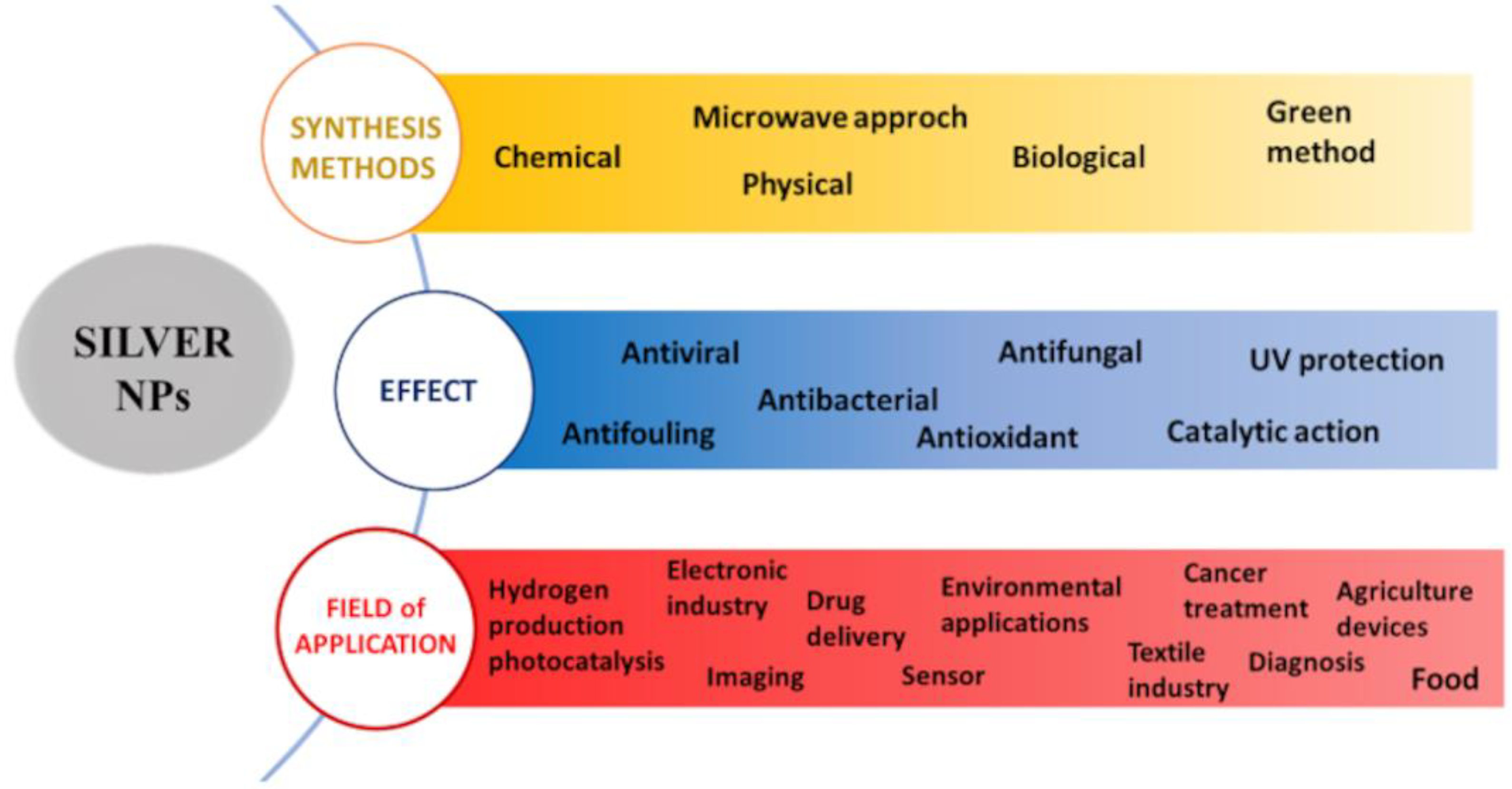
New antiviral drugs and new preventive antiviral strategies are a target of intense scientific interest. Thanks to their peculiar properties, nanomaterials play an important role in this field, and, in particular, among metallic materials, silver nanoparticles were demonstrated to be effective against a wide range of viruses, in addition to having a strong antibacterial effect. Although the mechanism of antiviral action is not completely clarified, silver nanoparticles can directly act on viruses, and on their first steps of interaction with the host cell, depending on several factors, such as size, shape, functionalization and concentration.
