; Brown, F.C.; Porter, H.K. Synthesis and Fungistatic Activity of Some 3-Hydroxybiphenyl Derivatives. J. Am. Chem. Soc. 1954, 76, 2357–2362.
- McOmie, J.F.W.; Watts, M.L.; West, D.E. Demethylation of Aryl Methyl Ethers by Boron Tribromide. Tetrahedron 1968, 24, 2289–2292.
- Kormos, C.M.; Jin, C.; Cueva, J.P.; Runyon, S.P.; Thomas, J.B.; Brieaddy, L.E.; Mascarella, S.W.; Navarro, H.A.; Gilmour, B.P.; Carroll, F.I. Discovery of N--4-phenoxybenzamide analogues as selective kappa opioid receptor antagonists. J. Med. Chem. 2013, 56, 4551–4567.
- Carroll, F.I.; Carlezon Jr, W.A. Development of κ Opioid Receptor Antagonists. J. Med. Chem. 2013, 56, 2178–2195.
- Puls, K.; Olivé-Marti, A.L.; Pach, S.; Pinter, B.; Erli, F.; Wolber, G.; Spetea, M. In Vitro, In Vivo and In Silico Characterization of a Novel Kappa-Opioid Receptor Antagonist. Pharmaceuticals 2022, 15, 680.
- Yamamoto, Y.; Tago, T.; Toyohara, J.; Saito, Y.; Yamamoto, F. Radiosynthesis and in Vivo and ex Vivo Evaluation of Isomeric methoxy Analogs of Nimesulide as Brain Cyclooxygenase-2-Targeted Imaging Agents. Biol. Pharm. Bull. 2022, 45, 94–103.
- Yamamoto, Y.; Hisa, T.; Arai, J.; Saito, Y.; Yamamoto, F.; Mukai, T.; Ohshima, T.; Maeda, M.; Ohkubo, Y. Isomeric Methoxy Analogs of Nimesulide for Development of Brain Cyclooxygenase-2 (COX-2)-Targeted Imaging Agents: Synthesis, In Vitro COX-2-Inhibitory Potency, and Cellular Transport Properties. Bioorg. Med. Chem. 2015, 23, 6807–6814.
- Lindley, J. Copper Assisted Nucleophilic Substitution of Aryl Halogen. Tetrahedron 1984, 40, 1433–1456. [Google Scholar] [CrossRef]
- Vagin, S.I.; Reichardt, R.; Klaus, S.; Rieger, B. Conformationally flexible dimeric salphen complexes for bifunctional catalysis. J. Am. Chem. Soc. 2010, 132, 14367–14369.
- Thomas, A.M.; Sujatha, A.; Anilkumar, G. Recent Advances and Perspectives in Copper-Catalyzed Sonogashira Coupling Reactions. RSC Adv. 2014, 4, 21688–21698.
- Min, H.; Palani, T.; Park, K.; Hwang, J.; Lee, S. Copper-Catalyzed Direct Synthesis of Diaryl 1, 2-Diketones from Aryl Iodides and Propiolic Acids. J. Org. Chem. 2014, 79, 6279–6285.
- Bryan, J.M.; George, W.K. Copper mediated formation of carbon-heteroatom bonds using organoboron reagents and ultrasound. Heterocycles Int. J. Rev. Commun. Heterocycl. Chem. 2015, 90, 271–297.
- Mann, F.G.; Stewart, F.H. The Action of Magnesium and of Grignard Reagents on Certain Benzyl Ethers. Part I. The Action of Magnesium on o-, m-, and p-Alkoxy-and-phenoxy-methylbenzyl Chlorides. J. Chem. Soc. 1954, 2826–2832.
- Taniguchi, T.; Imoto, M.; Takeda, M.; Nakai, T.; Mihara, M.; Iwai, T.; Ito, T.; Mizuno, T.; Nomoto, A.; Ogawa, A. Hydrolysis of Diazonium Salts Using a Two-Phase System (CPME and Water). Heteroat. Chem. 2015, 26, 411–416.
- Duvvuru, B.; Amankulova, D.; Gauden, S.; Haffemayer, T.; Clive, D.L. A mild alternative to the classical Ullmann coupling for preparation of 3-aryloxy phenols. Tetrahedron 2023, 133, 133264.
