Electronic cigarettes (e-cigarettes) are often considered a “safe substitute” for conventional cigarette cessation. The composition of the fluid is not always clearly defined and shows a large variation within brands and manufacturers. More than 80 compounds were detected in liquids and aerosols. E-cigarettes contain nicotine, and the addition of flavorings increases the toxicity of e-cigarette vapour in a significant manner. The heat generated by the e-cigarette leads to the oxidation and decomposition of its components, eventually forming harmful constituents in the inhaled vapour. The effects of these toxicants on male and female reproduction are well established in conventional cigarette smokers.
- e-cigarette
- oocyte
- sperm
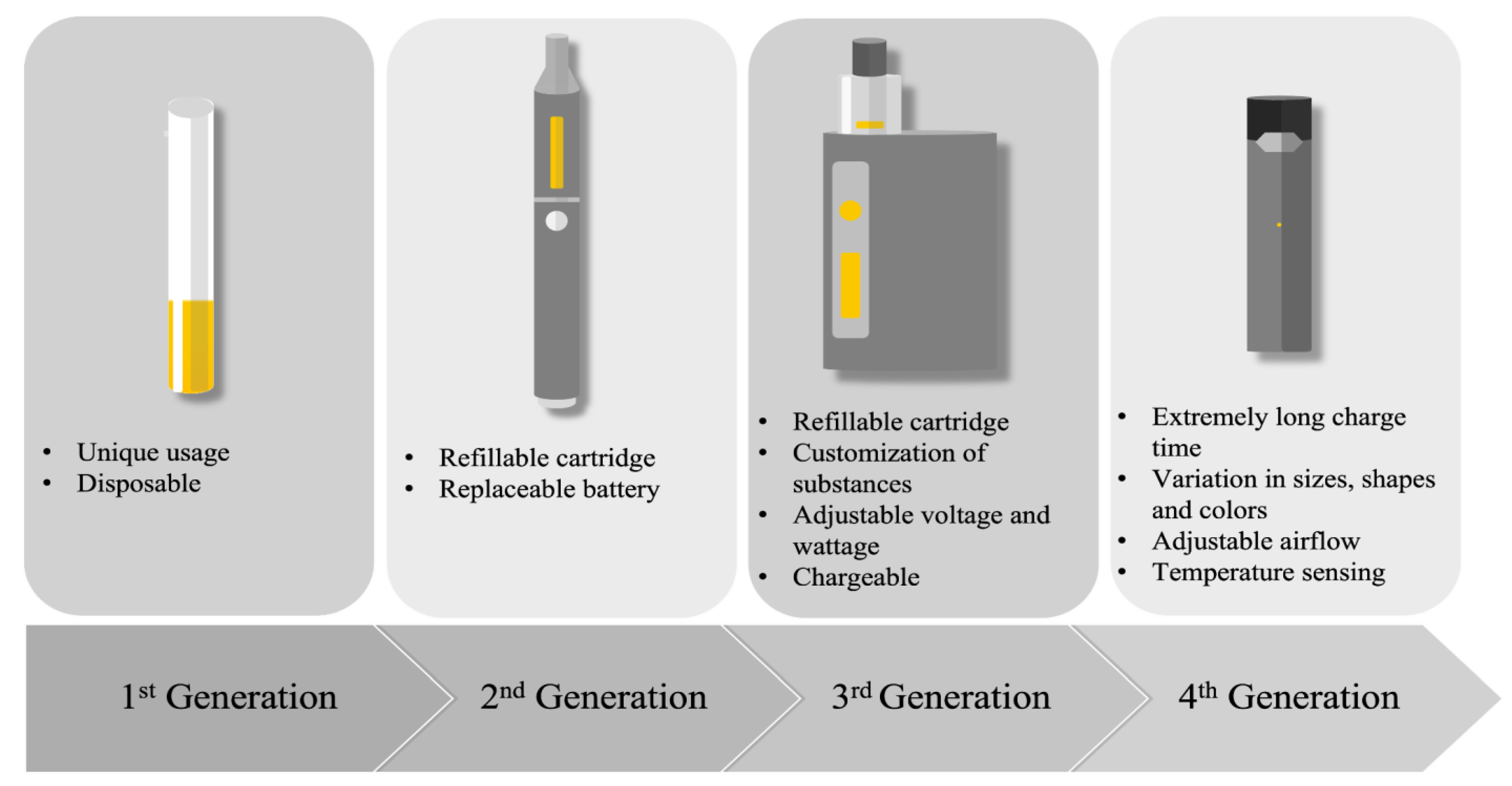