- silver
- applications
- antiviral
1. Introduction

| Field | Description of Application |
System | Tested Virus | Study Level | Ref. |
|---|---|---|---|---|---|
| Health sector | Potential therapeutic use | AgNPs | IFVA (H3N2) | Laboratory study | [9] |
| Potential therapeutic use | Tannic acid-modified AgNPs (TA-AgNP) | HSV-2 | Laboratory study | [10] | |
| Potential therapeutic use | TA-AgNPs into Carbopol 974P gel | HSV-1 and HSV-2 | Laboratory study | [11] | |
| Potential therapeutic use | TA-AgNPs | HSV-2 | Laboratory study | [12] | |
| Potential therapeutic use | AgNPs | RSV | Laboratory study | [13] | |
| Potential therapeutic use | AgNPs functionalized with PVP (ArgovitTM) | RVFV | Prototype | [14] | |
| Prevention | AgNPs (ArgovitTM) | SARS-CoV-2 | Prototype | [15] | |
| Coating for surfaces | Protective hybrid coatings with AgNPs realized by means of sol gel | HIV-1, DENV, HSV-1, IFVA, CoxB3 | Prototype | [16] | |
| Coating for condoms for the prevention of sexually transmitted viruses | AgNP coating by immersion | HIV, HSV-1, HSV-2 | Prototype | [17] | |
| Surgical mask doped with AgNPs by immersion | AgNPs obtained by electrochemical method and incorporating an aqueous solution | IFVA (H5N1) | Prototype | [18] | |
| Co-sputtered disposable mask | Silver nanoclusters/silica composite coating by co-sputtering method | SARS-CoV-2 | Prototype | [19] | |
| Part of respiratory and surgical masks | Graphene–silver nanocomposite | FCoV, IBDV | Prototype | [20] | |
| Veterinary sector | Potential therapeutic use | AgNPs synthesized by chemical reduction method | IBDV | Laboratory study | [21] |
| Potential therapeutic use | Biologically synthesized AgNPs | PPRV | Laboratory study | [22] | |
| Potential therapeutic use | AgNPs | RVFV | Laboratory study | [14] | |
| Potential therapeutic use | AgNPs | CVD | Laboratory study | [23] | |
| Potential therapeutic use | AgNPs | WSSV | Laboratory study | [24][25] | |
| Potential therapeutic use | AgNPs from aqueous extracts of clove | NDV | Laboratory study | [26] | |
| Water and air filtration systems | Water treatment | AgNPs produced via Lactobacillus fermentum (Biogenic Ag0) | UZ1 (bacteriophage), MNV-1 | Prototype | [27] |
