1. Whey Intake Improved Insulin Secretion and Postprandial Glycemia
Milk-derived whey as well as casein proteins can produce insulin secretion in obese, pre-diabetic, and also type 2 diabetes individuals [
24,
25,
26,
27,
28,
29]. Studies in humans have shown that whey protein decreases postprandial glycemia and could be used in medical/nutritional therapy to regulate blood sugar [
30,
31]. In diabetic subjects, whey intake has been associated with a reduction of postprandial hyperglycemia [
32,
33]. Even a small 15 g dose of whey protein consumed shortly before mixed-macronutrient meals stimulates insulin release, improves postprandial glycemia (−13%), and increases satiety in T2DM subjects (
p < 0.05) [
34].
Various studies have similarly reported positive effects of whey protein on insulin secretion [
35]. The intake of 50 g WPI associated with maltodextrin increased insulin production by 96% versus maltodextrin alone in pre-diabetic adults (
p < 0.05) and a 21% decrease in postprandial blood glucose after protein meals (
p < 0.0001) [
36]. Interestingly, for practical nutrition, the addition of whey (27.6 g) to high-glycemic-index meals (such as bread and mashed potatoes and meatballs) increases insulin release (31% for breakfast and 57% for lunch, both
p < 0.05) and diminishes postprandial blood glucose (−21%,
p < 0.05) excursion in type 2 diabetic subjects [
37].
In lean and healthy subjects, whey consumption has also been shown to decrease blood sugar [
36,
38]. Between whey, tuna, turkey, and egg albumin, the measure of postprandial glucose and insulin concentrations in 22 lean, healthy men provided the best results for whey [
24]. Blood glucose was significantly lower for whey meal than for turkey (
p < 0.023) and eggs (
p < 0.001), indicating a faster glucose uptake in cells, but not with the tuna meal. Blood insulin was also significantly higher for whey compared to tuna, turkey, and eggs (all
p < 0.001).
Moreover, whey protein may have beneficial effects on some symptoms of metabolic syndrome and improve cardiovascular risk factors [
35,
39,
40]. Metabolic syndrome is a combination of hyperglycemia, hypertension, excess body fat around the waist, and abnormal cholesterol or triglyceride levels, increasing the risk of diabetes, heart disease, and stroke [
41]. Moreover, a meta-analysis of 22 randomized controlled trials (RCTs), using the Cochrane method for the elimination of bias, showed that whey intake decreased insulin significantly in patients with metabolic syndrome (weighted mean difference (WMD): −0.94; 95% CI: −1.68, −0.21) but did not have an effect on fasting plasma glucose levels [
42]. Meanwhile subgroup analyses showed a significant reduction in fasting plasma glucose levels and other meta-analyses including in obese participants who had also shown improvement in fasting plasma glucose levels after whey protein intake [
43,
44].
2. Effects on Insulin Resistance and Glycated Hemoglobin
A meta-analysis of 22 randomized controlled trials (RCTs) highlighted a significant decrease in glycated hemoglobin (HbA1c) with whey intake in patients with metabolic syndrome (WMD: −0.15; 95% CI: −0.29, −0.01) and in the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) (WMD: −0.20; 95% CI: −0.36, −0.05) [
42]. Another meta-analysis of 30 RCTs suggested that dairy intake, in particular, low-fat dairy products, has a positive action on HOMA-IR (mean difference (MD) of −1.21; 95% CI −1.74 to −0.67;
p < 0.00001;
I2 = 92%) [
45].
Dairy protein consumption before a meal decreases food intake and, in association with carbohydrates, decreases glycemia by insulin-dependent as well as insulin-independent mechanisms [
46,
47].
Interestingly, whey can also be effective for controlling blood sugar parameters and inflammation before surgery. Fasting before surgery, which can be prolonged from 10 up to 16 h, can induce hyperglycemia due to a limitation of insulin action by the effect of counter-regulatory hormone action. Whey protein in a drink associated with carbohydrates has been shown to minimize the postoperative insulin resistance (HOMA-IR) and associated acute inflammation vs. carbohydrates alone (2.75 ± 0.72 vs. 5.74 ± 1.16;
p < 0.05) [
48].
