Cardiovascular diseases (CVDs) are among the most impactful illnesses globally. The available therapeutic option has several side effects, including hypotension, bradycardia, arrhythmia, and alteration in different ion concentrations. Recently, bioactive compounds from natural sources, including plants, microorganisms, and marine creatures, have gained a lot of interest. Marine sources serve as reservoirs for new bioactive metabolites with various pharmacological activities. The marine-derived compound such as omega-3 acid ethyl esters, xyloketal B, asperlin, and saringosterol showed promising results in several CVDs.
- marine drugs
- cardiovascular diseases
- atherosclerosis
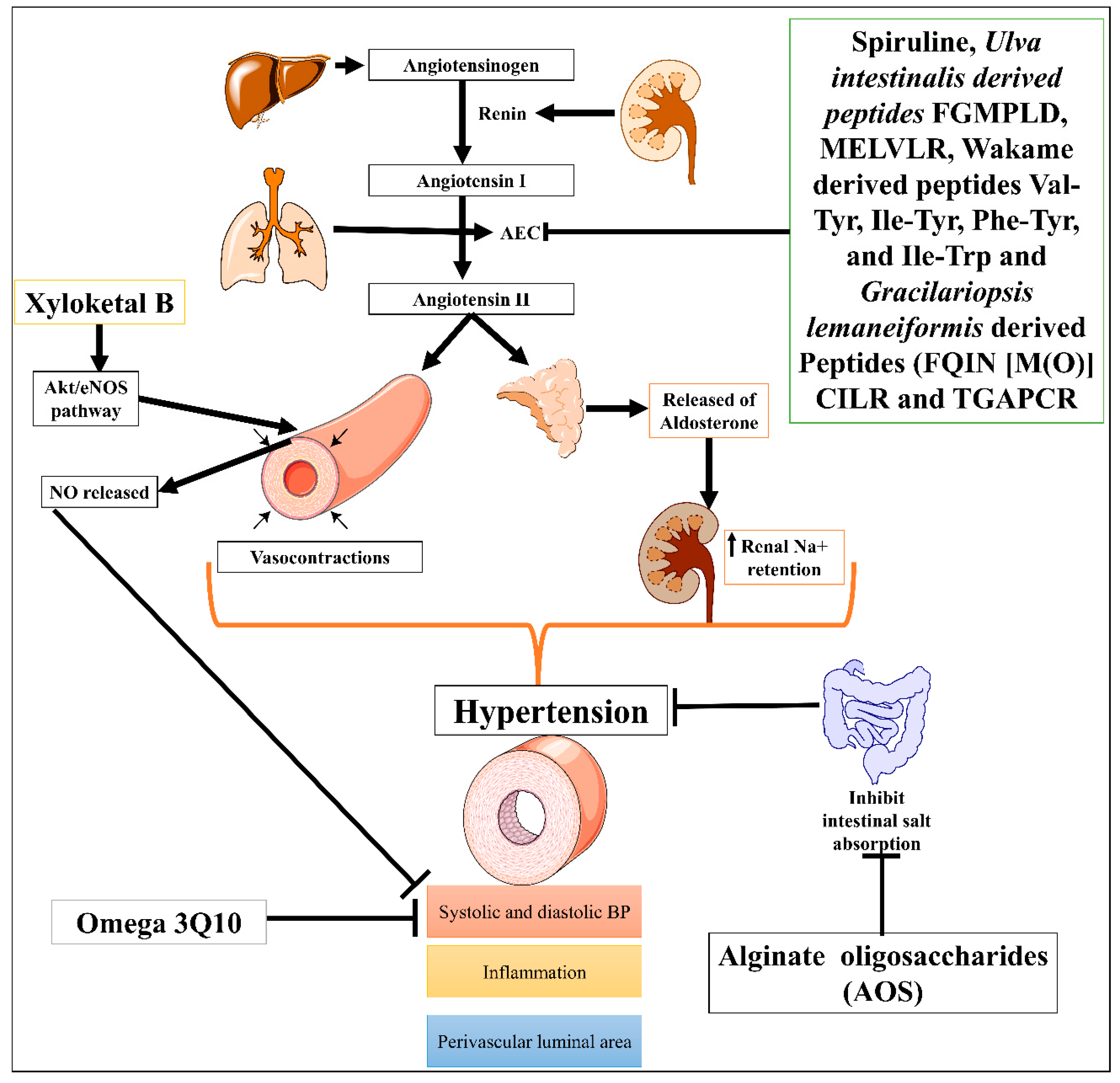
1. Hypertension
| S. No. | CVDs | Marine Drug Name | Species | Dose, Route and Time | MOA | Model Inducing Agents | Outcomes/Biological Effects | References |
|---|---|---|---|---|---|---|---|---|
| 1. | Hypertension | Protein hydrolysate Ulva intestinalis derived peptides FGMPLD and MELVLR | In vitro | 2.5 mg/mL of each hydrolysate | Inhibit ACE | ACE-induced hypertension | Antihypertensive effect | [9] |
| Wakame (Undaria pinnatifida) derived peptides (Val-Tyr, Ile-Tyr, Phe-Tyr, and Ile-Trp) | Rats | 1 mg/kg | Inhibit ACE | Spontaneously hypertensive rats | Antihypertensive effect | [8] | ||
| Low molecular mass potassium alginate (L-PA) | Rats | 250, 500 mg/kg, once orally for 30 days | Increased the excretion of sodium salt | Deoxycorticosterone acetate (DOCA)-salt-induced hypertension | Antihypertensive effect | [15] | ||
| Alginate oligosaccharides (AOS) | Rats | 5, 10 and 20 mg/kg for 5 weeks | Suppressed intestinal absorption of salts leads to vasodilatory effect | Monocrotaline (MCT)-induced pulmonary hypertension | Decrease P-selectin expression in serum, pulmonary tissue, and pulmonary arteries | [10] | ||
| Gracilariopsis lemaneiformis derived Peptides (FQIN [M(O)] CILR and TGAPCR) | 10 mg/kg, orally for 24 hrs. | Inhibit angiotensin-converting enzyme (ACE) | ACE-induced hypertension | Antihypertensive effects, reduced both systolic and diastolic blood pressure | [7][16] | |||
| Xyloketal B | 20 mg/kg/day, 20 for 12 weeks | Promoted endothelial NO release and protected against atherosclerosis through the Akt/eNOS pathway. | Phenylephrine (Phe)-induced contractions cause hypertension | Antihypertensive effect, Decrease the systolic and diastolic blood pressure, vasorelaxant effect, anti-inflammatory and anti-atherosclerotic effects |
[12] | |||
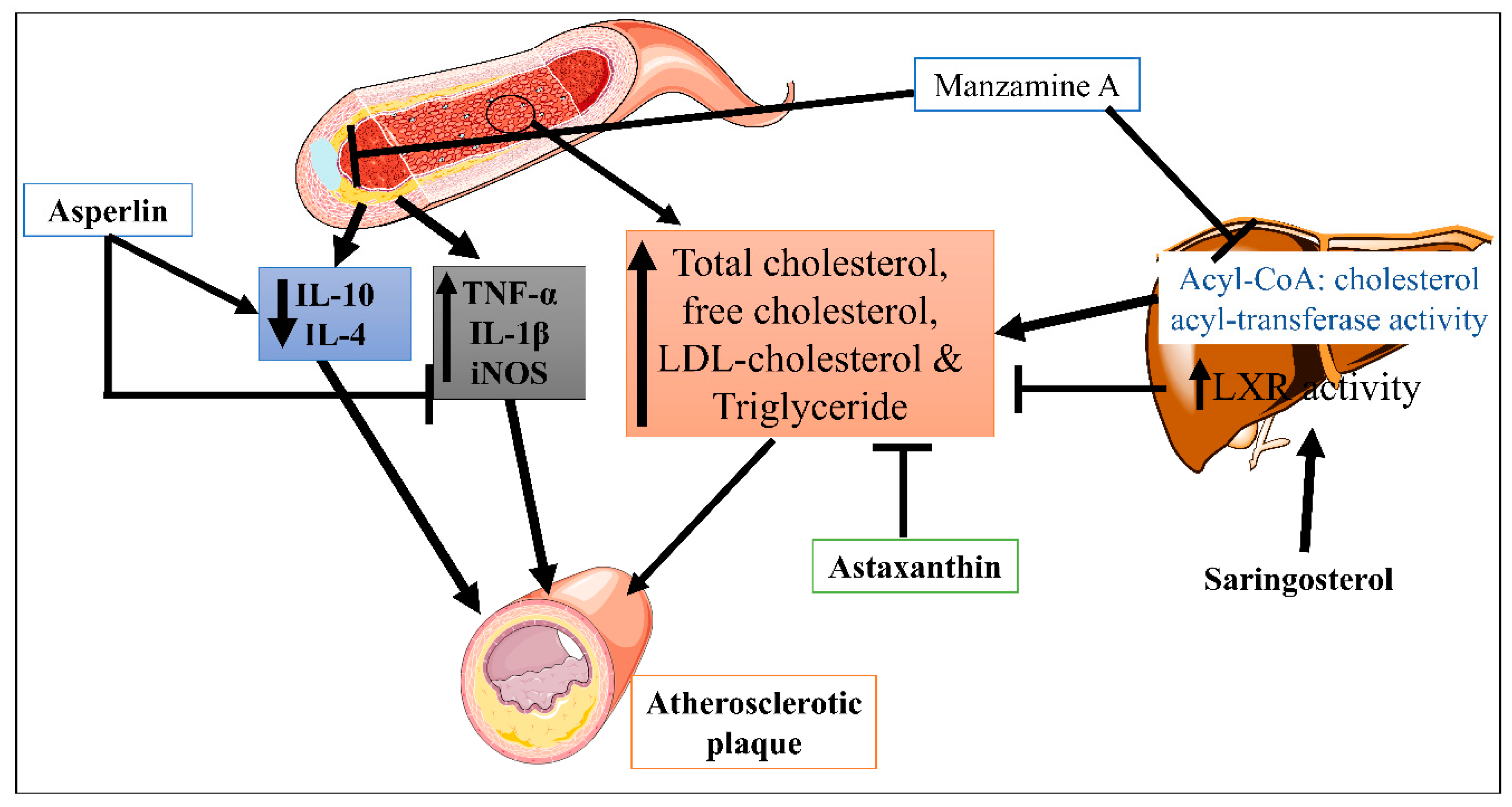
| 2. | Atherosclerosis | Asperlin | Mice | 80 mg/kg/day, orally for 12 weeks | Inhibit the pro-inflammatory markers | In vitro (LPS-induced foam cell formation in macrophages) and in vivo (high-fat diet-induced-atherosclerosis lesion in ApoE−/− mice) | Athero-protection via decreasing the expression levels of iNOS, IL-1β, and TNF-α, and increased the expression of IL-10 and IL-4, | [17] |
| Xyloketal B | 7, 14 and 28 mg/kg/day, orally for 16 weeks | Inhibit the oxidative endothelial dysfunction and increase nitric oxide (NO) bioavailability | High-fat diet-induced atherosclerotic lesion | Strong antioxidant actions, reduced the levels of vascular oxidative stress, improving the impaired endothelium integrity and NO-dependent aortic vasorelaxation in atherosclerotic | [18] | |||
| Saringosterol | Mice | 50 mg/kg/day, orally for 2 weeks | Altered the liver X receptor (LXR)-regulated gene expression | High-fat diet-induced atherosclerosis | Decrease cholesterol level and anti-atherogenic effect | [19] | ||
| Manzamine | ApoE-/- deficient mice |
30 mg/kg/day, orally for 80 days | Inhibited the acyl-CoA: cholesterol acyl-transferase (ACAT) activity | Decrease the level of total, free and LDL-cholesterol, and triglycerides | [20] | |||
