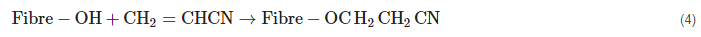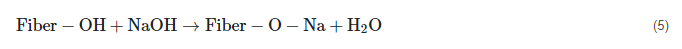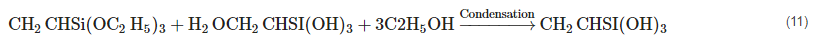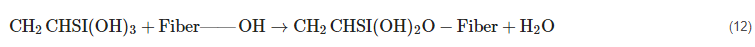1. Modifications in Physical Treatment
The most typical physical processes utilized to alter the surface of NF [
93] are corona, heat treatments, plasma, fiber, ultraviolet (UV), electron radiation, and pounding. The primary requirements for physical processing are the filamentization of the bundles of fibers and exterior alteration of the fibers to increase compatibility through the polymer matrix. By changing the fibers’ surface properties while keeping their structural makeup unaltered, these treatments increase fiber–matrix grip [
94]. Contrarily, because of the equipment used in exterior alteration processes, physical treatments are more pricy than chemical treatments [
95].
The increase in mechanical and thermal characteristics is proportional to the chemical concentration used and the exposure period. In a few cases, combining treatments with two distinct chemicals resulted in superior mechanical and thermal characteristics to the separate treatments. Improved wettability, interface adhesion, and fiber–matrix roughness were seen in NF with physical and chemical surface changes. Furthermore, the majority of treatments seek to reduce natural fiber hydrophilicity and moisture absorption. The ideal fiber modification approach will be determined by the composite end-use application and the fiber matrix employed. Understanding fiber–matrix interfacial characteristics and bonding processes is critical for optimizing NFRC and hybrid composite applications [
95].
2. Chemical Treatments
Cellulose, hemicelluloses, and lignin make up the majority of these lignocellulosic fibers. The major element that gives the fiber its strength is cellulose. Many different chemicals are used to treat NF. The primary goal is to improve the fiber cellulose content while eliminating unwanted contaminants, for example, oil and wax [
96,
97]. Chemical treatments are a typical method for improving fiber–matrix interface adhesion by chemical attachment or minimizing fiber water immersion and mechanically interconnecting at the boundary [
96,
97]. According to the literature, there are many fiber-changing methods with different levels of effectiveness. Chemical methods frequently outperform physical methods in terms of improving properties. This section covers the details of chemical treatments. The nature of the hydrophobic matrix and the fiber’s hydrophilic character produce the bulk of difficulties with natural fiber composites. Due to this inherent mismatch between the matrix and the fiber, mechanical performance is constrained by weak interfacial bonding, and long-term properties are impacted by low moisture resistance [
96,
97].
An essential factor in achieving enhanced fiber-reinforcement composite properties is the connection strength between the polymer matrix and the fiber in the composite. The fiber absorbs a remarkably high quantity of moisture because of polar clusters and dangling hydroxyl in the fiber, which lead to poor interfacial contact between the hydrophobic matrix polymers and the fiber [
98,
99]. To create composites in addition to adequate strength characteristics, fibers were chemically treated to minimize their hydrophilic behavior and moisture absorption [
98,
99]. The goal of these operations is to reduce the number of voids and amorphous areas in the fibers. Because of the enhanced packing of cellulose chains during lignin breakdown, chemically processed fiber-reinforced composites offer better strength properties over untreated fiber-reinforced composites.