| Water treatment under UV radiation | AgNPs via photochemical reduction of silver nitrate on Aeroxide TiO2 P25 and Anatase TiO2 | MS2 (bacteriophage) | Laboratory study | [28] | |
| Water treatment | Fe2O3/Ag NPs coating on fiber glass | MS2 (bacteriophage) | Laboratory study | [29] | |
| Water treatment | AgNP-doped and Ag/Cu NP-doped activated carbon by impregnation | T4 (bacteriophage) | Laboratory study | [30] | |
| Water treatment | Magnetic hybrid colloid-AgNPs | MNV, ɸX174, AdV2 | Laboratory study | [31] | |
| Water treatment | Colloidal and immobilized Ag nanoparticles on a glass substrate | MS2 and T4 (bacteriophages) | Laboratory study | [32] | |
| Air filtration | PP nonwoven substrate with a layer of PA6 electrospun nanofiber, impregnated with AgNPs | PDCoV | Prototype | [33] | |
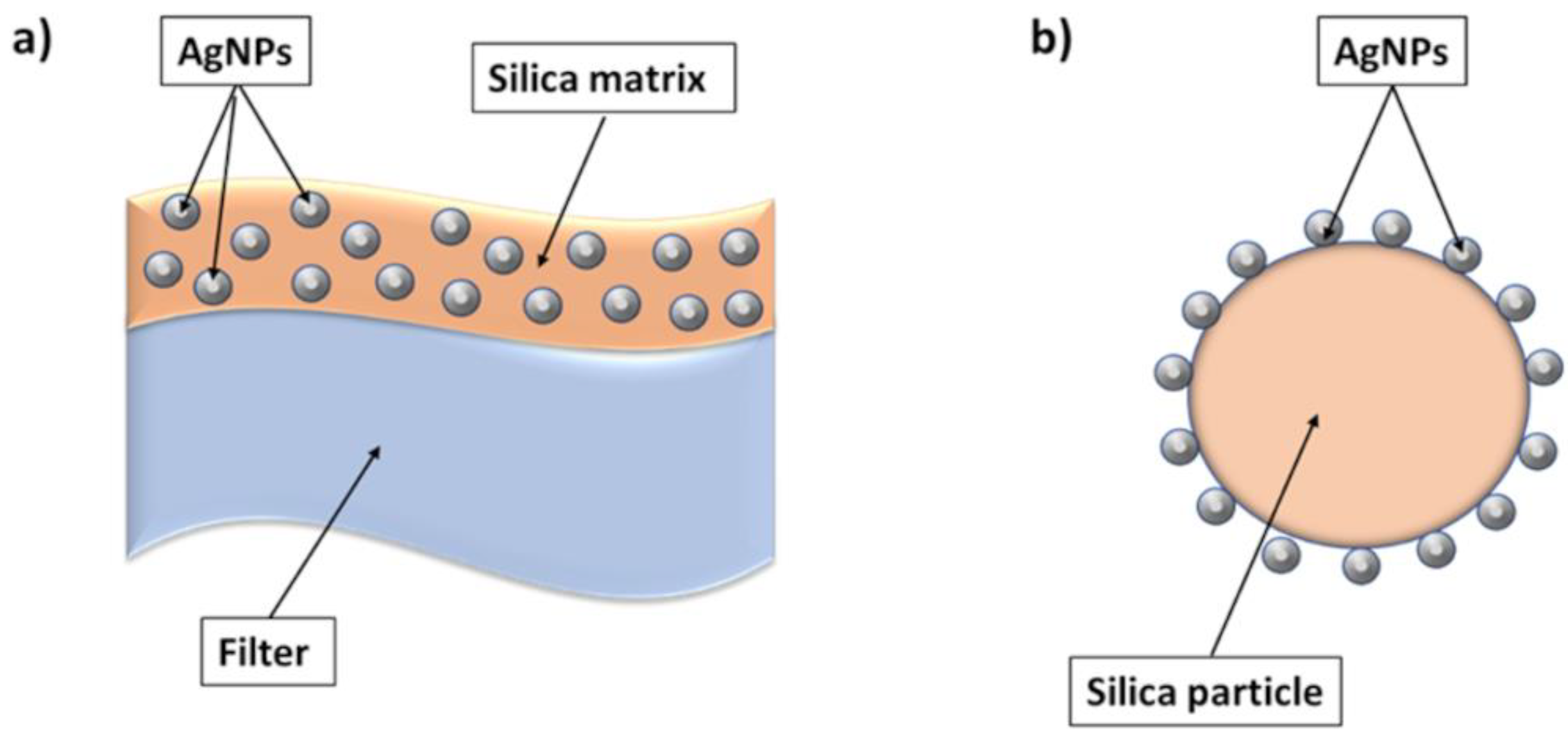
| Air filtration | Silver nanoclusters/silica composite coating on air filter | IFVA, RSV | Laboratory study | [34] | |
| Air filtration | Silver nanoparticle-coated silica particle | IFVA, MS2 (bacteriophage) | Laboratory study/Prototype | [34][35][36] | |
| Air filtration | Nano-Ag0/titania-chitosan | MS2 (bacteriophage) | Laboratory study | [37] | |
| Food packaging |
Packaging | Polymeric film–AgNPs | FCV and Murine Norovirus (MNV) | Prototype | [38] |
| Textile industry |
Antiviral clothing | AgNPs and phospholipid vesicles in the Viroblock/ViroFormulaTM |
FluVA, SARS-Cov 2 | Commercial product | [39] |
| Antiviral clothing | Electrospun nanofibers with ZnO Nanorods and Ag NPs | BCV, Bovine Parainfluenza Virus Type 3 (BPIV3) | Laboratory study | [15] | |
| Disinfectant for polyester/viscose spunlace wipes for use in surface disinfection |
AgNPs prepared by reducing agent or aqueous solution of PVA in the presence of glucose or photochemical reaction | MERS-CoV | Prototype | [40] |
2. Application in the Human Health Sector
3. Veterinary Applications of Silver Nanoparticles
4. Applications of Silver Nanoparticles in Water and Air Filtration Systems
4.1. Water Treatment
4.2. Air Filtration Systems
5. Applications of Silver Nanoparticles in the Food Industry
6. Applications of Silver Nanoparticles in the Textile Industry
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms11030629
References
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681.