| Astaxanthin | ApoE-/- deficient mice |
0.03% (equivalent to approx. 200 mg/day in humans), orally for 4 weeks | By increasing the expression of LDL receptor (LDLR) | High-fat diet (high fat 15% and high cholesterol 0.2%)-induced atherosclerosis | Decrease the level of total triglyceride, and cholesterol | [21] | ||
| Vitamin E | Rabbit | 450 mg/1000 g chow fed orally for 6-weeks | Decrease creatine kinase elevation | High cholesterol-enriched diet induced atherosclerosis | Lowered aortic TBARS levels, favorable prostanoid generation, and diminished atherosclerotic lesions | [22] | ||
| Fascaplysin | BALB/c mice | 5 mg/kg, intraperitoneally 19 h and 1 h before inducing thrombus | Inhibited kinase enzyme, and decreased GPIIb/IIIa activation | Photochemical-induced thrombus | Anti-platelet, and anti-thrombus effect via inhibiting GPIIb/IIIa integrin complex | [23] | ||
| Isaridin E | C57BL/6J mice | 12.5, 25, 50 and 100 mg/kg, orally at 1, 24 and 48 h before FeCl3-Induced thrombus | Inhibited adenosine diphosphate | FeCl3-induced thrombus | Antithrombotic, and antiplatelet effect in atherosclerosis | [24] | ||
| 3. | Myocardial Infarction (MI) | Cyanobacterial extract (CBE) and CBE+ GNPs | Rats | 200 mg/kg/day, intraperitoneally for 14 days | Inhibit the depletion of the anti-oxidant enzymes (GRx and SOD) | Isoproterenol-induced MI | Decrease ST and QT segments, heart rate, and serum activities of creatine phosphokinase (CPK), reduced systolic and diastolic blood pressure | [25] |
| Docosahexaenoic acid (DHA) | Pig | 45 mg or 1 mg/kg, infused in pericardial space for 40 min. | Inhibited Ca2+ and Na+/Ca2+ exchanger currents and prevented intracellularly Ca2+ concentration | Sternotomy method was used to expose the heart and induce MI | Decrease fatal arrhythmias and infarct sizes, decrease heart rates and reduce ventricular arrhythmia scores during ischemia. | [26][27] | ||
| 4. | Cardiac Stroke | Xyloketal B | Mice | 50 mg/kg intraperitoneally 0, 1 and 2 h. after ischemia | By suppressing TLR4/NF-κB/ROS signaling pathway | Transient middle cerebral artery occlusion-induced stroke | Decrease ROS production, focal cerebral ischemia, and reduce infarction volume. | [28] |
| 5. | Cardiac Arrythmia | Botulinum toxin-chitosan nanoparticles (BTN) | Rat | 5 U/kg, subepicardial injection for 14 days | Decreased the activation of Ca2+, K+ and Na+ channels | Calcium chloride-, barium chloride- and electrically induced arrhythmia | Inhibit ventricular fibrillation, reduce the incidence of ventricular arrhythmias | [29] |
| Eicosapentaenoic acid (EPA) | Dog | 5–15 μmol/L, intravenous infusion for 50–60 min. | Inhibition of Ca2+ and Na+/Ca2+ exchanger currents increase Ca2+ concentrations intracellularly | High Ca2+, ouabain, lysophosphatidylcholine, acylcarnitine, β-adrenergic agonist, and Ca2+ ionophore-induced arrhythmia | Inhibit cardiac arrhythmia through inhibition of fatal ischemia, prevents tachyarrhythmias | [26][30] | ||
| 6. | Heart value disease | Fucoxanthin (Fx) | Dog | 60 mg/kg twice daily for 2 years | Reduced oxidative stress-induced DNA damage | H2O2-induced oxidative stress-induced heart value damages | Strong antioxidant, anti-inflammatory, and antitumor properties, improved cell survival and, protective effect against calcification | [31] |
| 7. | Cardiac dysfunction | Zeaxanthin (ZH) | Rats | 250 μg/kg, orally for 4 weeks | Elevated retinoid receptor alpha (RAR-α) expression in cardiac tissue | d-galactose-induced cardiac dysfunction | Improve serum levels of homocysteine, creatinine kinase isoenzyme and lactate dehydrogenase, increase the cardiac contents of glucose transporter-4 and superoxide dismutase, decrease inducible nitric oxide synthetase and interleukin-6 | [32] |
2. Atherosclerosis