3. Mechanisms of Whey and Dairy Proteins Associated with Decrease in Postprandial Glycemia
3.1. Activity of Amino Acids on Insulin Secretion
It has been confirmed that the insulinotropic effect of dairy proteins is associated with certain amino acids, in particular the branched-chain amino acids (BCAAs) who seem to be of vital importance, especially leucine, isoleucine, valine, lysine, and threonine, inducing insulin secretion with leucine, reportedly having the greatest effect acutely [
49,
50,
51]. Leucine activates glutamate dehydrogenase activity in β-cells, which leads to an increase in Krebs cycle activity, oxygen consumption by these cells, and then to increased insulin production [
49]. Leucine and high protein intake also seem to modulate AMP-activated protein kinase (AMPK) and mTOR and influence hypothalamic neuropeptides, reducing the expression of orexigenic neuropeptides (NPY) and AgRP (Agouti-related peptide) and increasing anorexigenic neuropeptide pro-opiomelanocortin (POMC) [
51].
Whey protein is an exceptional source of BCAAs, which are easily and quickly digested, leading to a rapid increase in BCAA leads in the circulation and insulin release, which may improve postprandial hyperglycemia (
Figure 1) [
42]. Glutamate and alanine can also participate in insulin secretion coupling, not alone but by amplifying the stimulation by glucose [
50]. Cysteine could also be implicated in this process [
52].
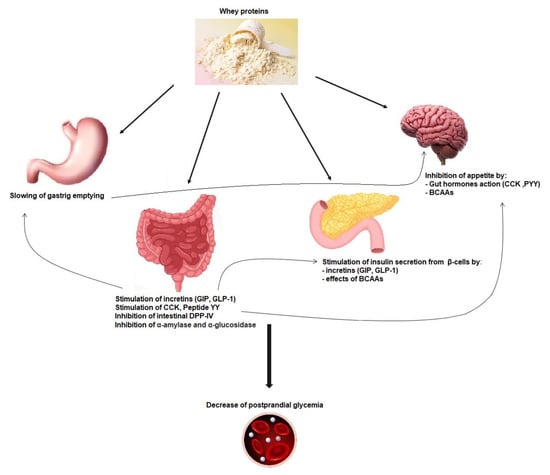
Figure 1. Mechanisms implicated in whey protein activity on postprandial glycemia reduction. GIP: glucose-dependent insulinotropic polypeptide; GLP-1: glucagon-like-peptide-1; CCK: cholecystokinin; PYY: peptide YY; DPP-IV: dipeptidyl peptidase-IV; BCAAs: branched-chain amino acids.
3.2. Incretin Secretion and Insulin Secretion
Dairy-protein-derived peptides can also increase the insulin secretion effect through dipeptidyl peptidase-4 (DPP-4) inhibitory activity in the proximal gut, preventing the incretin glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like-peptide-1 (GLP-1) degradation [
40] (
Figure 1) [
53]. Indeed a major part of secreted insulin is a result of the action of both GIP and GLP-1. However, because of the cleaving activity of DPP-4, GIP and GLP-1 have a half-life of only 1–2 min [
54]. Thus, inhibiting DPP-IV activity is considered as a way of treatment in T2DM [
55]. Incretins also increase the sensitivity of β-cells to glucose, stimulate β-cell proliferation, and protect these cells against apoptosis [
56].
When provided shortly prior to a meal, whey dose-dependently reduces (using from 9 to 18 g) the postprandial glycemia (
p < 0.0001) and increases GLP-1 levels (
p < 0.0001) [
57]. Bioactive substances in whey, among which are IGs, Lf, α-La, and glutamine, have been shown to increase incretin hormones and to inhibit dipeptidyl peptidase-IV [
31,
42,
58,
59]. Other studies have shown a strong increase in GLP-1 concentrations after a whey drink when compared to a glucose or fructose drink (from 25 to 50 g of whey,
p < 0.05) taken before a meal (30 min to 4 h) [
60,
61].
Some bioactive peptides should be responsible for influencing postprandial incretin responses [
62]. Indeed, whey and milk proteins are degraded during low-pH digestion in the stomach and by gastric pepsin and other peptidases. The resulting hydrolyzed proteins pass into the small intestine and are further split by pancreatic proteases into single amino acids and oligopeptides and finally by other enzymes from the brush-border enzymes into dipeptides, tripeptides, and amino acids. Bioactive peptides can contain from two to twenty amino acid residues or more. Some of these peptides have been identified in the gastrointestinal tract as well as in bloodstream after milk intake, but more studies are needed to fully characterize these peptides and their precise role in glycemia management [
62].