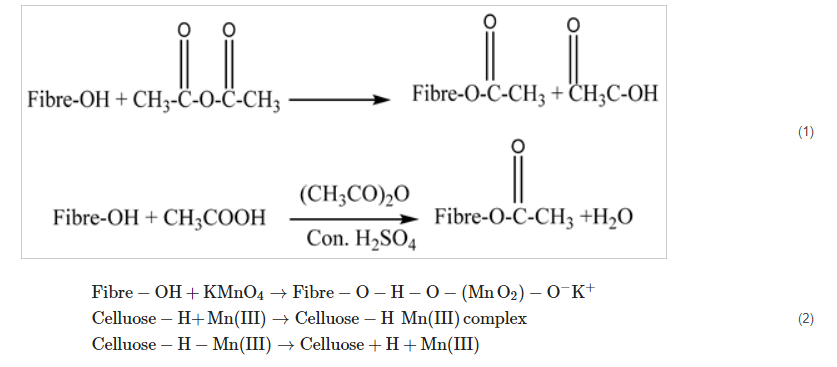
2.1. Acetylation Treatment
Chemicals containing a group of acetyls (CH
3CO), such as acetic anhydride and acetic acid, are added to produce acetylation. The cellulose fibers and the acetyl group interact, resulting in the loss of hydroxyl groups and a decreased capacity to absorb moisture. Acetylation produces a rugged surface topography with fewer voids than other chemical processes, which enhances interlocking properties [
100]. Acetylation can be performed with or without the assistance of an acid catalyst, according to Equations (1) and (2), with the inclusion of acetic anhydride resulting in a quicker reaction after the fibers have been soaked in acetic acid. Devoid of the usage of a co-solvent or a catalyst, the lignocellulosic material should be acetylated at 120–160 °C using a tiny amount of liquid acetic anhydride.
Potentially, small amounts of acetic acid might be used to open the cell wall and initiate the process. According to a tensile strength test, fibrillation happens when binding components are removed from the treated fibers and specific micropores start to appear [
101]. Additionally, when the level of acetylation rises, the fibrillation rises as well, leading to splits and deterioration of the fiber. Acetylated fiber composites also have improved resistance to UV radiation degradation, dimensional stability, and resistance to biological assault [
102]. Acetylation promotes dimensional stability by reducing water absorption [
103]. Additionally, acetylation has been demonstrated to increase the bond shear strength, materials’ stiffness, and tensile strength [
101,
104].
Equation (1) Acetylation along and Equation (2) devoid of acid catalyst [
101,
104].
2.2. Acrylation Treatment
Acrylic acid (AC) (CH
2=CHCOOH) is employed to increase polypropylene fiber adherence to their matrix. Hydroxyl radicals on cellulose interact with AC molecules to produce cellulose radicals for the polymerization medium. Peroxide radicals initiate the implanting of AC onto the matrix. Hydrogen atoms are from the hydroxyl groups eliminated from peroxide’s O-O bond in cellulose. As a result, as illustrated in Equation (3), these acids produce ester bonds with the hydroxyl groups (2). This fiber–matrix coupling procedure enhances the capacity for stress transmission at the interface and results in improving the composite properties. AC decreases fiber’s hydrophilicity [
105,
106]. At reflux temperature, aqueous 10% AC reacted with alkali-treated fibers resulting in a boost in flexural and tensile strengths by 13.9 and 42.2% in the jute–epoxy-phenolic resin composite [
107]. At 24 °C room temperature, the fibers were steeped in 10% NaOH for an hour. The wet product was then subjected to several concentrations of acrylic acid treatment. Additionally, the reaction was run for an hour at 50 °C, which had the effect of lowering the tensile modulus. On the other hand, high extensibility and increased impact resistance were noted [
108]. Compared to earlier processes, 40 weight percent fiber at 50 degrees Celsius produced a considerable quantity of moisture absorption capability (50%) [
108,
109]. In a NaOH solution, flax fibers were soaked for 30 min before spending two hours at 50 °C in an acrylic acid solution. This produced a fiber surface that was very smooth. It has a stronger tensile strength and less water absorption when compared to silane, permanganate, and sodium chloride [
110].
Equation (3). Acrylation treatment of the fiber [
108,
109].