3. Myocardial Infarction (MI)
4. Ischemic Heart Disease (IHD)
5. Cardiac Stroke
6. Cardiac Arrhythmia
7. Cardiac Dysfunction
8. Heart Valve Disease or Valvular Heart Disease
| Class | Marine Drugs | Marine Source | Biological Effects | References |
|---|---|---|---|---|
| Pigments (Xanthophyll carotenoid) |
Astaxanthin | Microalgae (Haematococcus pluvialis, Chlorella zofingiensis, and Chlorococcum sp.), fungi (red yeast Phaffia rhodozyma) crustacean, Shrimp, lobster, trout, krill, salmon, fungi, complex plants, seafood, flamingos, and quail |
Cardioprotective (atherosclerosis protective), antidepressant, antioxidant, anti-inflammatory, neuroprotective, anticancer, antidiabetic, gastrointestinal protective, and hepatoprotective. |
[75][76][77][78][79] |
| Fucoxanthin | Macroalgae (Undaria pinnatifida, Hijikia fusiformis and Sargassum fulvelum) | Cardioprotective, Antioxidant, thermogenesis, stroke prevention, anti-inflammatory, anticancer, and improved blood pressure and liver function. | [59] | |
| Soluble dietary fibers | Alginate/Alginic acid | Brown macroalgae (Pseudomonas and Azotobacter, Pseudomonas aeruginosa, Azotobacter chroococcum) | Cardioprotective (used in myocardial infarction), antimicrobial, anti-inflammatory, anticancer, and antidiabetic. | [79][80][81][82] |
| Carrageenan | Red macroalgae Chondrus armatus (Gigartinaceae), Eucheuma, Betaphycus, Kappaphycus, and Chondrus crispus |
Cardioprotective (used for ischemic heart disease), immunomodulator, anti-hypercholesterolaemic, anti-inflammatory, anticancer, and antivirus properties. | [83] | |
| Agar | Gelidium, Pterocladia, and Gracilaria gracilis (Rhodophyta) | Cardioprotective, anticoagulant, antiviral, antioxidative, anticancer, and immune-modulating activities. | [79][80][84] | |
| Fucoidans | Fucus vesiculosus and L. japonica | Cardioprotective, coagulant activity. | ||
| Ulvans | Ulva pertusua | Anti-oxidant activity. | ||
| Peptides | Leu-Lys-Gln-Glu-Leu-Glu-Asp-Leu-Leu-Glu- Lys-Gln-Glu | Crassostrea gigas | Anticancer, antihypertensive, anti-thrombosis, antioxidant, and anticoagulant properties. | [79][80] |
| Pepsin-hydrolyzed peptide (VECYGPNRPQF) | Seaweed (Chlorella vulgaris) | Potent antioxidant, anticancer, opioid agonists or antagonists, immunomodulatory, antithrombotic, anti-atherosclerotic, and antimicrobial activities. | [85] | |
| Antitumor polypeptide Y2 | Spirulina platensis | |||
| Phycobili protein byproduct | Porphyra columbina | Immunosuppressive effects through increasing IL-10 production and preventing the production of IFN-γ and TNF-α. | [86] | |
| Leu-Trp, Val-Tyr, Ile-Tyr, Phe-Tyr, and Ile-Tyr | U. pinnatifida | Antihypertensive effects. | [8] | |
| α and β subunits of phycoerythrin | Red seaweed (P. palmate) | ACE inhibition activity. | [87] | |
| Ile-Leu-Ala-Pro, Leu-Leu-Ala-Pro, and Met-Ala-Gly-Val-Asp-His-Ile | Macroalga (Palmaria palmata) |
Inhibited DPP-IV (ischemic cardiovascular disease marker). | [88] | |
| Ile-Pro and Ala-Phe-Leu | Chlorophyta U. rigida | ACE inhibition activity. | [16] | |
| Phlorotannins (phenolic compounds) | Phloroglucinol | Hyaleucerea fusiformis | Potent antioxidant effects, anti-inflammatory and anticancer effects, inhibit the hyaluronidase enzyme. | [79][80][89] |
| Phlorofucofuroeckol A | Eisenia bicyclis, Ecklonia cava (brown algae) | Antidiabetic, antihypertensive, antioxidant activity. | ||
| Minerals | Na, K, Mg, P, I, Zn, and Fe | Microalgae (Chlorococcum humicola and Chlorella vulgaris) | Used for the prevention and treatment of CVDs. | [79][80] |
| Na+/K+ ratio, Mg | Controls blood pressure, prevent metabolic syndrome and atherosclerosis. | |||
| NaCl | Increases arterial constriction and peripheral vascular resistance, increased blood pressure. | |||