However, in present hypertriglyceridemia, obesity and high-baseline GLP-1 levels tend to have poorer response to whey proteins [
63], and even positive results have been also observed [
25]. It could be linked to glucagon-induced increase with whey protein intake [
60]. Furthermore, hypertriglyceridemia augments the hyperglycemic effects of glucagon [
64].
Whey proteins seem also to inhibit other enzymes such as α-amylase and α-glucosidase [
53,
65,
66].
3.3. Gastric Emptying Effect on Postprandial Glycemia
The actions of whey proteins on gastric emptying, on postprandial glycemia, and on secretion of incretin hormones are linked together. In addition to its impact on insulinotropic effects, GLP-1 induced by whey intake is also able to slow down gastric emptying by relaxing the proximal stomach, reducing antral and duodenal motility, and increasing pyloric tone. This restrains energy intake and can inhibit glucagon secretion, which all together improve postprandial glycaemia (
Figure 1) [
67]. The function of the gastrointestinal tract is key for glucose homeostasis, especially during the postprandial phase, and slowing gastric emptying can diminish postprandial glycemic excursions in healthy and diabetic subjects (
Figure 1) [
58,
68,
69,
70]. Other gut hormones, namely, cholecystokinin (CCK) and peptide YY (PYY), can decrease gastric emptying and appetite [
71].
The importance of slowing gastric emptying is key in the decrease in postprandial glycemia observed when proteins are added to glucose intake [
72]. Likewise, a whey “preload” is able to slow gastric emptying of a following meal in both healthy [
73] and T2DM subjects [
74]. In diabetic subjects, GLP-1 was higher when whey was ingested (55 g) between −15 min and 90 min before the meal versus during the mean (
p < 0.001), even if the incremental area under the curve (iAUC) was not significantly different [
74], and both decrease postprandial glucose (363.7 ± 64.5 mmol · min
−1 · L
−1) and (406.3 ± 85.9 mmol · min
−1 · L
−1) compared with no whey (734.9 ± 98.9 mmol · min
−1 · L
−1;
p < 0.005).
3.4. Gut Hormones, Amino Acids, and Satiety
Satiety induced by proteins has been demonstrated, in an acute manner, with meals containing from 25 to 90% proteins, leading to a significant decrease in energy intake. It has also been shown with high protein content in ad libitum diets, lasting from a few days up to 6 months [
75]. Among the three macronutrients, protein has the greatest satiating action. After protein-containing nutrient intake, signals can be sent to the central nervous system (CNS) via gastric and gut peptide action and via the bloodstream after digestion. Indeed, satiety is induced by various mechanisms, which are both visceral (during digestion) and metabolic (inter-prandial phase) and directed towards the CNS directly at the level of the hypothalamus and indirectly mainly through the vagus nerve [
76]. Regarding meal size, the negative feedback control from gastrointestinal signals and bloodstream takes place in the dorsal vagal complex (brainstem) and in the hypothalamus.
The gut peptide hormones upregulated by whey consumption include CCK, PYY, GLP-1, and GPI (
Figure 1) [
56,
58,
73]. It has been proposed that high protein meals could induce the greatest production of PYY and the highest satiety feeling in obese as well as normal-weight human subjects [
74]. Ghrelin, an orexigenic peptide decreased after consumption of proteins; leptin; and insulin levels are also known to influence satiety [
58,
60,
74].
Although GLP-1 can have an action on peripheral organs through the circulation, it is of note also that GLP-1 can be produced by the pancreas and brain as well [
56].
Studies demonstrate that dairy and whey proteins decrease appetite better than other protein sources such as eggs, casein, or soy [
73,
77,
78,
79]. In the study from Hall et al., plasma CCK was increased by 60% (iAUC,
p < 0.005), GLP-1 by 65% (iAUC,
p < 0.05), and GIP by 36% (iAUC,
p < 0.01) following a 48 g whey preload when compared with casein, showing the particular potential of whey in this field [
73]. Whey, tuna, turkey, and egg albumin meals were compared in terms of appetite measures and energy intake in 22 lean healthy men. The best results were obtained for whey, with a significant reduction of mean energy intake at the ad libitum meal 4 h after (
p < 0.001) [
24]. Appetite rated by the subjects, postprandial insulin, and energy intake during the meal were strongly related.
This entry is adapted from the peer-reviewed paper 10.3390/nu15051294