- Kirubaharan, C.J.; Kalpana, D.; Lee, Y.S.; Kim, A.R.; Yoo, D.J.; Nahm, K.S.; Kumar, G.G. Biomediated Silver Nanoparticles for the Highly Selective Copper(II) Ion Sensor Applications. Ind. Eng. Chem. Res. 2012, 51, 7441–7446.
- Kale, S.K.; Parishwad, G.V.; Patil, A.S.N.H.A.S. Emerging Agriculture Applications of Silver Nanoparticles. ES Food Agrofor. 2021, 3, 17–22.
- Microban Silver Antimicrobial Technology. Microban. Available online: https://www.microban.com/antimicrobial-solutions/technologies/microban-silver-technology (accessed on 16 February 2022).
- Tecnologia SilverShield® Con Argento Antibatterico. Microban. Available online: https://www.microban.com/it/odor-control/technologies/silvershield (accessed on 16 February 2022).
- Abbigliamento Intimo Tecnico in Argento. Available online: https://www.silverskin.it/?gclid=EAIaIQobChMIlvH7yO2D9gIVIJBoCR2l_g-rEAAYASAAEgKoJ_D_BwE (accessed on 16 February 2022).
- ICOSAN DEFEND AG-Pittura Igienizzante Con Ioni D’argento | Colorificio Postumia. Available online: https://www.colorificiopostumia.it/prodotto/icosan-defend-ag-pittura-igienizzante-con-ioni-dargento (accessed on 16 February 2022).
- Face Mask FFP2 with Silver ANTI-COVID19. Available online: https://www.nanosilver.eu/Face-mask-FFP2-with-silver-ANTI-COVID19 (accessed on 16 February 2022).
- Xiang, D.; Zheng, C.; Zheng, Y.; Li, X.; Yin, J.; O’Conner, M.; Marappan, M.; Miao, Y.; Xiang, B.; Duan, W.; et al. Inhibition of A/Human/Hubei/3/2005 (H3N2) Influenza Virus Infection by Silver Nanoparticles in Vitro and in Vivo. Int. J. Nanomed. 2013, 8, 4103.
- Orlowski, P.; Tomaszewska, E.; Gniadek, M.; Baska, P.; Nowakowska, J.; Sokolowska, J.; Nowak, Z.; Donten, M.; Celichowski, G.; Grobelny, J.; et al. Tannic Acid Modified Silver Nanoparticles Show Antiviral Activity in Herpes Simplex Virus Type 2 Infection. PLoS ONE 2014, 9, e104113.
- Szymańska, E.; Orłowski, P.; Winnicka, K.; Tomaszewska, E.; Bąska, P.; Celichowski, G.; Grobelny, J.; Basa, A.; Krzyżowska, M. Multifunctional Tannic Acid/Silver Nanoparticle-Based Mucoadhesive Hydrogel for Improved Local Treatment of HSV Infection: In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2018, 19, 387.
- Orłowski, P.; Kowalczyk, A.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Węgrzyn, A.; Grzesiak, J.; Celichowski, G.; Grobelny, J.; Eriksson, K.; Krzyzowska, M. Antiviral Activity of Tannic Acid Modified Silver Nanoparticles: Potential to Activate Immune Response in Herpes Genitalis. Viruses 2018, 10, 524.
- Morris, D.; Ansar, M.; Speshock, J.; Ivanciuc, T.; Qu, Y.; Casola, A.; Garofalo, R. Antiviral and Immunomodulatory Activity of Silver Nanoparticles in Experimental RSV Infection. Viruses 2019, 11, 732.
