Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Cellular senescence is a physiological mechanism that has both beneficial and detrimental consequences. Senescence limits tumorigenesis, lifelong tissue damage, and is involved in different biological processes, such as morphogenesis, regeneration, and wound healing.
- cellular senescence
- age-related disorders
- SASP
- senolytic drugs
1. Introduction
It is definitely clear that living longer is a leading risk factor for increasing vulnerability to the development of the most prevalent pathologies, including cardiovascular diseases, neurodegenerative diseases, and cancer [1]. Increasing longevity presents a huge challenge for medical care, as aging progression is diverse and is not absolutely correlated to chronological age. Although a preventive medicine would have been the most suitable to prevent age related disease, pertinent biomarkers were not available to precisely measure precisely physiological age. However, new insights into the cellular and molecular events playing a role in biological aging led to the definition of a restricted set of biomarkers as potential targets to fight tissue aging deteriorations [2,3]. One emerging factor is the accumulation of senescent cells in tissues and organs, now considered as a common denominator in a vast majority of age-related pathologies [4,5]. Senescent cells are especially abundant at sites of age-related pathologies, and a great deal of evidence from mouse models demonstrated a causal role of senescent cells in several age-related diseases, such as sarcopenia, lordokyphosis, cataracts, osteoporosis, aged hematopoietic system, vasomotor dysfunction, atherosclerosis, neurodegeneration, idiopathic lung fibrosis, osteoarthritis, hepatic steatosis, and hair loss [6,7,8,9,10,11,12,13,14], demonstrated by genetic approaches the clearance of p16-expressing senescent cells in mice models [15,16]. As this approach gave rise to a global delay of the onset of deterioration of several organs and the appearance of the corresponding age-related diseases, it led to a new paradigm considering aging as a disease and not only as a biological process. Targeting senescence seems to be a promising approach for treatment and prevention of aging to further promote an increased health longevity [17,18].
The fundamental characteristic of senescent cells is an irreversible cell cycle arrest that occurs in normal proliferating cells in response to various forms of cellular stress. Telomere shortening, UV radiation, increased levels of ROS species, oncogene activation, direct DNA damage, and other forms of stress that elicit activation of the DNA damage response pathway can lead to senescence [19,20,21,22]. Senescence was originally understood as a physiological cellular response aiming to prevent propagation of damaged cells in the organism [4,23,24,25,26,27,28,29]. Later, a number of additional beneficial functions, such as tissue repair, wound healing, and embryonic development, were discovered [5].
In addition to the protective role of cellular senescence, the long-term presence of senescent cells was revealed to be detrimental to the organism [5,30]. Indeed, the proliferative incompetency of senescent cells is accompanied by a resistance to apoptosis, as well as the secretion of a complex plethora of factors, referred to as the senescence-associated secretory phenotype (SASP). It contains, chemokines, cytokines, tissue damaging proteases and growth factors, and proinflammatory factors that normally assist in their removal by the immune system [31,32]. Studies on diverse animal models indicate that multiple components of the immune system, including NK cells, T cells, and macrophages, are involved in controlling the presence of senescent cells in tissues [26,33,34,35,36,37]. The efficacy of this removal is variable among tissues and pathological conditions, and the mechanisms and rules regulating the homeostasis of senescent cells are yet to be fully understood. In the elderly, senescent cells and their SASP increasingly accumulate in tissues and contribute to the establishment of a chronic inflammation, so called “inflammaging”, due to continuous secretion of proinflammatory cytokines [24,38,39] considered as a feature of the majority of age-related diseases [40].
2. Senescence in Physiological Processes
Despite the detrimental effects of persistent cellular senescence and SASP being widely documented, these systems play crucial and appropriate roles in normal physiological processes, such as embryogenesis, wound healing, and fibrosis (Figure 1) [4,25,42,43]. Senescence is essential for the healthy functioning of several physiological processes, including embryonic development, wound healing, and fibrosis. This kind of senescence, also known as “acute senescence” [5], appears to be strictly controlled; it only affects a certain subset of tissue cells, which are then quickly eliminated by the immune system.
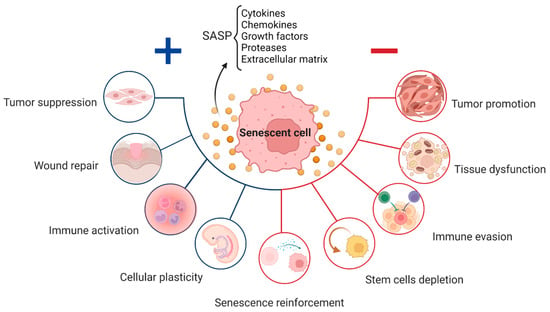
Figure 1. Role of senescent cells throughout life: positive (blue) and negative (red) effect depending on the level of senescence and SASP components released, including cytokines, chemokines, and other molecules, which affects the neighboring cells.
Cellular senescence is carefully regulated during embryogenesis, starts at certain periods and sites, and is involved in morphogenesis, tissue remodeling, and cell population balance [42,43]. Embryos at mid to late stages of development display senescent cells in very specific anatomical structures and locations throughout the embryo. Positive areas include the developing limbs, the tip of the tail, the otic and brain vesicles, the fusing sternum midline, the gut endoderm, and the closing neural tube. In each of these cases, senescent cells must be efficiently removed by macrophages to allow proper tissue formation and maturation. This is important, as suppression of cellular senescence in mice results in developmental abnormalities, highlighting the crucial function of cellular senescence in proper embryogenesis [4].
