Peanuts are the seeds of a legume crop grown for nuts and oil production. Peanut allergy has gained significant attention as a public health issue due to its increasing prevalence, high rate of sensitization, severity of the corresponding allergic symptoms, cross-reactivity with other food allergens, and lifelong persistence. Given the importance of peanuts in several sectors and taking into consideration the criticality of their high allergic potential, strategies aiming at mitigating their allergenicity are urgently needed. In this regard, most of the processing methods used to treat peanuts are categorized as either thermal or thermomechanical techniques. They differ in their effectiveness in alleviating the allergenicity, and in their capacity in preserving the structural integrity of the treated peanuts. Research data on this matter may open further perspectives for future relevant investigation ultimately aiming at producing hypoallergenic peanuts.
- peanut allergy
- immunoreactivity
- allergenicity mitigation
- thermomechanical processing
1. Peanut Allergy: Prevalence, Persistence, and Severity

2. Peanut Allergens and Their Mechanisms of Action
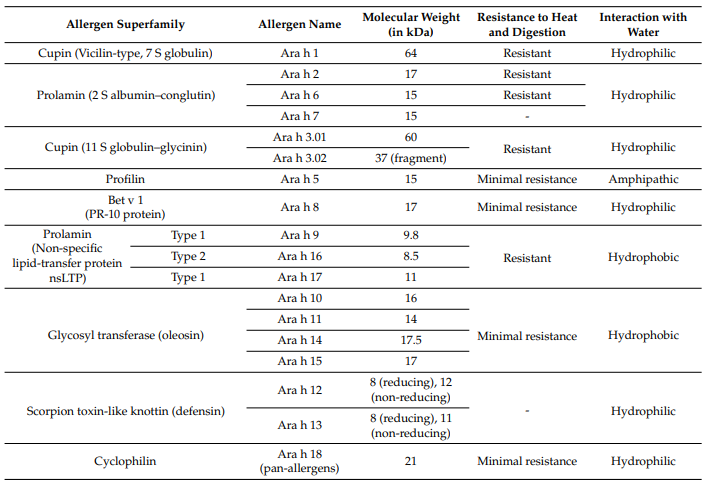
Table 1. Physicochemical properties of peanut allergens
3. Thermomechanical Processing of Peanuts
Figure 2. Thermomechanical treatments of peanuts
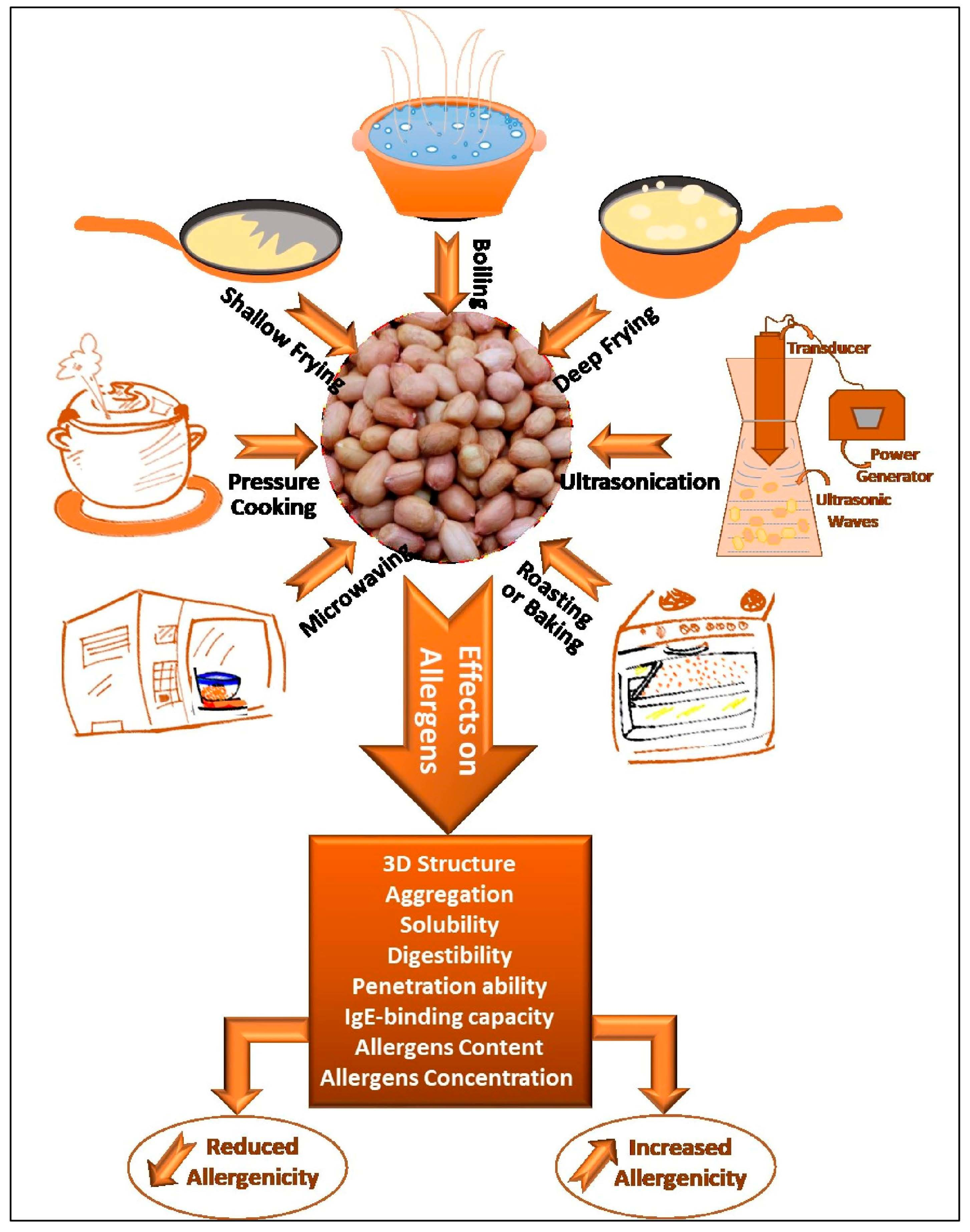
3.1. Boiling
3.2. Roasting/Baking
3.3. Microwaving
3.4. Ultrasonication
3.5. Frying
3.6. High-Pressure Steaming/Autoclaving
4. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/foods12061253
References
- Smejkal, G.; Kakumanu, S.; Cannady-Miller, A. Increasing the Solubility and Recovery of Ara H3 Allergen from Raw and Roasted Peanut. In Nutrition in Health and Disease—Our Challenges Now and Forthcoming Time; IntechOpen: London, UK, 2019.