- Borrego, B.; Lorenzo, G.; Mota-Morales, J.D.; Almanza-Reyes, H.; Mateos, F.; López-Gil, E.; de la Losa, N.; Burmistrov, V.A.; Pestryakov, A.N.; Brun, A.; et al. Potential Application of Silver Nanoparticles to Control the Infectivity of Rift Valley Fever Virus In Vitro and In Vivo. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1185–1192.
- Almanza-Reyes, H.; Moreno, S.; Plascencia-López, I.; Alvarado-Vera, M.; Patrón-Romero, L.; Borrego, B.; Reyes-Escamilla, A.; Valencia-Manzo, D.; Brun, A.; Pestryakov, A.; et al. Evaluation of Silver Nanoparticles for the Prevention of SARS-CoV-2 Infection in Health Workers: In Vitro and In Vivo. PLoS ONE 2021, 16, e0256401.
- Hodek, J.; Zajícová, V.; Lovětinská-Šlamborová, I.; Stibor, I.; Müllerová, J.; Weber, J. Protective Hybrid Coating Containing Silver, Copper and Zinc Cations Effective against Human Immunodeficiency Virus and Other Enveloped Viruses. BMC Microbiol. 2016, 16, 56.
- Fayaz, A.M.; Ao, Z.; Girilal, M.; Chen, L.; Xiao, X.; Kalaichelvan, P.T.; Yao, X. Inactivation of Microbial Infectiousness by Silver Nanoparticles-Coated Condom: A New Approach to Inhibit HIV-and HSV-Transmitted Infection. Int. J. Nanomed. 2012, 7, 5007.
- Valdez-Salas, B.; Beltran-Partida, E.; Cheng, N.; Salvador-Carlos, J.; Valdez-Salas, E.A.; Curiel-Alvarez, M.; Ibarra-Wiley, R. Promotion of Surgical Masks Antimicrobial Activity by Disinfection and Impregnation with Disinfectant Silver Nanoparticles. Int. J. Nanomed. 2021, 16, 2689–2702.
- Balagna, C.; Perero, S.; Percivalle, E.; Nepita, E.V.; Ferraris, M. Virucidal Effect against Coronavirus SARS-CoV-2 of a Silver Nanocluster/Silica Composite Sputtered Coating. Open Ceram. 2020, 1, 100006.
- Chen, Y.-N.; Hsueh, Y.-H.; Hsieh, C.-T.; Tzou, D.-Y.; Chang, P.-L. Antiviral Activity of Graphene–Silver Nanocomposites against Non-Enveloped and Enveloped Viruses. Int. J. Environ. Res. Public Health 2016, 13, 430.
- Pangestika, R.; Ernawati, R. Antiviral Activity Effect of Silver Nanoparticles (Agnps) Solution Against the Growth of Infectious Bursal Disease Virus on Embryonated Chicken Eggs with Elisa Test. KnE Life Sci. 2017, 3, 536.
- Khandelwal, N.; Kaur, G.; Chaubey, K.K.; Singh, P.; Sharma, S.; Tiwari, A.; Singh, S.V.; Kumar, N. Silver Nanoparticles Impair Peste Des Petits Ruminants Virus Replication. Virus Res. 2014, 190, 1–7.
- Bogdanchikova, N.; Vazquez-Munoz, R.; Huerta-Saquero, A.; Pena-Jasso, A.; Aguilar-Uzcanga, G.; Picos-Díaz, P.; Pestryakov, A.; Burmistrov, V.; Martynyuk, O.; Vazquez Gomez, R.; et al. Silver Nanoparticles Composition for Treatment of Distemper in Dogs. Int. J. Nanotechnol. 2016, 13, 227.
- Camacho-Jiménez, L.; Álvarez-Sánchez, A.R.; Mejía-Ruíz, C.H. Silver Nanoparticles (AgNPs) as Antimicrobials in Marine Shrimp Farming: A Review. Aquac. Rep. 2020, 18, 100512.
- Juarez-Moreno, K.; Mejía-Ruiz, C.H.; Díaz, F.; Reyna-Verdugo, H.; Re, A.D.; Vazquez-Felix, E.F.; Sánchez-Castrejón, E.; Mota-Morales, J.D.; Pestryakov, A.; Bogdanchikova, N. Effect of Silver Nanoparticles on the Metabolic Rate, Hematological Response, and Survival of Juvenile White Shrimp Litopenaeus vannamei. Chemosphere 2017, 169, 716–724.