| K+ | Decreases the blood pressure, preventing problems associated with high blood pressure. | |||
| Lipids | Eicosapentanoic acid | Microalga Nannochloropsis gaditana (NG) | Reduced inflammatory genes expression and inhibits platelets. | [80][90] |
| Arachidonic acid | Mortierella alpina (saprophytic, oleaginous soil fungus) | Activates the immune functions, pro-inflammatory properties, maintaining homeostasis, anticancer, cardioprotective, anti-psoriasis, anti-arteriosclerosis, and antiulcer properties. | [80][91] | |
| Sulphated fucans | Fucoidan | Brown seaweeds (Sargassum ilicifolium and Sargassum angustifolium) | Reduces lipid deposition in atherosclerosis, hypolipidemic effect controls obesity. CVDs |
[92][93] |
| Marine Neurotoxins | Tetrodotoxin (TTX) | Sea-slug Pleurobranchaea maculata and pufferfish Takifugu niphobles | Visceral analgesic, local anesthetic, controls cardiac contractions. |
[65][94][95][96] |
| Non-peptide neurotoxin | Saxitoxin (STX) | Dinoflagellates species from the genera Alexandrium, Gymnodinium, Centrodinium and Pyrodinium | Wound healing, corneal analgesic, controls myocardial impulse generation. |
[65][96][97] |
| Fungus | Xyloketal B | Mangrove fungus xylaria species | [98] |
This entry is adapted from the peer-reviewed paper 10.3390/md21030193
References
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global Epidemiology, Health Burden and Effective Interventions for Elevated Blood Pressure and Hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802.
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide Trends in Hypertension Prevalence and Progress in Treatment and Control from 1990 to 2019: A Pooled Analysis of 1201 Population-Representative Studies with 104 Million Participants. Lancet 2021, 398, 957–980.
- Erdmann, K.; Cheung, B.W.Y.; Schröder, H. The Possible Roles of Food-Derived Bioactive Peptides in Reducing the Risk of Cardiovascular Disease. J. Nutr. Biochem. 2008, 19, 643–654.
- Yokoyama, K.; Chiba, H.; Yoshikawa, M. Peptide Inhibitors for Angiotensin I-Converting Enzyme from Thermolysin Digest of Dried Bonito. Biosci. Biotechnol. Biochem. 1992, 56, 1541–1545.
- Wijesekara, I.; Kim, S.K. Angiotensin-I-Converting Enzyme (ACE) Inhibitors from Marine Resources: Prospects in the Pharmaceutical Industry. Mar. Drugs 2010, 8, 1080–1093.
- Abachi, S.; Bazinet, L.; Beaulieu, L. Antihypertensive and Angiotensin-i-Converting Enzyme (ACE)-Inhibitory Peptides from Fish as Potential Cardioprotective Compounds. Mar. Drugs 2019, 17, 613.
- Deng, Z.; Liu, Y.; Wang, J.; Wu, S.; Geng, L.; Sui, Z.; Zhang, Q. Antihypertensive Effects of Two Novel Angiotensin I-Converting Enzyme (Ace) Inhibitory Peptides from Gracilariopsis Lemaneiformis (Rhodophyta) in Spontaneously Hypertensive Rats (SHRs). Mar. Drugs 2018, 16, 299.
- Sato, M.; Hosokawa, T.; Yamaguchi, T.; Nakano, T.; Muramoto, K.; Kahara, T.; Funayama, K.; Kobayashi, A.; Nakano, T. Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Wakame (Undaria Pinnatifida) and Their Antihypertensive Effect in Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2002, 50, 6245–6252.
- Sun, S.; Xu, X.; Sun, X.; Zhang, X.; Chen, X.; Xu, N. Preparation and Identification of ACE Inhibitory Peptides from the Marine Macroalga Ulva Intestinalis. Mar. Drugs 2019, 17, 179.
- Hu, Y.; Feng, Z.; Feng, W.; Hu, T.; Guan, H.; Mao, Y. AOS Ameliorates Monocrotaline-Induced Pulmonary Hypertension by Restraining the Activation of P-Selectin/P38MAPK/NF-ΚB Pathway in Rats. Biomed. Pharmacother. 2019, 109, 1319–1326.
- Shen, T.; Xing, G.; Zhu, J.; Zhang, S.; Cai, Y.; Li, D.; Xu, G.; Xing, E.; Rao, J.; Shi, R. Effects of 12-Week Supplementation of Marine Omega-3 PUFA-Based Formulation Omega3Q10 in Older Adults with Prehypertension and/or Elevated Blood Cholesterol. Lipids Health Dis. 2017, 16, 253.
