Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Tyrosyl-DNA phosphodiesterase 1 (TDP1) is an important repair enzyme that removes various covalent adducts from the 3′ end of DNA. Particularly, covalent complexes of topoisomerase 1 (TOP1) with DNA stabilized by DNA damage or by various chemical agents are an examples of such adducts.
- natural compound
- derivative
- TDP1 inhibitors
1. Introduction
Tyrosyl-DNA phosphodiesterase 1 (TDP1) is a repair enzyme for “stalled” DNA -topoisomerase 1 (TOP1) cleavage complexes and other 3′ end DNA lesions. TDP1 plays a crucial role in the repair of DNA lesions formed by antitumor drugs such as the TOP1 inhibitors camptothecin, topotecan (a TOP1 poison in clinical use), and irinotecan; therefore, TDP1 is a promising target for adjunctive cancer treatment.
Because homologs of TDP1 have been present in organisms throughout most of evolution [1], there is a real possibility that natural biomolecules could have evolved to interact with this protein as well. In addition, a combination of antitumor and tumor-chemosensitizing properties of drugs can lead to an enhanced therapeutic effect, and many natural compounds have intrinsic antitumor properties. For instance, since the 1980s, approximately 40% of all approved anticancer drugs worldwide have been either natural substances or compounds derived from pharmacophores of natural substances [2]. Natural substances and compounds derived from natural pharmacophores are known to effectively disrupt many critical processes involved in the pathogenesis of many cancer types [3].
2. Tyrosyl-DNA Phosphodiesterase 1
TDP1 was discovered in the yeast Saccharomyces cerevisiae as an enzyme that hydrolyzes the covalent bond between a tyrosine residue and a 3′-phosphate group of DNA. Because the only known enzyme that forms the 3′-phosphotyrosyl linkage in vivo is DNA topoisomerase I (TOP1), some authors proposed that the detected activity is involved in the repair of lesions in DNA resulting from the formation of irreversible Top1–DNA complexes [4]. Later, it was shown [5] that some predicted but unidentified genes of eukaryotic organisms contain a sequence that plays a role in the hydrolysis of the covalent bond between a tyrosine residue and a 3′-phosphate group of DNA. This led to the hypothesis that TDP1 or its homologs are present in all eukaryotic organisms that had been studied [5].
A crystallographic study has revealed that human TDP1 is a monomer and consists of two topologically similar α-β-α domains, and each domain carries a conserved amino acid sequence, so-called HKN motif characteristic of TDP1 orthologs (for human TDP1: H263 K265 N283 and H493 K495 N516) [6][7]. These two HKN motifs are brought together in a tertiary structure, thereby forming a catalytic site located inside a narrow asymmetric substrate-binding channel, which has a positive charge for binding single-stranded DNA [6][7]. DNA stabilization is implemented via both hydrophobic and polar interactions, and therefore TDP1 is able to recognize a substrate regardless of its nucleotide sequence. This observation is consistent with the fact that TOP1–DNA complexes can form on different sequences, and TDP1 should perform the catalysis efficiently regardless of the nucleotide composition of this sequence [6][8].
TDP1 can carry out the catalytic process independently of cofactors or metals. TDP1 hydrolyzes the TOP1–DNA complex in two steps with the formation of a transient covalent complex [5] (Figure 1). The first step is a nucleophilic attack of the phosphotyrosyl bond of TOP1–DNA by His263 provided by the N-terminal HKN motif. Residue His493 from the opposite HKN motif acts as an acid and donates a proton to TOP1s leaving the tyrosine group (Figure 1A). A transient covalent phosphamide bond arises between His263 and the 3′ end of the DNA (Figure 1B). A nucleophilic attack by a water molecule activated by His493 hydrolyzes the phosphohistidine intermediate and free TDP1 (Figure 1C) [7]. The outcome is DNA with a free 3′-phosphate end (Figure 1D) which needs subsequent processing by polynucleotide kinase phosphatase (PNKP) [9][10].