- Mehmood, Y.; Farooq, U.; Yousaf, H.; Riaz, H.; Mahmood, R.K.; Nawaz, A.; Abid, Z.; Gondal, M.; Malik, N.S.; Barkat, K.; et al. Antiviral Activity of Green Silver Nanoparticles Produced Using Aqueous Buds Extract of Syzygium aromaticum. Pak. J. Pharm. Sci. 2020, 33, 839–845.
- De Gusseme, B.; Sintubin, L.; Baert, L.; Thibo, E.; Hennebel, T.; Vermeulen, G.; Uyttendaele, M.; Verstraete, W.; Boon, N. Biogenic Silver for Disinfection of Water Contaminated with Viruses. Appl. Environ. Microbiol. 2010, 76, 1082–1087.
- Liga, M.V.; Bryant, E.L.; Colvin, V.L.; Li, Q. Virus Inactivation by Silver Doped Titanium Dioxide Nanoparticles for Drinking Water Treatment. Water Res. 2011, 45, 535–544.
- Nangmenyi, G.; Li, X.; Mehrabi, S.; Mintz, E.; Economy, J. Silver-Modified Iron Oxide Nanoparticle Impregnated Fiberglass for Disinfection of Bacteria and Viruses in Water. Mater. Lett. 2011, 65, 1191–1193.
- Shimabuku, Q.L.; Arakawa, F.S.; Fernandes Silva, M.; Ferri Coldebella, P.; Ueda-Nakamura, T.; Fagundes-Klen, M.R.; Bergamasco, R. Water Treatment with Exceptional Virus Inactivation Using Activated Carbon Modified with Silver (Ag) and Copper Oxide (CuO) Nanoparticles. Environ. Technol. 2017, 38, 2058–2069.
- Park, S.; Park, H.H.; Kim, S.Y.; Kim, S.J.; Woo, K.; Ko, G. Antiviral Properties of Silver Nanoparticles on a Magnetic Hybrid Colloid. Appl. Environ. Microbiol. 2014, 80, 2343–2350.
- Bharti, S.; Mukherji, S.; Mukherji, S. Antiviral Application of Colloidal and Immobilized Silver Nanoparticles. Nanotechnology 2021, 32, 205102.
- Ju, Y.; Han, T.; Yin, J.; Li, Q.; Chen, Z.; Wei, Z.; Zhang, Y.; Dong, L. Bumpy Structured Nanofibrous Membrane as a Highly Efficient Air Filter with Antibacterial and Antiviral Property. Sci. Total Environ. 2021, 777, 145768.
- Balagna, C.; Francese, R.; Perero, S.; Lembo, D.; Ferraris, M. Nanostructured Composite Coating Endowed with Antiviral Activity against Human Respiratory Viruses Deposited on Fibre-Based Air Filters. Surf. Coat. Technol. 2021, 409, 126873.
- Park, S.; Ko, Y.-S.; Lee, S.J.; Lee, C.; Woo, K.; Ko, G. Inactivation of Influenza A Virus via Exposure to Silver Nanoparticle-Decorated Silica Hybrid Composites. Environ. Sci. Pollut. Res. 2018, 25, 27021–27030.
- Joe, Y.H.; Park, D.H.; Hwang, J. Evaluation of Ag Nanoparticle Coated Air Filter against Aerosolized Virus: Anti-Viral Efficiency with Dust Loading. J. Hazard. Mater. 2016, 301, 547–553.
- Wang, I.-J.; Chen, Y.-C.; Su, C.; Tsai, M.-H.; Shen, W.-T.; Bai, C.-H.; Yu, K.-P. Effectiveness of the Nanosilver/TiO 2-Chitosan Antiviral Filter on the Removal of Viral Aerosols. J. Aerosol Med. Pulm. Drug Deliv. 2021, 34, 293–302.
- Castro-Mayorga, J.L.; Randazzo, W.; Fabra, M.J.; Lagaron, J.M.; Aznar, R.; Sánchez, G. Antiviral Properties of Silver Nanoparticles against Norovirus Surrogates and Their Efficacy in Coated Polyhydroxyalkanoates Systems. LWT-Food Sci. Technol. 2017, 79, 503–510.
- Pelet, T.; Wallach, D.F.H. Composition for Inactivating an Enveloped Virus. U.S. Patent 9526700B2, 27 December 2016.