- Zhao, L.Y.; Li, J.; Huang, X.Q.; Wang, G.H.; Lv, X.F.; Meng, W.F.; Chen, W.L.; Pang, J.Y.; Lin, Y.C.; Sun, H.S.; et al. Xyloketal B Exerts Antihypertensive Effect in Renovascular Hypertensive Rats via the NO-SGC-CGMP Pathway and Calcium Signaling. Acta Pharmacol. Sin. 2018, 39, 875–884.
- Martínez-Sámano, J.; De Oca, A.T.M.; O.-Bocardo, O.I.L.; Torres-Durán, P.V.; Juárez-Oropeza, M.A. Spirulina Maxima Decreases Endothelial Damage and Oxidative Stress Indicators in Patients with Systemic Arterial Hypertension: Results from Exploratory Controlled Clinical Trial. Mar. Drugs 2018, 16, 496.
- Nuhu, A.A. Spirulina (Arthrospira): An Important Source of Nutritional and Medicinal Compounds. J. Mar. Biol. 2013, 2013, 325636.
- Chen, Y.Y.; Ji, W.; Du, J.R.; Yu, D.K.; He, Y.; Yu, C.X.; Li, D.S.; Zhao, C.-Y.; Qiao, K. yun Preventive Effects of Low Molecular Mass Potassium Alginate Extracted from Brown Algae on DOCA Salt-Induced Hypertension in Rats. Biomed. Pharmacother. 2010, 64, 291–295.
- Cho, C.H.; Lu, Y.A.; Kim, M.Y.; Jeon, Y.J.; Lee, S.H. Therapeutic Potential of Seaweed–Derived Bioactive Compounds for Cardiovascular Disease Treatment. Appl. Sci. 2022, 12, 1025.
- Zhou, Y.; Chen, R.; Liu, D.; Wu, C.; Guo, P.; Lin, W. Asperlin Inhibits LPS-Evoked Foam Cell Formation and Prevents Atherosclerosis in ApoE−/− Mice. Mar. Drugs 2017, 15, 358.
- Zhao, L.Y.; Li, J.; Yuan, F.; Li, M.; Zhang, Q.; Huang, Y.Y.; Pang, J.Y.; Zhang, B.; Sun, F.Y.; Sun, H.S.; et al. Xyloketal B Attenuates Atherosclerotic Plaque Formation and Endothelial Dysfunction in Apolipoprotein E Deficient Mice. Mar. Drugs 2015, 13, 2306–2326.
- Yan, Y.; Niu, Z.; Wang, B.; Zhao, S.; Sun, C.; Wu, Y.; Li, Y.; Ying, H.; Liu, H. Saringosterol from Sargassum Fusiforme Modulates Cholesterol Metabolism and Alleviates Atherosclerosis in ApoE-Deficient Mice. Mar. Drugs 2021, 19, 485.
- Eguchi, K.; Fujiwara, Y.; Hayashida, A.; Horlad, H.; Kato, H.; Rotinsulu, H.; Losung, F.; Mangindaan, R.E.P.; De Voogd, N.J.; Takeya, M.; et al. Manzamine A, a Marine-Derived Alkaloid, Inhibits Accumulation of Cholesterol Ester in Macrophages and Suppresses Hyperlipidemia and Atherosclerosis In Vivo. Bioorganic Med. Chem. 2013, 21, 3831–3838.
- Yang, Y.; Seo, J.M.; Nguyen, A.; Pham, T.X.; Park, H.J.; Park, Y.; Kim, B.; Bruno, R.S.; Lee, J. Astaxanthin-Rich Extract from the Green Alga Haematococcus Pluvialis Lowers Plasma Lipid Concentrations and Enhances Antioxidant Defense in Apolipoprotein E Knockout Mice. J. Nutr. 2011, 141, 1611–1617.
- Chen, M.F.; Hsu, H.C.; Liau, C.S.; Lee, Y.T. The Role of Vitamin E on the Anti-Atherosclerotic Effect of Fish Oil in Diet-Induced Hypercholesterolemic Rabbits. Prostaglandins Other Lipid Mediat. 1999, 57, 99–111.
- Ampofo, E.; Später, T.; Müller, I.; Eichler, H.; Menger, M.D.; Laschke, M.W. The Marine-Derived Kinase Inhibitor Fascaplysin Exerts Anti-Thrombotic Activity. Mar. Drugs 2015, 13, 6774–6791.
- Pan, N.; Li, Z.C.; Li, Z.H.; Chen, S.H.; Jiang, M.H.; Yang, H.Y.; Liu, Y.S.; Hu, R.; Zeng, Y.W.; Dai, L.H.; et al. Antiplatelet and Antithrombotic Effects of Isaridin E Isolated from the Marine-Derived Fungus via Downregulating the PI3K/Akt Signaling Pathway. Mar. Drugs 2022, 20, 23.
- Bakir, E.M.; Younis, N.S.; Mohamed, M.E.; El Semary, N.A. Cyanobacteria as Nanogold Factories: Chemical and Anti-Myocardial Infarction Properties of Gold Nanoparticles Synthesized by Lyngbya Majuscula. Mar. Drugs 2018, 16, 217.
- Xiao, Y.F.; Sigg, D.C.; Ujhelyi, M.R.; Wilhelm, J.J.; Richardson, E.S.; Iaizzo, P.A. Pericardial Delivery of Omega-3 Fatty Acid: A Novel Approach to Reducing Myocardial Infarct Sizes and Arrhythmias. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, 2212–2218.
- Desnoyers, M.; Gilbert, K.; Rousseau, G. Cardioprotective Effects of Omega-3 Polyunsaturated Fatty Acids: Dichotomy between Experimental and Clinical Studies. Mar. Drugs 2018, 16, 234.
- Pan, N.; Lu, L.Y.; Li, M.; Wang, G.H.; Sun, F.Y.; Sun, H.S.; Wen, X.J.; Cheng, J.D.; Chen, J.W.; Pang, J.Y.; et al. Xyloketal B Alleviates Cerebral Infarction and Neurologic Deficits in a Mouse Stroke Model by Suppressing the ROS/TLR4/NF-ΰ B Inflammatory Signaling Pathway. Acta Pharmacol. Sin. 2017, 38, 1236–1247.
- Sergeevichev, D.; Fomenko, V.; Strelnikov, A.; Dokuchaeva, A.; Vasilieva, M.; Chepeleva, E.; Rusakova, Y.; Artemenko, S.; Romanov, A.; Salakhutdinov, N.; et al. Botulinum Toxin-Chitosan Nanoparticles Prevent Arrhythmia in Experimental Rat Models. Mar. Drugs 2020, 18, 410.