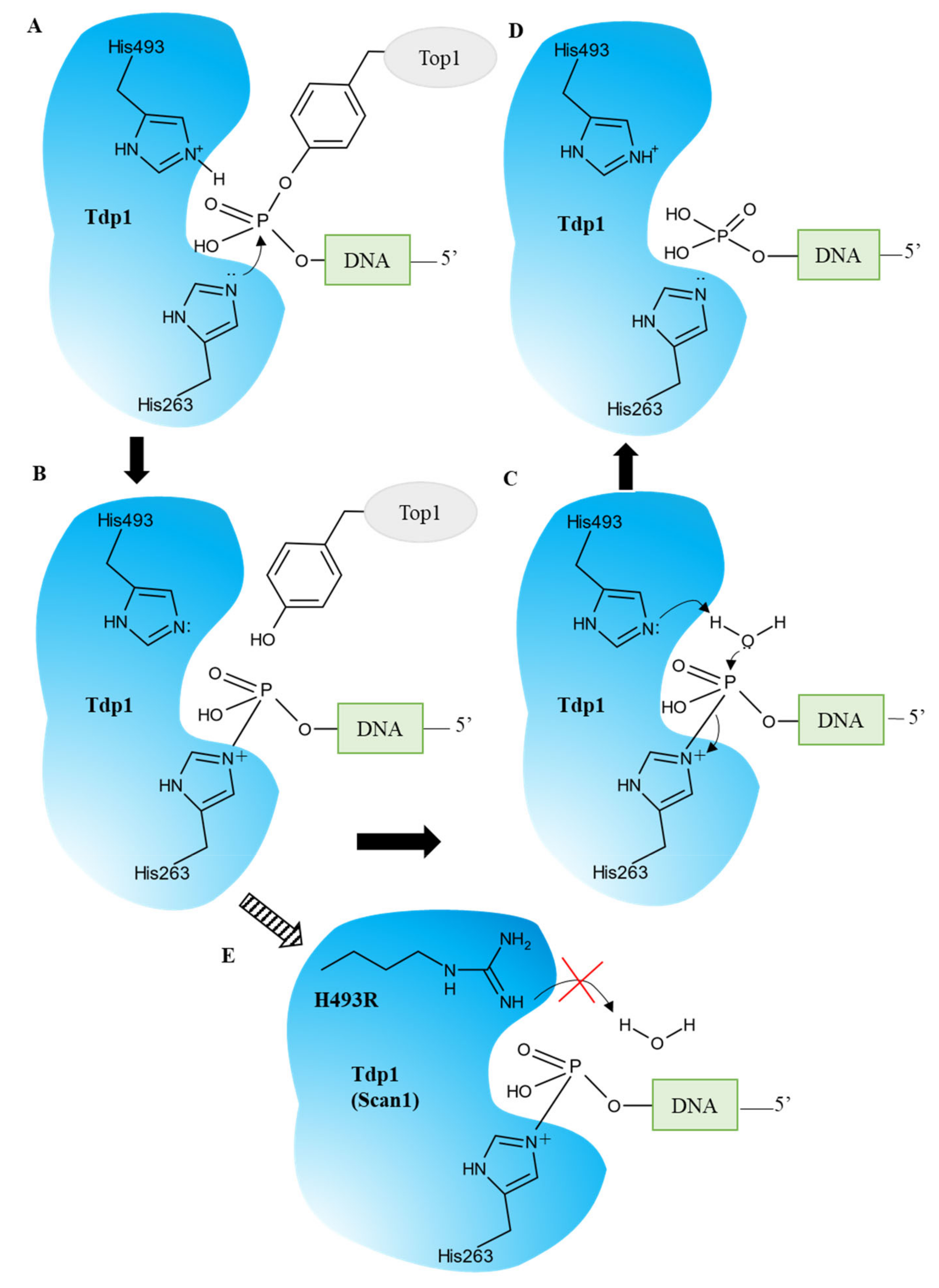
Figure 1. TDP1′s catalytic cycle. (A) The nucleophilic attack of the phosphodiester backbone by the imidazole N2 atom of H263. H493 donates a proton to the tyrosyl moiety of the leaving group. (B) The phosphohistidine covalent intermediate. (C) The second nucleophilic attack mediated by a water molecule activated by H493. (D) The emergence of the final 3′-phosphate product and free TDP1. (E) The SCAN1 mutation (H493R) leads to the accumulation of the TDP1–DNA intermediate and a fall in the TDP1 turnover rate.