2.3. Grafting Using Acrylonitrile
Free radicals produced by acryliconitrile (AN) dehydrate and oxidize the cellulose molecules in the fiber. Activated free-radical sites interact with the monomer of the matrix on the fiber surface. Either the available radicals can be produced in reaction media via a redox process and be carried there or the backbone can be directly oxidized by transition metal ions (such as Ce
4+, Cr
6+, and V
5+) [
111]. The Equation depicts the entire response (4)
Equation (4). Acrylonitrile grafting treatment of the fiber [
111].
The amount of lignin in the fiber has a large impact on the degree of grafting. Lignin, in general, slows polymerization and functions as an inhibitor at greater lignin levels. Grafting can increase several properties, for example, moisture absorption, solubility, chemical and heat resistance, and swelling behavior in various solvents [
2,
111]. The combination of wet fibers of Agave americana and nitric acid (0.277 mol L
−1) containing ceric ammonium nitrate was agitated at the ideal temperature and time interval after the addition of monomer components. The grafted fibers were thoroughly cleaned with distilled water after the homopolymer was removed using dimethylformamide, and they were then dried at 60 °C to get the findings shown below. As the graft percentage grew, so did the resistance to moisture, acid, and base. Graft copolymerization significantly boosted the thermal stability of the fibers [
111].
Oil palm-phenol formaldehyde fibers that had been alkali bleached (2 percent for 30 min) and oxidized (0.02 mL
−1 KMnO
4 for 10 min) had also been rinsed in water before being combined in a 30:1 ratio with 1 percent H
2SO
4 containing acrylonitrile. Additionally, it spent 120 min at 50 °C in a thermostatic water bath, where the results showed stronger elastic nature due to higher strain values and a small improvement in stiffness 28 [
2]. In comparison to previous treatments, fiber at 40 weight percent at 50 °C generated a greater moisture absorption capacity (200%) [
111]. For 10 min, a known amount of butyl acetate, potassium permanganate, concentrated HNO
3, glacial acetic acid, and 0.1 g of pineapple leaf were combined in a container. Before the examination of fibers, it is extracted and completely immersed in N-N dimethylformamide. The findings demonstrate that modified fibers have a poorer graft yield than untreated fibers [
2].
2.4. Alkaline Treatment
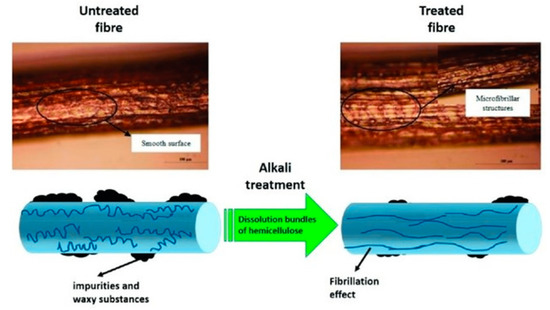
The application of a known concentration of sodium hydroxide (NaOH) onto the NF is known as alkaline treatment (mercerization). Sodium hydroxide interacts with alkali-sensitive hydroxyl groups (OH) on the exterior of fibers, resulting in the release of water particles. The moisture absorption propensity decreases as the number of hydroxyl groups decreases. It also dissolves hemicellulose, pectin, lignin, and another waxy layer in small concentrations. The chemical route between fiber and alkali is depicted in Equation (5), and
Figure 1 displays the influence of alkali treatment on NF [
112].
Figure 1. Alkali treatment on natural fiber at 100 µm [
112].
Equation (5). Alkali treatment on natural fiber.
Alkali treatment boosts mechanical interlocking at the interface by generating a bumpy surface texture, which improves the adherence of the fiber surface characteristics. This is achieved by disabling hydrogen bonding and removing synthetic and natural impurities from the network structure. Additionally, fiber has increased thermodynamic stability [
2,
102,
113,
114]. The alkali procedure removes microvoids from the surface, making it more uniform and boosting its ability to transfer stress [
2,
102,
113,
114]. When fibers are considered with sodium hydroxide (NaOH), the aspect ratio and fiber diameter both decrease, which improves the fiber–matrix interactions because more surface area is created because it increases effective surface area and is good at removing waxy coatings. For the pretreatment of fibers, mercerization is widely used prior to supplementary substances being applied for strengthening. Treatment with alkali or NaOH greatly improves mechanical, thermal, and water retention properties [
2,
102,
113,
114].