- Masilamani, M.; Commins, S.; Shreffler, W. Determinants of Food Allergy. Immunol. Allergy Clin. N. Am. 2012, 32, 11–33.
- Asai, Y.; Eslami, A.; van Ginkel, C.D.; Akhabir, L.; Wan, M.; Ellis, G.; Ben-Shoshan, M.; Martino, D.; Ferreira, M.A.; Allen, K.; et al. Genome-Wide Association Study and Meta-Analysis in Multiple Populations Identifies New Loci for Peanut Allergy and Establishes C11orf30/EMSY as a Genetic Risk Factor for Food Allergy. J. Allergy Clin. Immunol. 2018, 141, 991–1001.
- Asai, Y.; Eslami, A.; van Ginkel, C.D.; Akhabir, L.; Wan, M.; Yin, D.; Ellis, G.; Ben-Shoshan, M.; Marenholz, I.; Martino, D.; et al. A Canadian Genome-Wide Association Study and Meta-Analysis Confirm HLA as a Risk Factor for Peanut Allergy Independent of Asthma. J. Allergy Clin. Immunol. 2018, 141, 1513–1516.
- Chang, C.; Wu, H.; Lu, Q. The Epigenetics of Food Allergy. In Epigenetics in Allergy and Autoimmunity; Springer: Singapore, 2020; pp. 141–152.
- Shah, F.; Shi, A.; Ashley, J.; Kronfel, C.; Wang, Q.; Maleki, S.J.; Adhikari, B.; Zhang, J. Peanut Allergy: Characteristics and Approaches for Mitigation. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1361–1387.
- Cabanillas, B.; Jappe, U.; Novak, N. Allergy to Peanut, Soybean, and Other Legumes: Recent Advances in Allergen Characterization, Stability to Processing and IgE Cross-Reactivity. Mol. Nutr. Food Res. 2018, 62, 1700446.
- Koppelman, S.J.; Jayasena, S.; Luykx, D.; Schepens, E.; Apostolovic, D.; de Jong, G.A.H.; Isleib, T.G.; Nordlee, J.; Baumert, J.; Taylor, S.L.; et al. Allergenicity Attributes of Different Peanut Market Types. Food Chem. Toxicol. 2016, 91, 82–90.
- Marsh, J.T.; Palmer, L.K.; Koppelman, S.J.; Johnson, P.E. Determination of Allergen Levels, Isoforms, and Their Hydroxyproline Modifications among Peanut Genotypes by Mass Spectrometry. Front. Allergy 2022, 3, 872714.