A natural mutation, H493R, in the TDP1 gene leads to a neurodegenerative disease called spinocerebellar ataxia syndrome with axonal neuropathy (SCAN1), and the cause of this disease is not a loss of function or activity of TDP1 but a gain of function: the formation of stable covalent complexes of the mutant enzyme with DNA (Figure 1E) [11][12]. It has been hypothesized that inhibition of the mutant enzyme for preventing the formation of these complexes can improve the clinical condition of patients and/or slow down the development of the disease.
2.1. TDP1 as a Target for Anticancer Therapy
Originally, TDP1 was discovered as an enzyme capable of hydrolyzing the 3′-phosphotyrosyl bond between TOP1 and a 3′-end of DNA [4]. This enzyme has been extensively studied, and it has been found that, aside from the hydrolysis of the 3′-phosphotyrosyl bond of TOP1, TDP1 can cleave a wide range of physiological and pharmacological 3′-blocking lesions [11] and has the ability to cleave 5′-phosphotyrosyl bonds [13][14].
The use of TDP1 as a target of anticancer therapy for enhancing the action of TOP1 inhibitors was proposed by Nash’s research group [4] as early as 1996. Later, it was shown that overexpression of TDP1 is associated with chromosomal instability [15] and is observed in such types of cancer as non-small-cell lung cancer [16] and colorectal cancer [17] as well as in cell lines derived from breast cancer [18] and some rhabdomyosarcomas [19]. Additionally, overexpression of TDP1 protects cells from both camptothecin and its derivatives aimed at suppressing TOP1 activity [20] and from etoposide, which is intended to suppress the activity of TOP2 [20], which forms 5′-phosphotyrosyl bonds. Human cell lines with a TDP1 gene mutation that reduces enzymatic activity and TDP1 knockout mice are hypersensitive to camptothecin [21][22][23][24]. Conversely, during elevated expression of TDP1, camptothecin or its derivatives cause fewer DNA lesions [20][25][26][27][28].
It has also been demonstrated that suppression of TDP1 activity causes hypersensitivity of cells not only to camptothecin and its derivatives but also to other DNA-damaging agents [11]. For instance, inhibition of TDP1 activity enhances the sensitivity of cells to anticancer drugs temozolomide [29] and bleomycin as well as to hydrogen peroxide, ionizing radiation, and methyl methanesulfonate [13]. In glioblastoma cells resistant to temozolomide-based chemotherapy, TDP1 depletion induces noticeable sensitization to this drug [29].
All of the above indicates that the suppression of the activity of TDP1 can increase the cytotoxicity of various antitumor drugs aimed at damaging tumor DNA and can help in the fight against drug-resistant tumors. A possible therapeutic benefit of the combined use of such substances and TDP1 inhibitors is stronger suppression of cancer cell growth and/or a reduction in the dose of conventional chemotherapy.
It was shown in [24][30] that a TDP1 gene knockout in vertebrate systems did not affect the survival of the organism; accordingly, it can be theorized that a decrease in the activity of TDP1 will be tolerated by patients fairly well during chemotherapy. Furthermore, SCAN1 patients have spinocerebellar atrophy (causing ataxia that debuts in the second decade of life) but show no increase in cancer predisposition, myocardial toxicity, immunodeficiency, or other problems related to impaired DNA repair [1].