1. E-Cigarettes: Types, Usage
2. Reprotoxicological Profile of E-Cigarette Components
2.1. Nicotine
2.2. Flavouring Compounds
2.3. Heavy Metals
2.4. E-Cigarette Vapour
3. Evidence of the Impact of E-Cigarette Exposure on Reproduction
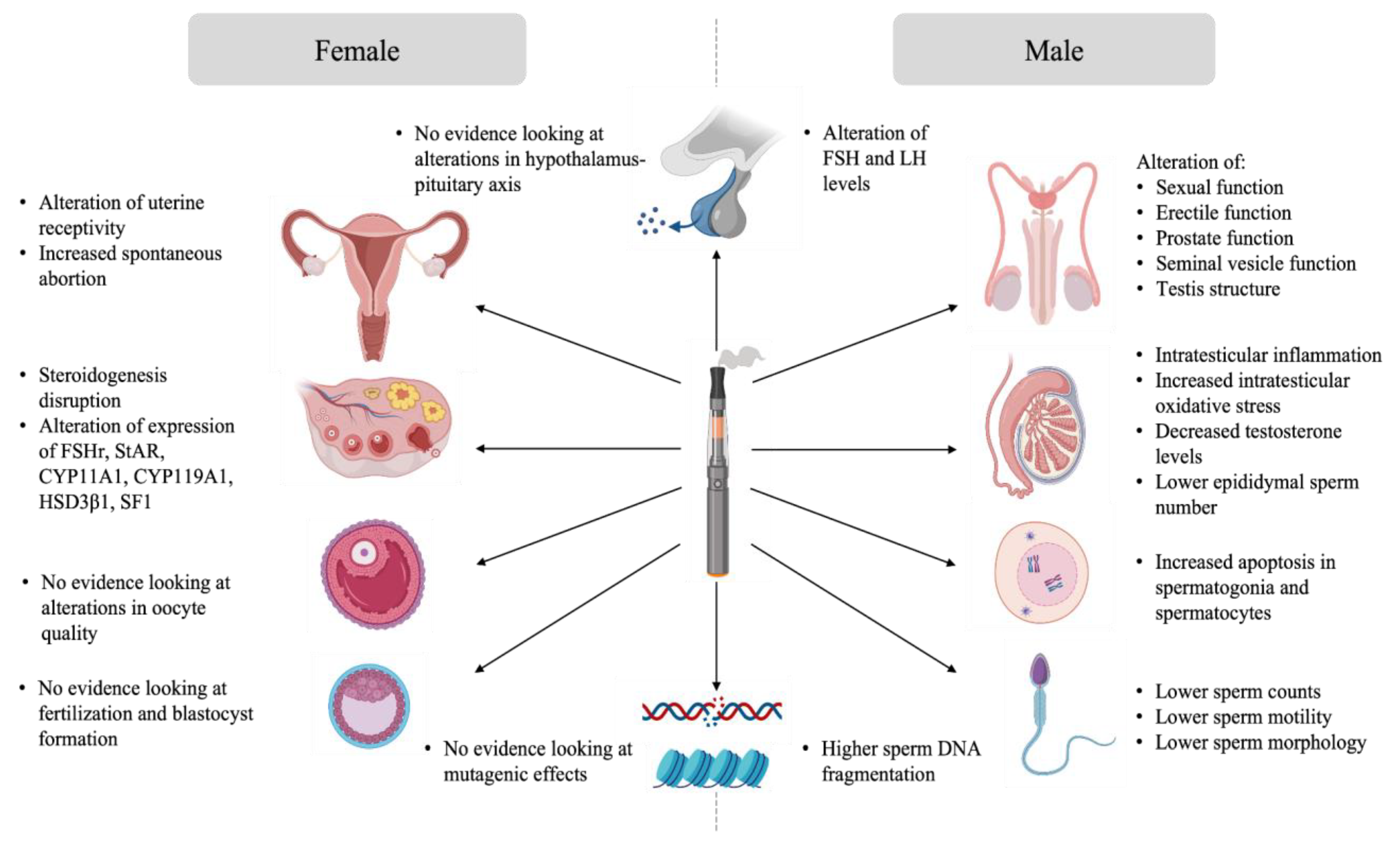
3.1. Evidence Analysis of the Impact of E-Cigarette on Male Reproduction
3.2. Evidence Analysis of the Impact of E-Cigarette on Female Reproduction
3.3. Evidence Analysis of the Impact of E-Cigarette on Assisted Reproductive Technologies Outcomes
This entry is adapted from the peer-reviewed paper 10.3390/life13030827
References
- Bertholon, J.F.; Becquemin, M.H.; Annesi-Maesano, I.; Dautzenberg, B. Electronic cigarettes: A short review. Respiration 2013, 86, 433–438.