The ideal soaking time and concentration of NaOH solution are those that produce the best mechanical properties. Mechanical qualities have been shown to improve with increasing concentration. Any concentration beyond the recommended level caused harm to the fibers by enlightening them. For improved crystallinity, wettability, amorphous area reduction, and fiber diameter, Tridax procumbens fiber was treated with 5% NaOH [
36,
115]. The flexural strength and tensile modulus of the NFRCs are also enhanced by alkaline treatment [
116,
117,
118].
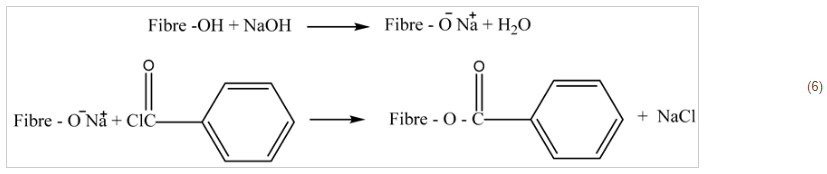
Benzoylation is an efficient technique for increasing the fibers’ thermal stability and hydrophobicity. The benzoyl (C
6H
5C
14O) group, found in the most often used chemical in this treatment, is what causes the treated fiber to be less hydrophilic and interact more favorably with the hydrophobic matrix. Equation (6) indicates that during the operation, an alkali is used to treat the fibers. When extractable fiber components, for example, oils, lignin, and waxes are eliminated, additional reactive hydroxyl groups are visible on the surface [
87,
134]. These filaments receive BP therapy. The fiber hydroxyl groups are now substituted through benzoyl groups. The surface of the fiber is made rougher by benzoylation, which leads to fibrillation and enhances strength contact along with the polymer matrix. This dramatically increases hydrophobicity and matrix adherence. In a tensile test, advanced fiber led to fiber fracture rather than fiber de-bonding [
87,
134]. With this method, moisture absorption is reduced while Young’s modulus and tensile strength are enhanced. Additionally, it enhances hardness, eliminates voids, and boosts Tg and thermal stability [
135,
136,
137,
138,
139]. Jute fibers that had undergone alkali treatment were immersed in benzoyl chloride (C
6H
5CH
2Cl) solution for 15 min before being soaked in ethanol for an hour before being combined with epoxy, improving the storage modulus and thermal stability.
The flax fibers remained dipped in NaOH solution (10%) for fifteen minutes and mixed with (C6H5CH2Cl) before immersing in ethanol for 60 min; the results show LDPE had the best tensile and impact strength, while HDPE had the highest impact severities. The alkali-treated sisal fibers remained drenched in BP and acetone for 30 min, yielding tensile strength saturation at 6% BP.
When compared to silane and peroxide treatments, it resulted in reduced water absorption [
93,
140]. The fiber surface was found to be smooth. After steeping in a 2% NaOH solution for 30 min, chopped banana NFs (6 mm) were vigorously stirred with benzoyl chloride for a further 30 min. Tensile strength and modulus were both enhanced significantly by 13% and 5%, respectively, while thermal conductivity increased noticeably. However, it is still less than that of fibers treated with alkali and silane [
93,
140].
Equation (6). Benzoylation treatment of natural fiber.
2.6. Etherification
By grafting bifunctional monomers, etherification is a chemical process that makes it simple for fiber to react with the matrix polymer chain. Regarding fibers etherified, the thermal steadiness of alfa fiber-reinforced polypropylene composites was considerably improved. Cellulosic fibers are enhanced by being ethylated, which increases their usefulness and acceptance in a number of applications [
83]. Sodium hydroxide enhances the benzyl chloride, acrylonitrile, alkyl halides, formaldehyde, and nucleophilic addition of epoxides by producing an alleged medium species including the fiber. Epoxides, like epichlorohydrin, have a stretched, three- or four-membered oxygen-rich ring that removes electrons from surrounding carbons. Because of their structure, epoxides are extremely reactive with substances containing alcohol, such as cellulose [
141]. The interaction of epichlorohydrin with wood preservatives such as pentachlorophenol, according to Rowell and Chen, results in wood alteration [
141]. Ohkoshi [
142] merged dual surfaces of wood that had been altered by allyl bromide etherification by hot pressing.