2.2. Approaches to the Search for Inhibitors and Methods for Determining the Activity of the Inhibitors
One of the approaches in the search for TDP1 inhibitors is a virtual screening of known natural metabolites and their derivatives. A popular model for virtual docking of inhibitors is the PDB structure of a quaternary complex, 1NOP [6], TDP1 crystallized in the presence of a tyrosine-containing peptide, a single-stranded DNA oligonucleotide, and vanadate. This complex mimics the transition state in the first step of the catalytic reaction. Researchers also employ PDB structure 1MU7: a human TDP1–tungstate complex [7]. Tungstate is an analog of phosphate in the transition state of the enzyme’s active site. Among the studied potential inhibitors of TDP1, compounds having an intrinsic antitumor activity, preferably inhibitors of TOP1, receive special attention. The combination of inhibitory properties toward both enzymes within a single molecule is attractive because TDP1 inhibitors have been proposed as adjunctive drugs for antitumor therapy based on TOP1 poisons. The traditional method of TDP1 activity testing is the electrophoretic separation of the substrate from the product of phosphotyrosine cleavage from the 3′ end of an oligonucleotide [7]. This method is extremely low-throughput and does not permit an accurate assessment of the kinetic parameters of the reaction catalyzed by TDP1, although this technique is still used for semiquantitative assessment of the effects of various compounds on the activity of this enzyme.
For fast screening of libraries of compounds, investigators have developed several colorimetric and fluorescent assays of TDP1 activity, such as, for example, a fluorescence-based assay involving oligonucleotide and nucleotide substrates containing 3′-(4-methylumbelliferone)-phosphate [31]. All these assays are based on the ability of TDP1 to cleave off various 3′ end DNA adducts, including dyes [8][31][32], and to cleave a phosphodiester bond in molecules other than DNA [33][34]. These methods are simple and inexpensive, the activity of the enzyme either increases absorption at a certain wavelength [31][33] or induces fluorescence [34], or a fluorophore/quencher pair is separated, where fluorescence intensity also strengthens [32][35][36]. Several exotic, complicated, and expensive high-throughput methods of screening for inhibitors of TDP1 should also be mentioned:
-
The electrochemiluminescent (ECL) assay based on ruthenium labels (BV-TAG™) designed to emit light upon stimulation [37].
-
AlphaScreen technology [38], which is based on the separation of a donor/acceptor pair located at different ends of an oligonucleotide. Biotin at the 5′ end of the oligonucleotide is attached to the donor through a complex with streptavidin. The donor emits singlet oxygen when irradiated with light at 680 nm. FITC (fluorescein isothiocyanate) residue at the 3′ end of the oligonucleotide in complex with an anti-FITC antibody is the acceptor. The latter, when colliding with singlet oxygen, emits light of 580–620 nm wavelength. An intact substrate, when mixed with the donor streptavidin and anti-FITC antibody (acceptor), gives a strong AlphaScreen signal, whereas cleavage of the substrate by TDP1 weakens the signal.
-
Gyrasol assay technology [39]. This technology is based on a small-molecule nonfluorescent trivalent metal ion sensor designed to bind to the phosphate backbone of a DNA oligonucleotide. There is a fluorophore at the 3′ end of the oligonucleotide. The fluorescence is suppressed by electron transfer quenching. When the distance to the fluorophore increases, the distance becomes too long for the quenching, and the TDP1 activity can be monitored as an increase in fluorescence intensity.
This entry is adapted from the peer-reviewed paper 10.3390/ijms24065781
References
- Takashima, H.; Boerkoel, C.F.; John, J.; Saifi, G.M.; Salih, M.A.M.; Armstrong, D.; Mao, Y.; Quiocho, F.A.; Roa, B.B.; Nakagawa, M.; et al. Mutation of TDP1, Encoding a Topoisomerase I-Dependent DNA Damage Repair Enzyme, in Spinocerebellar Ataxia with Axonal Neuropathy. Nat. Genet. 2002, 32, 267–272.
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803.
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of Natural Products on Developing New Anti-Cancer Agents. Chem. Rev. 2009, 109, 3012–3043.
- Yang, S.W.; Burgin, A.B.; Huizenga, B.N.; Robertson, C.A.; Yao, K.C.; Nash, H.A. A Eukaryotic Enzyme That Can Disjoin Dead-End Covalent Complexes between DNA and Type I Topoisomerases. Proc. Natl. Acad. Sci. USA 1996, 93, 11534–11539.