- Bonku, R.; Yu, J. Health Aspects of Peanuts as an Outcome of Its Chemical Composition. Food Sci. Hum. Wellness 2020, 9, 21–30.
- Geng, Q.; Zhang, Y.; Song, M.; Zhou, X.; Tang, Y.; Wu, Z.; Chen, H. Allergenicity of Peanut Allergens and Its Dependence on the Structure. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1058–1081.
- Blankestijn, M.A.; Knulst, A.C.; Knol, E.F.; Le, T.-M.; Rockmann, H.; Otten, H.G.; Klemans, R.J.B. Sensitization to PR-10 Proteins Is Indicative of Distinctive Sensitization Patterns in Adults with a Suspected Food Allergy. Clin. Transl. Allergy 2017, 7, 42.
- Eiwegger, T.; Rigby, N.; Mondoulet, L.; Bernard, H.; Krauth, M.-T.; Boehm, A.; Dehlink, E.; Valent, P.; Wal, J.M.; Mills, E.N.C.; et al. Gastro-Duodenal Digestion Products of the Major Peanut Allergen Ara h 1 Retain an Allergenic Potential. Clin. Exp. Allergy 2006, 36, 1281–1288.
- Jappe, U.; Schwager, C. Relevance of Lipophilic Allergens in Food Allergy Diagnosis. Curr. Allergy Asthma Rep. 2017, 17, 61.
- Fuhrmann, V.; Huang, H.-J.; Akarsu, A.; Shilovskiy, I.; Elisyutina, O.; Khaitov, M.; van Hage, M.; Linhart, B.; Focke-Tejkl, M.; Valenta, R.; et al. From Allergen Molecules to Molecular Immunotherapy of Nut Allergy: A Hard Nut to Crack. Front. Immunol. 2021, 12, 742732.
- Toomer, O.T. Nutritional Chemistry of the Peanut (Arachis hypogaea). Crit. Rev. Food Sci. Nutr. 2018, 58, 3042–3053.
- Mittag, D.; Akkerdaas, J.; Ballmer-Weber, B.K.; Vogel, L.; Wensing, M.; Becker, W.-M.; Koppelman, S.J.; Knulst, A.C.; Helbling, A.; Hefle, S.L.; et al. Ara h 8, a Bet v 1–Homologous Allergen from Peanut, Is a Major Allergen in Patients with Combined Birch Pollen and Peanut Allergy. J. Allergy Clin. Immunol. 2004, 114, 1410–1417.
- Koppelman, S.J.; Vlooswijk, R.A.A.; Knippels, L.M.J.; Hessing, M.; Knol, E.F.; van Reijsen, F.C.; Bruijnzeel-Koomen, C.A.F.M. Quantification of Major Peanut Allergens Ara h 1 and Ara h 2 in the Peanut Varieties Runner, Spanish, Virginia, and Valencia, Bred in Different Parts of the World. Allergy 2001, 56, 132–137.
- Zhang, T.; Shi, Y.; Zhao, Y.; Tang, G.; Niu, B.; Chen, Q. Boiling and Roasting Treatment Affecting the Peanut Allergenicity. Ann. Transl. Med. 2018, 6, 357.
- Prodić, I.; Smiljanić, K.; Simović, A.; Radosavljević, J.; Ćirković Veličković, T. Thermal Processing of Peanut Grains Impairs Their Mimicked Gastrointestinal Digestion While Downstream Defatting Treatments Affect Digestomic Profiles. Foods 2019, 8, 463.
- Meng, S.; Li, J.; Chang, S.; Maleki, S.J. Quantitative and Kinetic Analyses of Peanut Allergens as Affected by Food Processing. Food Chem. X 2019, 1, 100004.
- Tao, B.; Bernardo, K.; Eldi, P.; Chegeni, N.; Wiese, M.; Colella, A.; Kral, A.; Hayball, J.; Smith, W.; Forsyth, K.; et al. Extended Boiling of Peanut Progressively Reduces IgE Allergenicity While Retaining T Cell Reactivity. Clin. Exp. Allergy 2016, 46, 1004–1014.
- Zhang, T.; Shi, Y.; Zhao, Y.; Wang, J.; Wang, M.; Niu, B.; Chen, Q. Different Thermal Processing Effects on Peanut Allergenicity. J. Sci. Food Agric. 2019, 99, 2321–2328.
- Novak, N.; Maleki, S.J.; Cuadrado, C.; Crespo, J.F.; Cabanillas, B. Interaction of Monocyte-Derived Dendritic Cells with Ara h 2 from Raw and Roasted Peanuts. Foods 2020, 9, 863.