- Hamouda, T.; Ibrahim, H.M.; Kafafy, H.H.; Mashaly, H.M.; Mohamed, N.H.; Aly, N.M. Preparation of Cellulose-Based Wipes Treated with Antimicrobial and Antiviral Silver Nanoparticles as Novel Effective High-Performance Coronavirus Fighter. Int. J. Biol. Macromol. 2021, 181, 990–1002.
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of Silver Nanoparticles with HIV-1. J. Nanobiotechnol. 2005, 3, 6.
- Galdiero, S.; Rai, M.; Gade, A.; Falanga, A.; Incoronato, N.; Russo, L.; Galdiero, M.; Gaikwad, S.; Ingle, A. Antiviral Activity of Mycosynthesized Silver Nanoparticles against Herpes Simplex Virus and Human Parainfluenza Virus Type 3. Int. J. Nanomed. 2013, 8, 4303.
- El-Mohamady, R.S.; Ghattas, T.A.; Zawrah, M.F.; Abd El-Hafeiz, Y.G.M. Inhibitory Effect of Silver Nanoparticles on Bovine Herpesvirus-1. Int. J. Vet. Sci. Med. 2018, 6, 296–300.
- Hu, R.L.; Li, S.R.; Kong, F.J.; Hou, R.J.; Guan, X.L.; Guo, F. Inhibition Effect of Silver Nanoparticles on Herpes Simplex Virus 2. Genet. Mol. Res. 2014, 13, 7022–7028.
- Sharmin, S.; Rahaman, M.M.; Sarkar, C.; Atolani, O.; Islam, M.T.; Adeyemi, O.S. Nanoparticles as Antimicrobial and Antiviral Agents: A Literature-Based Perspective Study. Heliyon 2021, 7, e06456.
- Shao, W.; Liu, X.; Min, H.; Dong, G.; Feng, Q.; Zuo, S. Preparation, Characterization, and Antibacterial Activity of Silver Nanoparticle-Decorated Graphene Oxide Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 6966–6973.
- Sinclair, T.R.; van den Hengel, S.K.; Raza, B.G.; Rutjes, S.A.; de Roda Husman, A.M.; Peijnenburg, W.J.G.M.; Roesink, H.D.W.; de Vos, W.M. Surface Chemistry-Dependent Antiviral Activity of Silver Nanoparticles. Nanotechnology 2021, 32, 365101.
- Baron, M.D.; Parida, S.; Oura, C.A.L. Peste Des Petits Ruminants: A Suitable Candidate for Eradication? Vet. Rec. 2011, 169, 16–21.
- Khalid, M.; Khalid, N.; Ahmed, I.; Hanif, R.; Ismail, M.; Janjua, H.A. Comparative Studies of Three Novel Freshwater Microalgae Strains for Synthesis of Silver Nanoparticles: Insights of Characterization, Antibacterial, Cytotoxicity and Antiviral Activities. J. Appl. Phycol. 2017, 29, 1851–1863.
- Abbaszadegan, M.; Lechevallier, M.; Gerba, C. Occurrence of Viruses in US Groundwaters. J. Am. Water Works Assoc. 2003, 95, 107–120.
- Hamza, I.A.; Jurzik, L.; Stang, A.; Sure, K.; Überla, K.; Wilhelm, M. Detection of Human Viruses in Rivers of a Densly-Populated Area in Germany Using a Virus Adsorption Elution Method Optimized for PCR Analyses. Water Res. 2009, 43, 2657–2668.
- Wong, M.; Kumar, L.; Jenkins, T.M.; Xagoraraki, I.; Phanikumar, M.S.; Rose, J.B. Evaluation of Public Health Risks at Recreational Beaches in Lake Michigan via Detection of Enteric Viruses and a Human-Specific Bacteriological Marker. Water Res. 2009, 43, 1137–1149.
- Yates, M.V.; Malley, J.; Rochelle, P.; Hoffman, R. Effect of Adenovirus Resistance on UV Disinfection Requirements: A Report on the State of Adenovirus Science. J. Am. Water Work. Assoc. 2006, 98, 93–106.
- Liberti, L.; Notarnicola, M.; Petruzzelli, D. Advanced Treatment for Municipal Wastewater Reuse in Agriculture. UV Disinfection: Parasite Removal and by-Product Formation. Desalination 2003, 152, 315–324.