The shear strength of the preserved wood has been found to be comparable to that of unaffected wood. Furthermore, the effect of allylation on specific wood constituents [
143] and the exterior alkylated wood grafted with styrene [
142] were investigated. A wood byproduct that could be pushed into films or squeezed out into objects that could be molded was created when benzyl chloride was used in thermoplastic wood. Above 25% of NaOH concentration and temperatures over 90 °C, it was crucial to prepare the wood in order to lessen hydrolysis of the wood’s constituents. Wood and bagasse thermo-plasticization was obtained at lower reaction temperatures and with lower alkali pretreatment concentrations. Due to a lower degree of polymerization, wood pulp was shown to be more reactive in a subsequent cyanoethylation study with both cotton and wood pulp [
144,
145].
2.7. Treatment with Isocyanates
Isocyanates are organic mixtures with the N-R=O=C isocyanate functional group. These groups react strongly with hydroxyl groups, resulting in the production of urethane linkages, as illustrated in Equation (7). These are employed in the elimination of hydroxyl sorts through lignin and cellulose. Additionally, the isocyanate interacts with the hydroxyl groups in cellulose to produce urea by combining with the moisture on the fibers. The main disadvantage of alkali treatment is that it causes cellulose hydroxyl groups to lose their hydrogen bonds, making them more reactive than earlier [
123]. Isocyanate treatment is seen to be a suitable option for alkalization due to its superior effectiveness at eliminating free hydroxyl groups from the fiber’s surface. As a consequence, isocyanate treatment improves strength properties while lowering water absorption [
146].
Equation (7). Isocyanate treatment of natural fiber.
Before being treated with isocyanate using dibutyltin dilaurate as an impetus at 125 °C for 1 h in a composite made of flax-epoxy, jute, and hemp, the nonwoven fiber mat was permitted to develop in DMF for thirty minutes. The carpets were extensively washed in hot DMF after the process and dried in a 105 °C oven, yielding a product with enhanced firmness and a 17 percent reduction in influence strength [
147]. The results show that at 40 weight percent, fiber at 50 °C had a better dampness assimilation limit (280%) than other medications. The filaments were treated with a soluble base, then immersed in chloroform including toluene diisocyanate. Dibutyltin dilaurate was applied stepwise, and the mixture was unsettled for two hours and cleaned by refluxing with oil palm-pineapple fiber [
109]. The alkali-treated fibers were then treated with CCl
4 and a little quantity of dibutyltin dilaurate catalyst. The urethane derivative was poured into the flask stepwise, with steady swirling, until it was completely dissolved. The reaction was permitted to remain for 1 h, and the findings show that the dielectric constant decreased after the treatment [
148]. Fibers outperformed alkaline and untreated fibers in conditions of tensile strength, and when matched to alkaline-treated fibers, the elongation at break was doubled [
123]. Pre-dried fibers were treated with DIC in another study undertaken by Wulin et al. [
149]; prior to the fibrous cellulose–PP composite, the finished product had 30 wt% fibers.