- Weisberg, E. Smoking and reproductive health. Clin. Reprod. Fertil. 1985, 3, 175–186.
- Jandíková, H.; Dušková, M.; Stárka, L. The influence of smoking and cessation on the human reproductive hormonal balance. Physiol. Res. 2017, 66, S323–S331.
- Ochedalski, T.; Lachowicz-Ochedalska, A.; Dec, W.; Czechowski, B. Examining the effects of tobacco smoking on levels of certain hormones in serum of young men. Ginekol. Pol. 1994, 65, 87–93.
- Vine, M.F. Smoking and male reproduction: A review. Int. J. Androl. 1996, 19, 323–337.
- Harte, C.B.; Meston, C.M. Acute Effects of Nicotine on Physiological and Subjective Sexual Arousal in Nonsmoking Men: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Sex. Med. 2008, 5, 110–121.
- El Golli, N.; Rahali, D.; Jrad-Lamine, A.; Dallagi, Y.; Jallouli, M.; Bdiri, Y.; Ba, N.; Lebret, M.; Rosa, J.; El May, M.; et al. Impact of electronic-cigarette refill liquid on rat testis. Toxicol. Mech. Methods 2016, 26, 417–424.
- Rahali, D.; Jrad-Lamine, A.; Dallagi, Y.; Bdiri, Y.; Ba, N.; El May, M.; El Fazaa, S.; El Golli, N. Semen Parameter Alteration, Histological Changes and Role of Oxidative Stress in Adult Rat Epididymis on Exposure to Electronic Cigarette Refill Liquid. Chin. J. Physiol. 2018, 61, 75–84.
- Vivarelli, F.; Canistro, D.; Cirillo, S.; Cardenia, V.; Rodriguez-Estrada, M.T.; Paolini, M. Impairment of testicular function in electronic cigarette (e-cig, e-cigs) exposed rats under low-voltage and nicotine-freeconditions. Life Sci. 2019, 228, 53–65.
- Wawryk-Gawda, E.; Zarobkiewicz, M.K.; Chłapek, K.; Chylinska-Wrzos, P.; Jodłowska-Jedrych, B. Histological changes in the reproductive system of male rats exposed to cigarette smoke or electronic cigarette vapor. Toxicol. Environ. Chem. 2019, 101, 404–419.
- Pacifici, R.; Altieri, I.; Gandini, L.; Lenzi, A.; Pichini, S.; Rosa, M.; Zuccaro, P.; Dondero, F. Nicotine, cotinine, and trans-3-hydroxycotinine levels in seminal plasma of smokers: Effects on sperm parameters. Ther. Drug Monit. 1993, 15, 358–363.
- Sharma, R.; Harlev, A.; Agarwal, A.; Esteves, S.C. Cigarette Smoking and Semen Quality: A New Meta-analysis Examining the Effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur. Urol. 2016, 70, 635–645.
- Vine, M.F.; Tse, C.K.; Hu, P.; Truong, K.Y. Cigarette smoking and semen quality. Fertil. Steril. 1996, 65, 835–842.
- Harlev, A.; Agarwal, A.; Gunes, S.O.; Shetty, A.; du Plessis, S.S. Smoking and Male Infertility: An Evidence-Based Review. World J. Men Health 2015, 33, 143–160.
- El Mulla, K.F.; Köhn, F.M.; El Beheiry, A.H.; Schill, W.B. The effect of smoking and varicocele on human sperm acrosin activity and acrosome reaction. Hum. Reprod. 1995, 10, 3190–3194.
- Soares, S.; Simón, C.; Remohi, J.; Pellicer, A. Cigarette smoking affects uterine receptiveness. Hum. Reprod. 2006, 22, 543–547.
- Heger, A.; Sator, M.; Walch, K.; Pietrowski, D. Smoking Decreases Endometrial Thickness in IVF/ICSI Patients. Geburtshilfe Und Frauenheilkd. 2018, 78, 78–82.
- Orzabal, M.R.; Lunde-Young, E.R.; Ramirez, J.I.; Howe, S.Y.; Naik, V.D.; Lee, J.; Heaps, C.L.; Threadgill, D.W.; Ramadoss, J. Chronic exposure to e-cig aerosols during early development causes vascular dysfunction and offspring growth deficits. Transl. Res. 2019, 207, 70–82.
- Szumilas, K.; Szumilas, P.; Grzywacz, A.; Wilk, A. The Effects of E-Cigarette Vapor Components on the Morphology and Function of the Male and Female Reproductive Systems: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 6152.
- Akar, Y.; Ahmad, N.; Khalıd, M. The effect of cadmium on the bovine in vitro oocyte maturation and early embryo development. Int. J. Veter Sci. Med. 2018, 6, S73–S77.