- Tian, Y.; Rao, H.; Zhang, K.; Tao, S.; Xue, W.T. Effects of Different Thermal Processing Methods on the Structure and Allergenicity of Peanut Allergen Ara h 1. Food Sci. Nutr. 2018, 6, 1706–1714.
- Wang, T.; Huang, Y.; Tang, X.; Wang, H.; Li, B.; Meng, X.; Jiang, S. Effects of Different Cooking Methods on Peanut Allergenicity. Food Biosci. 2022, 47, 101757.
- Salve, A.R.; LeBlanc, J.G.; Arya, S.S. Effect of Processing on Polyphenol Profile, Aflatoxin Concentration and Allergenicity of Peanuts. J. Food Sci. Technol. 2021, 58, 2714–2724.
- Cohen, C.; Zhao, W.; Beaudette, L.; Lejtenyi, D.; Jean-Claude, B.; Mazer, B. High-Pressure and Temperature Autoclaving of Peanuts Reduces the Proportion of Intact Allergenic Proteins. Authorea Prepr. 2021.
- Đukić, T.; Smiljanić, K.; Mihailović, J.; Prodić, I.; Apostolović, D.; Liu, S.-H.; Epstein, M.M.; van Hage, M.; Stanić-Vučinić, D.; Ćirković Veličković, T. Proteomic Profiling of Major Peanut Allergens and Their Post-Translational Modifications Affected by Roasting. Foods 2022, 11, 3993.
- Prodic, I.; Stanic-Vucinic, D.; Apostolovic, D.; Mihailovic, J.; Radibratovic, M.; Radosavljevic, J.; Burazer, L.; Milcic, M.; Smiljanic, K.; van Hage, M.; et al. Influence of Peanut Matrix on Stability of Allergens in Gastric-Simulated Digesta: 2S Albumins Are Main Contributors to the IgE Reactivity of Short Digestion-Resistant Peptides. Clin. Exp. Allergy 2018, 48, 731–740.
- di Stasio, L.; Tranquet, O.; Picariello, G.; Ferranti, P.; Morisset, M.; Denery-Papini, S.; Mamone, G. Comparative Analysis of Eliciting Capacity of Raw and Roasted Peanuts: The Role of Gastrointestinal Digestion. Food Res. Int. 2020, 127, 108758.
- Corzo-Martínez, M.; Villamiel, M.; Moreno, F.J. Impact of High-Intensity Ultrasound on Protein Structure and Functionality during Food Processing. In Ultrasound in Food Processing; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 417–436.
- Li, H.; Yu, J.; Ahmedna, M.; Goktepe, I. Reduction of Major Peanut Allergens Ara h 1 and Ara h 2, in Roasted Peanuts by Ultrasound Assisted Enzymatic Treatment. Food Chem. 2013, 141, 762–768.
- Meng, S. Quantitative Analysis of Allergens in Peanut Varieties and Assessment of Effects of Food Processing on Peanut Allergens. Ph.D. Thesis, 2018. 3696. Available online: https://scholarsjunction.msstate.edu/td/3696 (accessed on 2 November 2022).
- Bavaro, S.L.; di Stasio, L.; Mamone, G.; de Angelis, E.; Nocerino, R.; Canani, R.B.; Logrieco, A.F.; Montemurro, N.; Monaci, L. Effect of Thermal/Pressure Processing and Simulated Human Digestion on the Immunoreactivity of Extractable Peanut Allergens. Food Res. Int. 2018, 109, 126–137.
- Zhang, J.; Hong, Y.; Cai, Z.; Huang, B.; Wang, J.; Ren, Y. Simultaneous Determination of Major Peanut Allergens Ara H1 and Ara H2 in Baked Foodstuffs Based on Their Signature Peptides Using Ultra-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Anal. Methods 2019, 11, 1689–1696.
- Faisal, S.; Zhang, J.; Meng, S.; Shi, A.; Li, L.; Wang, Q.; Maleki, S.J.; Adhikari, B. Effect of High-Moisture Extrusion and Addition of Transglutaminase on Major Peanut Allergens Content Extracted by Three Step Sequential Method. Food Chem. 2022, 385, 132569.
- Bavaro, S.L.; Orlando, A.; de Angelis, E.; Russo, F.; Monaci, L. Investigation on the Allergen Profile of the Soluble Fraction of Autoclaved Peanuts and Its Interaction with Caco-2 Cells. Food Funct. 2019, 10, 3615–3625.
- Zhang, Y.; Wu, Z.; Li, K.; Li, X.; Yang, A.; Tong, P.; Chen, H. Allergenicity Assessment on Thermally Processed Peanut Influenced by Extraction and Assessment Methods. Food Chem. 2019, 281, 130–139.