- Interthal, H.; Pouliot, J.J.; Champoux, J.J. The Tyrosyl-DNA Phosphodiesterase TDP1 Is a Member of the Phospholipase D Superfamily. Proc. Natl. Acad. Sci. USA 2001, 98, 12009–12014.
- Davies, D.R.; Interthal, H.; Champoux, J.J.; Hol, W.G.J. Crystal Structure of a Transition State Mimic for TDP1 Assembled from Vanadate, DNA, and a Topoisomerase I-Derived Peptide. Chem. Biol. 2003, 10, 139–147.
- Davies, D.R.; Interthal, H.; Champoux, J.J.; Hol, W.G. Insights into substrate binding and catalytic mechanism of human tyrosyl-DNA phosphodiesterase (Tdp1) from vanadate and tungstate-inhibited structures. J. Mol. Biol. 2002, 324, 917–932.
- Pommier, Y.; Huang, S.-y.N.; Gao, R.; Das, B.B.; Murai, J.; Marchand, C. Tyrosyl-DNA-Phosphodiesterases (TDP1 and TDP2). DNA Repair 2014, 19, 114–129.
- Karimi-Busheri, F.; Lee, J.; Tomkinson, A.E.; Weinfeld, M. Repair of DNA Strand Gaps and Nicks Containing 3′-Phosphate and 5′-Hydroxyl Termini by Purified Mammalian Enzymes. Nucleic Acids Res. 1998, 26, 4395–4400.
- Wilson, D.M. Processing of Nonconventional DNA Strand Break Ends. Environ. Mol. Mutagen. 2007, 48, 772–782.
- Comeaux, E.Q.; Van Waardenburg, R.C.A.M. Tyrosyl-DNA Phosphodiesterase I Resolves Both Naturally and Chemically Induced DNA Adducts and Its Potential as a Therapeutic Target. Drug Metab. Rev. 2014, 46, 494–507.
- Interthal, H.; Chen, H.J.; Kehl-Fie, T.E.; Zotzmann, J.; Leppard, J.B.; Champoux, J.J. SCAN1 Mutant TDP1 Accumulates the Enzyme-DNA Intermediate and Causes Camptothecin Hypersensitivity. EMBO J. 2005, 24, 2224–2233.
- Murai, J.; Huang, S.Y.N.; Das, B.B.; Dexheimer, T.S.; Takeda, S.; Pommier, Y. Tyrosyl-DNA Phosphodiesterase 1 (TDP1) Repairs DNA Damage Induced by Topoisomerases I and II and Base Alkylation in Vertebrate Cells. J. Biol. Chem. 2012, 287, 12848–12857.
- Nitiss, K.C.; Malik, M.; He, X.; White, S.W.; Nitiss, J.L. Tyrosyl-DNA Phosphodiesterase (TDP1) Participates in the Repair of Top2-Mediated DNA Damage. Proc. Natl. Acad. Sci. USA 2006, 103, 8953–8958.
- Duffy, S.; Fam, H.K.; Wang, Y.K.; Styles, E.B.; Kim, J.H.; Ang, J.S.; Singh, T.; Larionov, V.; Shah, S.P.; Andrews, B.; et al. Overexpression Screens Identify Conserved Dosage Chromosome Instability Genes in Yeast and Human Cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 9967–9976.
- Liu, C.; Zhou, S.; Begum, S.; Sidransky, D.; Westra, W.H.; Brock, M.; Califano, J.A. Increased Expression and Activity of Repair Genes TDP1 and XPF in Non-Small Cell Lung Cancer. Lung Cancer 2007, 55, 303–311.
- Yu, J.; Shannon, W.D.; Watson, M.A.; McLeod, H.L. Gene Expression Profiling of the Irinotecan Pathway in Colorectal Cancer. Clin. Cancer Res. 2005, 11, 2053–2062.