- Kang, J.X.; Leaf, A. Prevention of Fatal Cardiac Arrhythmias by Polyunsaturated Fatty Acids. Am. J. Clin. Nutr. 2000, 71, 202S–207S.
- Chiang, Y.F.; Tsai, C.H.; Chen, H.Y.; Wang, K.L.; Chang, H.Y.; Huang, Y.J.; Hong, Y.H.; Ali, M.; Shieh, T.M.; Huang, T.C.; et al. Protective Effects of Fucoxanthin on Hydrogen Peroxide-Induced Calcification of Heart Valve Interstitial Cells. Mar. Drugs 2021, 19, 307.
- El-Baz, F.K.; Hussein, R.A.; Saleh, D.O.; Jaleel, G.A.R.A. Zeaxanthin Isolated from Dunaliella Salina Microalgae Ameliorates Age Associated Cardiac Dysfunction in Rats through Stimulation of Retinoid Receptors. Mar. Drugs 2019, 17, 290.
- Soehnlein, O.; Libby, P. Targeting Inflammation in Atherosclerosis–from Experimental Insights to the Clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610.
- Patil, N.P.; Le, V.; Sligar, A.D.; Mei, L.; Chavarria, D.; Yang, E.Y.; Baker, A.B. Algal Polysaccharides as Therapeutic Agents for Atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 153.
- Chen, Z.; Liu, J.; Fu, Z.; Ye, C.; Zhang, R.; Song, Y.; Zhang, Y.; Li, H.; Ying, H.; Liu, H. 24(S)-Saringosterol from Edible Marine Seaweed Sargassum Fusiforme Is a Novel Selective LXRβ Agonist. J. Agric. Food Chem. 2014, 62, 6130–6137.
- Munekata, P.E.S.; Pateiro, M.; Conte-Junior, C.A.; Domínguez, R.; Nawaz, A.; Walayat, N.; Fierro, E.M.; Lorenzo, J.M. Marine Alkaloids: Compounds with in Vivo Activity and Chemical Synthesis. Mar. Drugs 2021, 19, 374.
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A Potential Therapeutic Agent in Cardiovascular Disease. Mar. Drugs 2011, 9, 447–465.
- D’Alessandro, E.; Becker, C.; Bergmeier, W.; Bode, C.; Bourne, J.H.; Brown, H.; Buller, H.R.; Ten Cate-Hoek, A.J.; Ten Cate, V.; Van Cauteren, Y.J.M.; et al. Thrombo-Inflammation in Cardiovascular Disease: An Expert Consensus Document from the Third Maastricht Consensus Conference on Thrombosis. Thromb Haemost 2020, 120, 538–564.
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Platelet Activation and Prothrombotic Mediators at the Nexus of Inflammation and Atherosclerosis: Potential Role of Antiplatelet Agents. Blood Rev. 2021, 45, 100694.
- Palur Ramakrishnan, A.V.K.; Varghese, T.P.; Vanapalli, S.; Nair, N.K.; Mingate, M.D. Platelet Activating Factor: A Potential Biomarker in Acute Coronary Syndrome? Cardiovasc. Ther. 2017, 35, 64–70.
- Soni, R.; Muller, L.; Furet, P.; Schoepfer, J.; Stephan, C.; Zumstein-Mecker, S.; Fretz, H.; Chaudhuri, B. Inhibition of Cyclin-Dependent Kinase 4 (Cdk4) by Fascaplysin, a Marine Natural Product. Biochem. Biophys. Res. Commun. 2000, 275, 877–884.
- Jiang, M.; Wu, Z.; Wu, Q.; Yin, H.; Guo, H.; Yuan, S.; Liu, Z.; Chen, S.; Liu, L. Amphichoterpenoids A–C, Unprecedented Picoline-Derived Meroterpenoids from the Ascidian-Derived Fungus Amphichorda Felina SYSU-MS7908. Chin. Chem. Lett. 2021, 32, 1893–1896.
- Ren, R.; Azuma, Y.; Ojima, T.; Hashimoto, T.; Mizuno, M.; Nishitani, Y.; Yoshida, M.; Azuma, T.; Kanazawa, K. Modulation of Platelet Aggregation-Related Eicosanoid Production by Dietary F-Fucoidan from Brown Alga Laminaria Japonica in Human Subjects. Br. J. Nutr. 2013, 110, 880–890.
- Sakamoto, A.; Saotome, M.; Iguchi, K.; Maekawa, Y. Marine-Derived Omega-3 Polyunsaturated Fatty Acids and Heart Failure: Current Understanding for Basic to Clinical Relevance. Int. J. Mol. Sci. 2019, 20, 4025.
- Wu, Y.; Chang, T.; Chen, W.; Wang, X.; Li, J.; Chen, Y.; Yu, Y.; Shen, Z.; Yu, Q.; Zhang, Y. Release of VEGF and BMP9 from Injectable Alginate Based Composite Hydrogel for Treatment of Myocardial Infarction. Bioact. Mater. 2021, 6, 520–528.
- Wu, T.; Liu, W. Functional Hydrogels for the Treatment of Myocardial Infarction. NPG Asia Mater. 2022, 14, 1–15.
- Fan, C.; Joshi, J.; Li, F.; Xu, B.; Khan, M.; Yang, J.; Zhu, W. Nanoparticle-Mediated Drug Delivery for Treatment of Ischemic Heart Disease. Front. Bioeng. Biotechnol. 2020, 8, 687.
- Ogita, H.; Node, K.; Asanuma, H.; Sanada, S.; Takashima, S.; Minamino, T.; Soma, M.; Kim, J.; Hori, M.; Kitakaze, M. Eicosapentaenoic Acid Reduces Myocardial Injury Induced by Ischemia and Reperfusion in Rabbit Hearts. J. Cardiovasc. Pharmacol. 2003, 41, 964–969.
- Severino, P.; D’Amato, A.; Prosperi, S.; Magnocavallo, M.; Mariani, M.V.; Netti, L.; Birtolo, L.I.; De Orchi, P.; Chimenti, C.; Maestrini, V.; et al. Potential Role of ENOS Genetic Variants in Ischemic Heart Disease Susceptibility and Clinical Presentation. J. Cardiovasc. Dev. Dis. 2021, 8, 116.
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349.