2.8. Permanganate Treatment
In acetone, potassium permanganate (KMnO
4) produces extremely reactive MnO
4- ions; these ions form cellulose-manganate when they relate with the hydroxyl groups in cellulose. This triggers the graft copolymerization process, which results in the production of fiber with outstanding thermal stability. Generally, soaking (for 1–3 min) is undertaken after an alkaline pretreatment by applying various concentrations of KMnO
4 solution in acetone. In addition to removing the hydroxyl groups from the cellulose, it also interacts with the lignin in the cell wall and eliminates it. There is thus a high level of hydrophobicity. Fiber’s hydrophilic propensity reduces when KMnO
4 concentrations grow to an optimal amount [
89,
148]. Polar groups between fiber and matrix are produced at a concentration of 1%, resulting in cellulosic fiber breakdown [
89,
148]. The oxidation of permanganate etches the fiber surface, making it physically rougher; in this situation, the induced mechanical interlocking improves interfacial adhesion while simultaneously improving the contact space between the matrix and fiber [
123].
The strength of this fusing of a scratched matrix with the fibers induced by oxidation is comparable to chemical bonding observed with silane treatment [
134]. When NFRCs are permanganate-treated, their ductility, flexural strength, impact strength, thermal stability, and flexural modulus are all enhanced [
162,
163,
164]. KMnO
4 improves fiber roughness and converts cellulose hydroxyl groups to carboxyl and aldehyde groups [
162]. A sisal fiber was treated with alkali and soaked in acetone for 1 min in permanganate solutions (0.033 percent, 0.0625 percent, and 0.125 percent concentrations). The hydrophilicity of fiber reduced as the quantity of KMnO
4 increased. However, the maximum cellulose degradation was seen at a KMnO
4 concentration of 1% [
148]. In another study, ref. [
36], alkali-pretreated fibers were immersed in permanganate solution (conc. of 0.01%, 0.05%, and 0.1%) in acetone for about 2–3 min of the oil palm–PF composite, and in comparison to previous chemical treatments, the findings demonstrate the highest tensile strength and modulus.
A highly fibrillated structure results in an excellent fiber matrix. After 30 min of soaking in a 0.5 percent KMnO
4-acetone solution, the alkali-treated banana NF yielded a 16 percent yield. Thermal diffusivity rises [
131]. Modulus and tensile strength increased by 7.5% and 6.4%, respectively. Modulus and flexural strength increased by 10% and 5%, respectively, which is less than in alkali and silane treatments [
165]. The alkali-treated sisal fibers were immersed in KMnO
4 solutions of various dilutions in acetone for 60 s, and the results were quite successful. The cellulose decomposed quicker at 1% concentration, producing more arctic groups compared to unprocessed composites [
148]. Sisal fibers were bathed in a 0.055% permanganate acetone solution for 2 min, which resulted in greater inter-laminar shear strength, tensile characteristics, and flexural characteristics than silane-treated fibers; nonetheless, untreated fibers absorb less impact energy [
134].
2.9. Maleated Coupling Agents
The inclusion of maleated coupling agents improves the interfacial interaction between the matrix and fiber. MA interacts with the hydroxyl groups during grafting and eliminates them from the fiber cells [
94]. Before the treatment, the copolymer is warmed to 170 °C and then the esterification operation is carried out. Following this treatment, the cellulose fiber’s surface energy is significantly closer to that of the matrix. Enhanced wettability of the fiber causes an increase in interfacial adhesion [
36]. A separate research study looked at the utility of MAPP in improving thermal deflection temperature, flexural strength, tensile strength, and impact energy absorption when mixed with short Latania fiber at an ideal concentration of 2 wt% [
166]. Huang et al. proved the efficiency of wood–polypropylene composites in increasing flexural strength and decreasing water absorption in their paper [
167]. Maleated polyolefins (MaPOs) span the gap between polar and nonpolar species thanks to a unique confluence of properties. This, together with the fact that they are easy to make, contributes to MaPOs’ popularity [
168].
2.10. Treatment with Peroxide
The divalent O-O ion is a component of functional groups called organic peroxides (RO-OR). These peroxides are highly susceptible to oxidizing into free radicals. As seen in Equation (8), these extremely reactive available radicals react with hydrogen in the hydroxyl groups.
Furthermore, when the free radical combines with a matrix molecule, such as polyethylene (PE-H), the reaction described in Equation (9) occurs, yielding polyethylene (PE).