- Dean, R.A.; Fam, H.K.; An, J.; Choi, K.; Shimizu, Y.; Jones, S.J.M.; Boerkoel, C.F.; Interthal, H.; Pfeifer, T.A. Identification of a Putative TDP1 Inhibitor (CD00509) by in Vitro and Cell-Based Assays. J. Biomol. Screen. 2014, 19, 1372–1382.
- Fam, H.K.; Walton, C.; Mitra, S.A.; Chowdhury, M.; Osborne, N.; Choi, K.; Sun, G.; Wong, P.C.W.; O’Sullivan, M.J.; Turashvili, G.; et al. TDP1 and PARP1 Deficiency Are Cytotoxic to Rhabdomyosarcoma Cells. Mol. Cancer Res. MCR 2013, 11, 1179–1192.
- Barthelmes, H.U.; Habermeyer, M.; Christensen, M.O.; Mielke, C.; Interthal, H.; Pouliot, J.J.; Boege, F.; Marko, D. TDP1 Overexpression in Human Cells Counteracts DNA Damage Mediated by Topoisomerases I and II. J. Biol. Chem. 2004, 279, 55618–55625.
- El-Khamisy, S.F.; Katyal, S.; Patel, P.; Ju, L.; McKinnon, P.J.; Caldecott, K.W. Synergistic Decrease of DNA Single-Strand Break Repair Rates in Mouse Neural Cells Lacking Both TDP1 and Aprataxin. DNA Repair 2009, 8, 760–766.
- Das, B.B.; Antony, S.; Gupta, S.; Dexheimer, T.S.; Redon, C.E.; Garfield, S.; Shiloh, Y.; Pommier, Y. Optimal Function of the DNA Repair Enzyme TDP1 Requires Its Phosphorylation by ATM and/or DNA-PK. EMBO J. 2009, 28, 3667–3680.
- Hirano, R.; Interthal, H.; Huang, C.; Nakamura, T.; Deguchi, K.; Choi, K.; Bhattacharjee, M.B.; Arimura, K.; Umehara, F.; Izumo, S.; et al. Spinocerebellar Ataxia with Axonal Neuropathy: Consequence of a TDP1 Recessive Neomorphic Mutation? EMBO J. 2007, 26, 4732–4743.
- Katyal, S.; El-Khamisy, S.F.; Russell, H.R.; Li, Y.; Ju, L.; Caldecott, K.W.; McKinnon, P.J. TDP1 Facilitates Chromosomal Single-Strand Break Repair in Neurons and Is Neuroprotective in Vivo. EMBO J. 2007, 26, 4720–4731.
- Nivens, M.C.; Felder, T.; Galloway, A.H.; Pena, M.M.O.; Pouliot, J.J.; Spencer, H.T. Engineered Resistance to Camptothecin and Antifolates by Retroviral Coexpression of Tyrosyl DNA Phosphodiesterase-I and Thymidylate Synthase. Cancer Chemother. Pharmacol. 2004, 53, 107–115.
- Alagoz, M.; Gilbert, D.C.; El-Khamisy, S.; Chalmers, A.J. DNA Repair and Resistance to Topoisomerase I Inhibitors: Mechanisms, Biomarkers and Therapeutic Targets. Curr. Med. Chem. 2012, 19, 3874–3885.
- Perego, P.; Cossa, G.; Tinelli, S.; Corna, E.; Carenini, N.; Gatti, L.; De Cesare, M.; Ciusani, E.; Zunino, F.; Luison, E.; et al. Role of Tyrosyl-DNA Phosphodiesterase 1 and Inter-Players in Regulation of Tumor Cell Sensitivity to Topoisomerase I Inhibition. Biochem. Pharmacol. 2012, 83, 27–36.
- Meisenberg, C.; Ward, S.E.; Schmid, P.; El-Khamisy, S.F. TDP1/TOP1 Ratio as a Promising Indicator for the Response of Small Cell Lung Cancer to Topotecan. J. Cancer Sci. Ther. 2014, 6, 258–267.
- Alagoz, M.; Wells, O.S.; El-Khamisy, S.F. TDP1 Deficiency Sensitizes Human Cells to Base Damage via Distinct Topoisomerase I and PARP Mechanisms with Potential Applications for Cancer Therapy. Nucleic Acids Res. 2014, 42, 3089–3103.