- Papier, K.; Knuppel, A.; Syam, N.; Jebb, S.A.; Key, T.J. Meat Consumption and Risk of Ischemic Heart Disease: A Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 426–437.
- Park, J.H.; Lee, N.K.; Lim, H.J.; Mazumder, S.; Rethineswaran, V.K.; Kim, Y.J.; Jang, W.B.; Ji, S.T.; Kang, S.; Kim, D.Y.; et al. Therapeutic Cell Protective Role of Histochrome under Oxidative Stress in Human Cardiac Progenitor Cells. Mar. Drugs 2019, 17, 368.
- Dwivedi, R.; Pomin, V.H. Marine Antithrombotics. Mar. Drugs 2020, 18, 514.
- Zhong, R.; Wan, X.; Wang, D.; Zhao, C.; Liu, D.; Gao, L.; Wang, M.; Wu, C.J.; Nabavid, S.M.; Daglia, M.; et al. Polysaccharides from Marine Enteromorpha: Structure and Function. Trends Food Sci. Technol. 2020, 99, 11–20.
- Liang, B.; Cai, X.-Y.; Gu, N. Marine Natural Products and Coronary Artery Disease. Front. Cardiovasc. Med. 2021, 8, 739932.
- Akil, L.; Anwar Ahmad, H. Relationships between Obesity and Cardiovascular Diseases in Four Southern States and Colorado. J. Health Care Poor Underserved 2011, 22, 61–72.
- Bhatia, S.; Makkar, R.; Behl, T.; Sehgal, A.; Singh, S.; Rachamalla, M.; Mani, V.; Iqbal, M.S.; Bungau, S.G. Biotechnological Innovations from Ocean: Transpiring Role of Marine Drugs in Management of Chronic Disorders. Molecules 2022, 27, 1539.
- Zhao, J.; Cao, Q.; Xing, M.; Xiao, H.; Cheng, Z.; Song, S.; Ji, A. Advances in the Study of Marine Products with Lipid-Lowering Properties. Mar. Drugs 2020, 18, 390.
- Riccioni, G.; D’Orazio, N.; Franceschelli, S.; Speranza, L. Marine Carotenoids and Cardiovascular Risk Markers. Mar. Drugs 2011, 9, 1166–1175.
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential Anti-Atherosclerotic Properties of Astaxanthin. Mar. Drugs 2016, 14, 35.
- Chen, W.L.; Qian, Y.; Meng, W.F.; Pang, J.Y.; Lin, Y.C.; Guan, Y.Y.; Chen, S.P.; Liu, J.; Pei, Z.; Wang, G.L. A Novel Marine Compound Xyloketal B Protects against Oxidized LDL-Induced Cell Injury in Vitro. Biochem. Pharmacol. 2009, 78, 941–950.
- Blackwell, D.J.; Schmeckpeper, J.; Knollmann, B.C. Animal Models to Study Cardiac Arrhythmias. Circ. Res. 2022, 130, 1926–1964.
- Do, H.K.; Kogure, K.; Imada, C.; Noguchi, T.; Ohwada, K.; Simidu, U. Tetrodotoxin Production of Actinomycetes Isolated from Marine Sediment. J. Appl. Bacteriol. 1991, 70, 464–468.
- Hong, B.; He, J.; Le, Q.; Bai, K.; Chen, Y.; Huang, W. Combination Formulation of Tetrodotoxin and Lidocaine as a Potential Therapy for Severe Arrhythmias. Mar. Drugs 2019, 17, 685.
- Mackieh, R.; Abou-Nader, R.; Wehbe, R.; Mattei, C.; Legros, C.; Fajloun, Z.; Sabatier, J.M. Voltage-Gated Sodium Channels: A Prominent Target of Marine Toxins. Mar. Drugs 2021, 19, 562.
- Saravanan, P.; Davidson, N.C.; Schmidt, E.B.; Calder, P.C. Cardiovascular Effects of Marine Omega-3 Fatty Acids. Lancet 2010, 376, 540–550.
- Bali, J.; Thakur, R. Poison as Cure: A Clinical Review of Botulinum Toxin as an Invaluable Drug. J. Venom. Anim. Toxins Incl. Trop. Dis. 2005, 11, 412–421.
- Ertl, G.; Gaudran, P.; Neubauer, S.; Bauer, B.; Horn, M.; Hu, K.; Tian, R. Cardiac Dysfunction and Development of Heart Failure. Eur. Heart J. 1993, 14, 33–37.
- Rhee, E.J.; Plutzky, J. Retinoid Metabolism and Diabetes Mellitus. Diabetes Metab. J. 2012, 36, 167–180.
- Tang, X.H.; Gudas, L.J. Retinoids, Retinoic Acid Receptors, and Cancer. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 345–364.
- Shao, M.; Lu, L.; Wang, Q.; Ma, L.; Tian, X.; Li, C.; Li, C.; Guo, D.; Wang, Q.; Wang, W.; et al. The Multi-Faceted Role of Retinoid X Receptor in Cardiovascular Diseases. Biomed. Pharmacother. 2021, 137, 111264.
- Zhu, S.; Guleria, R.S.; Thomas, C.M.; Roth, A.; Gerilechaogetu, F.; Kumar, R.; Dostal, D.E.; Baker, K.M.; Pan, J. Loss of Myocardial Retinoic Acid Receptor α Induces Diastolic Dysfunction by Promoting Intracellular Oxidative Stress and Calcium Mishandling in Adult Mice. J. Mol. Cell. Cardiol. 2016, 99, 100–112.
- Guleria, R.S.; Singh, A.B.; Nizamutdinova, I.T.; Souslova, T.; Mohammad, A.A.; Kendall, J.A.; Baker, K.M.; Pan, J. Activation of Retinoid Receptor-Mediated Signaling Ameliorates Diabetes-Induced Cardiac Dysfunction in Zucker Diabetic Rats. J. Mol. Cell. Cardiol. 2013, 57, 106–118.
- Méresse, S.; Fodil, M.; Fleury, F.; Chénais, B. Fucoxanthin, a Marine-Derived Carotenoid from Brown Seaweeds and Microalgae: A Promising Bioactive Compound for Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 9273.
- Catanesi, M.; Caioni, G.; Castelli, V.; Benedetti, E.; D’angelo, M.; Cimini, A. Benefits under the Sea: The Role of Marine Compounds in Neurodegenerative Disorders. Mar. Drugs 2021, 19, 24.