The expression of p21 and other SASP components are two characteristics that embryonic senescent cells have in common with oncogene-induced senescent cells. The absence of p16 expression, DNA damage, and the release of IL-6 and IL-8 are some other variations between adult and embryonic senescence [43]. Embryonic senescence appears to be solely dependent on p21, indicating that it is not affected by p53, DNA damage, or other cell cycle inhibitors. The senescent cells in the embryo occur for a short period of time, undergo cell death, and are then eliminated by the immune system before birth [42,43]. As a result, throughout embryo development, TGF-β activates SMAD, which works in conjunction with FOXO proteins to regulate p21 expression and p21-mediated cell cycle arrest.
Later in life, cellular senescence is initiated during the first phases of tissue repair, suggesting that wound healing and tissue regeneration depend on a similar process of cellular senescence as seen during embryonic development [25,42].
Senescent fibroblasts and endothelial cells produce many proteins, including PDGF-AA, upon injury [25]. To begin wound healing, PDGF-AA stimulates myofibroblast differentiation and granulation tissue formation. Additionally, SASP-associated proteases aid in regulating excessive fibrosis [25]. Besides this, pharmacological cellular senescence suppression in vivo decreases wound healing [16,25]. Thus, the importance of cellular senescence in development and repair is highlighted by the necessity of senescent cells and SASP at the early stages of tissue remodeling [4].
It is important to point out that the development of tissue fibrosis has been shown to benefit from cellular senescence. Senescence has also been proposed to reduce fibrosis through the production of proteases during wound healing [25]. However, in the lung, it is considered to induce pulmonary fibrosis [25,44].
Within several days of a skin injury, senescent fibroblasts and endothelial cells emerge at the wound site; lack of senescent cells results in delayed wound healing. Treatment with recombinant PGDF-AA can prevent this delay, but severe fibrosis can still occur [25]. This, in turn, is limited by senescence of myofibroblasts, which, when not senescent, promote fibrosis by producing ECM. Aside from this, senescent cells release ECM-degrading proteases into the SASP [45].
Similarly, in the presence of a chronically damaged liver with severe fibrosis, aging of hepatic ECM-producing stellate cells can inhibit fibrosis, just as it does in wound healing (HSC). Active HSCs, as they become senescent, decrease ECM release and improve immune surveillance. HSC senescence can be triggered by insulin-like growth factor-1 (IGF-1) or CCN1, as well as by the anti-inflammatory factors IL-10 and IL-22, through the signal transducer and activator of transcription 3 (STAT3) and p53-dependent pathway.
During regeneration in salamanders, a large number of senescent cells were found in the limb. However, when the limb had fully recovered, these senescent cells disappeared [46]. Other experiments revealed that, when both normal and senescent cells were inserted into a salamander, the senescent cells also disappeared rapidly. This suggests that senescent cells could potentially be involved in the regeneration process and that salamanders possess a functional system capable of effectively clearing these cells. Macrophages, among other immune system components, may help in the removal of senescent cells from some tissues, as shown by previous studies. Demonstrating how senescent cells were surrounded by macrophages in living salamanders regenerated limbs supported this theory. Similarly, senescent cells were not eliminated as efficiently in some salamanders whose macrophages were destroyed by the toxic clodronate-loaded liposomes, as in salamanders with active macrophages [47]. Therefore, macrophages are a vital factor in the process of removing senescent cells from salamander tissues, and this mechanism of eliminating senescent cells may unlock the secret of the salamander’s ability to maintain regeneration during aging.
Because senescence play an important role in physiological processes, such as wound healing [25], tissue remodeling, and embryonic development [42,43], the emerging approaches of clearing senescent cells to ameliorate age-related tissue deteriorations have been questioned.
3. Senescence in Age-Related Diseases
Aging is the largest risk factor for developing many disorders, ranging from tissue degeneration to cancer. One pivotal driver of most of the major age-related pathologies is cellular senescence [2,5]. There are different types and mechanisms of senescent cells formation. Cellular senescence, as first described by Hayflick and Moorhead, referred to the finite lifespan of primary human fibroblasts in culture [48,49]. This observation describes only one particular type of cellular senescence that is caused by the attrition of telomeres, called replicative senescence (RS), a naturally occurring process that takes place every cell division [50]. Cellular senescence is induced by a variety of stress-causing, irreversible damages. These can include oncogene signaling, DNA damage, irradiation, or chemotherapy, causing the activation of tumor suppressor networks, including p53, p16, and p21, as well as cell cycle arrest [22,24,30,31,51]. In this case, it is categorized as a premature senescence. Thereby, senescence acts as intrinsic tumor suppressive mechanism that might prevent the proliferation of damaged cells. Irrespective of the stimulus, the senescence program is executed upon persistent activation of p53/p21 and/or RB/p16 tumor suppressor pathways, orchestrating the transition to and the maintenance of the senescent phenotype [24].
This entry is adapted from the peer-reviewed paper 10.3390/cells12060915
This entry is offline, you can click here to edit this entry!