- Sobsey, M.D.; Fuji, T.; Shields, P.A. Inactivation of Hepatitis A Virus and Model Viruses in Water by Free Chlorine and Monochloramine. Water Sci. Technol. 1988, 20, 385–391.
- Tree, J.A.; Adams, M.R.; Lees, D.N. Chlorination of Indicator Bacteria and Viruses in Primary Sewage Effluent. Appl. Environ. Microbiol. 2003, 69, 2038–2043.
- Möritz, M.; Peters, H.; Nipko, B.; Rüden, H. Capability of Air Filters to Retain Airborne Bacteria and Molds in Heating, Ventilating and Air-Conditioning (HVAC) Systems. Int. J. Hyg. Environ. Health 2001, 203, 401–409.
- Ferraris, M.; Balagna, C.; Perero, S. Method for the Application of an Antiviral Coating to a Substrate and Relative Coating. WO2019/082001, 2 May 2019.
- Balagna, C.; Irfan, M.; Perero, S.; Miola, M.; Maina, G.; Crosera, M.; Santella, D.; Simone, A.; Ferraris, M. Antibacterial Nanostructured Composite Coating on High Performance VectranTM Fabric for Aerospace Structures. Surf. Coat. Technol. 2019, 373, 47–55.
- Irfan, M.; Perero, S.; Miola, M.; Maina, G.; Ferri, A.; Ferraris, M.; Balagna, C. Antimicrobial Functionalization of Cotton Fabric with Silver Nanoclusters/Silica Composite Coating via RF Co-Sputtering Technique. Cellulose 2017, 24, 2331–2345.
- Balagna, C.; Perero, S.; Bosco, F.; Mollea, C.; Irfan, M.; Ferraris, M. Antipathogen Nanostructured Coating for Air Filters. Appl. Surf. Sci. 2020, 508, 145283.
- Joe, Y.H.; Woo, K.; Hwang, J. Fabrication of an Anti-Viral Air Filter with SiO2–Ag Nanoparticles and Performance Evaluation in a Continuous Airflow Condition. J. Hazard. Mater. 2014, 280, 356–363.
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver Nanoparticles in Polymeric Matrices for Fresh Food Packaging. J. King Saud Univ. Sci. 2016, 28, 273–279.
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; World Health Organization: Geneva, Switzerland, 2015.
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.-S.; Chen, G. Silver Nanoparticles: Synthesis, Properties, and Therapeutic Applications. Drug Discov. Today 2015, 20, 595–601.
- Pratsinis, A.; Hervella, P.; Leroux, J.-C.; Pratsinis, S.E.; Sotiriou, G.A. Toxicity of Silver Nanoparticles in Macrophages. Small 2013, 9, 2576–2584.
- Soares, T.; Ribeiro, D.; Proença, C.; Chisté, R.C.; Fernandes, E.; Freitas, M. Size-Dependent Cytotoxicity of Silver Nanoparticles in Human Neutrophils Assessed by Multiple Analytical Approaches. Life Sci. 2016, 145, 247–254.
- Zhang, Y.; Fan, W.; Sun, Y.; Chen, W.; Zhang, Y. Application of Antiviral Materials in Textiles: A Review. Nanotechnol. Rev. 2021, 10, 1092–1115.
- VIROFORMULATM: Wear a Better Future Now. Thomas Mason. Available online: https://www.thomasmason.co.uk/en/news/viroformula-wear-a-better-future-now/ (accessed on 16 February 2022).
- HeiQ Viroblock. HeiQ Materials AG. Available online: https://www.heiq.com/products/functional-textile-technologies/heiq-viroblock/ (accessed on 16 February 2022).
- Karagoz, S.; Kiremitler, N.B.; Sarp, G.; Pekdemir, S.; Salem, S.; Goksu, A.G.; Onses, M.S.; Sozdutmaz, I.; Sahmetlioglu, E.; Ozkara, E.S.; et al. Antibacterial, Antiviral, and Self-Cleaning Mats with Sensing Capabilities Based on Electrospun Nanofibers Decorated with ZnO Nanorods and Ag Nanoparticles for Protective Clothing Applications. ACS Appl. Mater. Interfaces 2021, 13, 5678–5690.