- Liu, X.; Shibata, T.; Hisaka, S.; Osawa, T. Astaxanthin Inhibits Reactive Oxygen Species-Mediated Cellular Toxicity in Dopaminergic SH-SY5Y Cells via Mitochondria-Targeted Protective Mechanism. Brain Res. 2009, 1254, 18–27.
- Fakhri, S.; Aneva, I.Y.; Farzaei, M.H.; Sobarzo-Sánchez, E. The Neuroprotective Effects of Astaxanthin: Therapeutic Targets and Clinical Perspective. Molecules 2019, 24, 2640.
- Ikeda, Y.; Tsuji, S.; Satoh, A.; Ishikura, M.; Shirasawa, T.; Shimizu, T. Protective Effects of Astaxanthin on 6-Hydroxydopamine-Induced Apoptosis in Human Neuroblastoma SH-SY5Y Cells. J. Neurochem. 2008, 107, 1730–1740.
- Suleria, H.A.R.; Gobe, G.; Masci, P.; Osborne, S.A. Marine Bioactive Compounds and Health Promoting Perspectives; Innovation Pathways for Drug Discovery. Trends Food Sci. Technol. 2016, 50, 44–55.
- Cardoso, S.M.; Pereira, O.R.; Seca, A.M.L.; Pinto, D.C.G.A.; Silva, A.M.S. Seaweeds as Preventive Agents for Cardiovascular Diseases: From Nutrients to Functional Foods. Mar. Drugs 2015, 13, 6838–6865.
- Spoială, A.; Ilie, C.I.; Ficai, D.; Ficai, A.; Andronescu, E. From Biomedical Applications of Alginate towards CVD Implications Linked to COVID-19. Pharmaceuticals 2022, 15, 318.
- Xu, Z.; Lam, M.T. Alginate Application for Heart and Cardiovascular Diseases. Springer Ser. Biomater. Sci. Eng. 2018, 11, 185–212.
- Sokolova, E.V.; Bogdanovich, L.N.; Ivanova, T.B.; Byankina, A.O.; Kryzhanovskiy, S.P.; Yermak, I.M. Effect of Carrageenan Food Supplement on Patients with Cardiovascular Disease Results in Normalization of Lipid Profile and Moderate Modulation of Immunity System Markers. PharmaNutrition 2014, 2, 33–37.
- Gioele, C.; Marilena, S.; Valbona, A.; Nunziacarla, S.; Andrea, S.; Antonio, M. Gracilaria Gracilis, Source of Agar: A Short Review. Curr. Org. Chem. 2017, 21, 380–386.
- Zhang, B.; Zhang, X. Separation and Nanoencapsulation of Antitumor Polypeptide from Spirulina Platensis. Biotechnol. Prog. 2013, 29, 1230–1238.
- Cian, R.E.; Martínez-Augustin, O.; Drago, S.R. Bioactive Properties of Peptides Obtained by Enzymatic Hydrolysis from Protein Byproducts of Porphyra Columbina. Food Res. Int. 2012, 49, 364–372.
- Furuta, T.; Miyabe, Y.; Yasui, H.; Kinoshita, Y.; Kishimura, H. Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Phycobiliproteins of Dulse Palmaria Palmata. Mar. Drugs 2016, 14, 32.
- Harnedy, P.A.; O’Keeffe, M.B.; Fitzgerald, R.J. Purification and Identification of Dipeptidyl Peptidase (DPP) IV Inhibitory Peptides from the Macroalga Palmaria Palmata. Food Chem. 2015, 172, 400–406.
- You, H.N.; Lee, H.A.; Park, M.H.; Lee, J.H.; Han, J.S. Phlorofucofuroeckol A Isolated from Ecklonia Cava Alleviates Postprandial Hyperglycemia in Diabetic Mice. Eur. J. Pharmacol. 2015, 752, 92–96.
- Lozano-Muñoz, I.; Muñoz, S.; Díaz, N.F.; Medina, A.; Bazaes, J.; Riquelme, C. Nutritional Enhancement of Farmed Salmon Meat via Non-GMO Nannochloropsis Gaditana: Eicosapentaenoic Acid (EPA, 20:5 n-3), Docosapentaenoic Acid (DPA, 22:5 n-3) and Vitamin D3 for Human Health. Molecules 2020, 25, 4615.
- Rayaroth, A.C.; Tomar, R.S.; Mishra, R.K. Arachidonic Acid Synthesis in Mortierella Alpina: Origin, Evolution and Advancements. Proc. Natl. Acad. Sci. India Sect. B–Biol. Sci. 2017, 87, 1053–1066.
- Agarwal, S.; Chauhan, K. Fucoidan: A Promising Target for Dyslipidemia-A Concise Review. Pharma Innov. J. 2019, 8, 62–67.
- Kordjazi, M.; Etemadian, Y.; Shabanpour, B.; Pourashouri, P. Chemical Composition Antioxidant and Antimicrobial Activities of Fucoidan Extracted from Two Species of Brown Seaweeds (Sargassum Ilicifolium and S.Angustifolium) around Qeshm Island. Iran. J. Fish. Sci. 2019, 18, 457–475.
- Mattei, C. Tetrodotoxin, a Candidate Drug for Nav1.1-Induced Mechanical Pain? Mar. Drugs 2018, 16, 72.
- González-Cano, R.; Tejada, M.Á.; Artacho-Cordón, A.; Nieto, F.R.; Entrena, J.M.; Wood, J.N.; Cendán, C.M. Effects of Tetrodotoxin in Mouse Models of Visceral Pain. Mar. Drugs 2017, 15, 188.
- Biessy, L.; Smith, K.F.; Wood, S.A.; Tidy, A.; van Ginkel, R.; Bowater, J.R.D.; Hawes, I. A Microencapsulation Method for Delivering Tetrodotoxin to Bivalves to Investigate Uptake and Accumulation. Mar. Drugs 2021, 19, 33.
- Durán-Riveroll, L.M.; Cembella, A.D. Guanidinium Toxins and Their Interactions with Voltage-Gated Sodium Ion Channels. Mar. Drugs 2017, 15, 303.
- Xiao, A.J.; Chen, W.; Xu, B.; Liu, R.; Turlova, E.; Barszczyk, A.; Sun, C.L.; Liu, L.; Deurloo, M.; Wang, G.L.; et al. Marine Compound Xyloketal B Reduces Neonatal Hypoxic-Ischemic Brain Injury. Mar. Drugs 2015, 13, 29–47.