Equation illustrates how the PE radicals may interact and cross-link or graft onto cellulose fibers (10)
Dicumyl peroxide ((C
6H
3C(CH
3)
2O)
2) and benzoyl peroxide (C
6H
5CO)
2O
2) are two organic peroxide treatments which are often applied. When composites are being cured, the peroxide breakdown proces takes place at the contact. Higher temperatures encourage the decomposition of peroxides [
108]. For a certain fiber composition, the composite’s tensile strength obtained its highest value at a critical peroxide concentration. At this stage, grafted polymer (matrix) molecules have been applied to the fibers, and further peroxide addition increases the cross-linking of the matrix molecules, but this does not have influence on the mechanical characteristics of the composite [
123]. Water absorption is reduced while thermal stability, interfacial adhesion, tensile strength, and elastic modulus between the fiber and matrix are enhanced by peroxide treatment.
An organic peroxide treatment that is often utilized is dicumyl peroxide ((C
6H
3C(CH
3)
2O)
2) and benzoyl peroxide (BP, (C
6H
5CO)
2O
2). The peroxide disintegration process happens at the contact during the drying of composites. The breakdown of peroxides is promoted at higher temperatures [
108]. For a particular fiber composition, there is a crucial peroxide dilution through which the composite tensile strength attains the maximum. In this phase, the fibers have been coated with grafted polymer (matrix) molecules, and consequent peroxide supplement adds to certain matrix cross-linking, which has no influence on composite strength properties [
123]. Peroxide treatment thereby decreases water absorption while boosting interfacial adhesion between the matrix and fiber, thermal stability, tensile strength, and modulus of elasticity [
85,
86,
87,
110,
170,
171].
2.11. Silane Treatment
As a coupling agent, silane (SiH4), a versatile chemical, is utilized to increase the fiber–matrix interface. Several phases of bond formation, condensation, and hydrolysis occur throughout the fiber treatment process. Silanol is produced in the presence of moisture. Equation (11) depicts the reaction process.
When silanol condenses, one side of the substance interacts with the functional group of the matrix, while the other side alters the cellulose hydroxyl group of the fiber to establish a chemical connection (12).
In general, silane treatment begins with a silane derivative (usually amine) in acetone/ alcohol/solution. When the fibers are immersed in the silane solution, they interact with the matrix more effectively compared to alkaline-treated fibers, resulting in higher thermal stability, flexural stiffness, tensile strength, and tensile modulus. Sisal fibers were treated for 2 h with a 2 wt% solution (pH 3.5, room temperature), resulting in an epoxy composite with better impact strength than alkalized fibers. Valadez et al. [
172] and Agrawal et al. [
173]. Additionally, silane solutions at concentrations of 0.033 and 1% were used to treat palm oil and henequen fibers, respectively. Crystallinity proportion and interfacial interactions were shown to be improved. Fibers were treated for 1 h with a 1 percent oligomeric siloxane 96 percent alcohol solution. Comparing alkali treatment alone to alkali treatment with silane treatment, modulus and strength improved by 7% and 12% [
174]. In another research study, the author used natural hemp and basalt fibers that had been treated with cyanate ester and benzoxazine, respectively. According to the researchers, flexural strength, micro-hardness, and thermal stability all increased [
175,
176]. Atiqah and co. studied sugar palm fibers treated with silane, alkaline, and a silane–alkaline combination. Improved fiber–matrix bonding was achieved by eliminating the outer layers, which include contaminants, fewer nodes, wax, and pectin [
177].
3. Biological (Fungi and Enzymes)
The above-mentioned chemical treatments for surface modification have several advantages, but they are hazardous and might cause difficulties for the environment. Enzyme therapy is a modern biochemical treatment procedure that is gaining popularity due to its environmental friendliness. Enzymes primarily target non-cellulosic components of fiber; also, enzymes may be regenerated and are cost-effective [
2,
32].
This entry is adapted from the peer-reviewed paper 10.3390/jcs7030120