- El-Khamisy, S.F.; Caldecott, K.W. DNA Single-Strand Break Repair and Spinocerebellar Ataxia with Axonal Neuropathy-1. Neuroscience 2007, 145, 1260–1266.
- Rideout, M.C.; Raymond, A.C.; Burgin, A.B., Jr. Design and Synthesis of Fluorescent Substrates for Human Tyrosyl-DNA Phosphodiesterase I. Nucleic Acids Res. 2004, 32, 4657–4664.
- Jensen, P.W.; Falconi, M.; Kristoffersen, E.L.; Simonsen, A.T.; Cifuentes, J.B.; Marcussen, L.B.; Frøhlich, R.; Vagner, J.; Harmsen, C.; Juul, S.; et al. Real-Time Detection of TDP1 Activity Using a Fluorophore-Quencher Coupled DNA-Biosensor. Biosens. Bioelectron. 2013, 48, 230–237.
- Cheng, T.-J.; Rey, P.G.; Poon, T.; Kan, C.-C. Kinetic Studies of Human Tyrosyl-DNA Phosphodiesterase, an Enzyme in the Topoisomerase I DNA Repair Pathway. Eur. J. Biochem. 2002, 269, 3697–3704.
- Tang, Z.-Y.; Zhang, Y.; Chen, Y.-T.; Yu, Q.; An, L.-K. The First Small Fluorescent Probe as Tyrosyl-DNA Phosphodiesterase 1 (TDP1) Substrate. Dye. Pigment. 2019, 169, 45–50.
- Zakharenko, A.; Khomenko, T.; Zhukova, S.; Koval, O.; Zakharova, O.; Anarbaev, R.; Lebedeva, N.; Korchagina, D.; Komarova, N.; Vasiliev, V.; et al. Synthesis and Biological Evaluation of Novel Tyrosyl-DNA Phosphodiesterase 1 Inhibitors with a Benzopentathiepine Moiety. Bioorganic Med. Chem. 2015, 23, 2044–2052.
- Zhang, X.R.; Wang, H.W.; Tang, W.L.; Zhang, Y.; Yang, H.; Hu, D.X.; Ravji, A.; Marchand, C.; Kiselev, E.; Ofori-Atta, K.; et al. Discovery, Synthesis, and Evaluation of Oxynitidine Derivatives as Dual Inhibitors of DNA Topoisomerase IB (TOP1) and Tyrosyl-DNA Phosphodiesterase 1 (TDP1), and Potential Antitumor Agents. J. Med. Chem. 2018, 61, 9908–9930.
- Antony, S.; Marchand, C.; Stephen, A.G.; Thibaut, L.; Agama, K.K.; Fisher, R.J.; Pommier, Y. Novel High-Throughput Electrochemiluminescent Assay for Identification of Human Tyrosyl-DNA Phosphodiesterase (TDP1) Inhibitors and Characterization of Furamidine (NSC 305831) as an Inhibitor of TDP1. Nucleic Acids Res. 2007, 35, 4474–4484.
- Marchand, C.; Lea, W.A.; Jadhav, A.; Dexheimer, T.S.; Austin, C.P.; Inglese, J.; Pommier, Y.; Simeonov, A. Identification of Phosphotyrosine Mimetic Inhibitors of Human Tyrosyl-DNA Phosphodiesterase I by a Novel AlphaScreen High-Throughput Assay. Mol. Cancer Ther. 2009, 8, 240–248.
- Walker, S.; Meisenberg, C.; Bibby, R.A.; Askwith, T.; Williams, G.; Rininsland, F.H.; Pearl, L.H.; Oliver, A.W.; El-Khamisy, S.; Ward, S.; et al. Development of an Oligonucleotide-Based Fluorescence Assay for the Identification of Tyrosyl-DNA Phosphodiesterase 1 (TDP1) Inhibitors. Anal. Biochem. 2014, 454, 17–22.
This entry is offline, you can click here to edit this entry!