- O’Neill, H.; Nutakor, A.; Magnus, E.; Bracey, E.; Williamson, E.; Harper, J. Effect of Electronic-Cigarette Flavourings on (I) Human Sperm Motility, Chromatin Integrity in Vitro and (II) Mice Testicular Function in Vivo. 2017. Available online: http://srf-reproduction.org/wp-content/uploads/2017/01/Fertility-2017-Final-Programme-and-Abstracts.pdf (accessed on 20 January 2023).
- Holden, L.L.; Truong, L.; Simonich, M.T.; Tanguay, R.L. Assessing the hazard of E-Cigarette flavor mixtures using zebrafish. Food Chem. Toxicol. 2020, 136, 110945.
- Thirión-Romero, I.; Pérez-Padilla, R.; Zabert, G.; Barrientos-Gutiérrez, I. Respiratory impacy of electronic cigarettes and “low-risk” tobacco. Rev. Investig. Clin. 2019, 71, 17–27.
- Marzec-Wróblewska, U.; Kamiński, P.; Lakota, P.; Szymański, M.; Wasilow, K.; Ludwikowski, G.; Kuligowska-Prusińska, M.; Odrowąż-Sypniewska, G.; Stuczyński, T.; Michałkiewicz, J. Zinc and iron concentration and SOD activity in human semen and seminal plasma. Biol. Trace Elem. Res. 2011, 143, 167–177.
- Marzec-Wróblewska, U.; Kamiński, P.; Łakota, P.; Szymański, M.; Wasilow, K.; Ludwikowski, G.; Jerzak, L.; Stuczyński, T.; Woźniak, A.; Buciński, A. Human Sperm Characteristics with Regard to Cobalt, Chromium, and Lead in Semen and Activity of Catalase in Seminal Plasma. Biol. Trace Elem. Res. 2019, 188, 251–260.
- Shi, X.; Chan, C.P.S.; Man, G.K.Y.; Chan, D.Y.L.; Wong, M.H.; Li, T.C. Associations between blood metal/ metalloid concentration and human semen quality and sperm function: A cross-sectional study in Hong Kong. J. Trace Elem. Med. Biol. 2021, 65, 126735.
- Zhang, X.F.; Gurunathan, S.; Kim, J.H. Effects of silver nanoparticles on neonatal testis development in mice. Int. J. Nanomed. 2015, 10, 6243–6256.
- Zhang, T.; Ru, Y.F.; Bin Wu, B.; Dong, H.; Chen, L.; Zheng, J.; Li, J.; Wang, X.; Wang, Z.; Wang, X.; et al. Effects of low lead exposure on sperm quality and sperm DNA methylation in adult men. Cell Biosci. 2021, 30, 110–150.
- Chen, C.; Li, B.; Huang, R.; Dong, S.; Zhou, Y.; Song, J.; Zeng, X.; Zhang, X. Involvement of Ca2+ and ROS signals in nickel-impaired human sperm function. Ecotoxicol. Environ. Saf. 2022, 231, 113181.
- Zhao, L.L.; Ru, Y.F.; Liu, M.; Tang, J.N.; Zheng, J.F.; Wu, B.; Gu, Y.H.; Shi, H.J. Reproductive effects of cadmium on sperm function and early embryonic development in vitro. PLoS ONE 2017, 12, e0186727.
- Roychoudhury, S.; Nath, S.; Massanyi, P.; Stawarz, R.; Kacaniova, M.; Kolesarova, A. Copper-induced changes in reproductive functions: In vivo and in vitro effects. Physiol. Res. 2016, 65, 11–22.
- Kong, L.; Tang, M.; Zhang, T.; Wang, D.; Hu, K.; Lu, W.; Wei, C.; Liang, G.; Pu, Y. Nickel Nanoparticles Exposure and Reproductive Toxicity in Healthy Adult Rats. Int. J. Mol. Sci. 2014, 15, 21253–21269.
- Yiqin, C.; Yan, S.; Peiwen, W.; Yiwei, G.; Qi, W.; Qian, X.; Panglin, W.; Sunjie, Y.; Wenxiang, W. Copper exposure disrupts ovarian steroidogenesis in human ovarian granulosa cells via the FSHR/CYP19A1 pathway and alters methylation patterns on the SF-1 gene promoter. Toxicol. Lett. 2022, 356, 11–20.
- Anttila, A.; Sallmén, M. Effects of parental occupational exposure to lead and other metals on spontaneous abortion. J. Occup. Environ. Med. 1995, 37, 915–921.
- Vosoughi, S.; Khavanin, A.; Salehnia, M.; Mahabadi, H.A.; Shahverdi, A.; Esmaeili, V. Adverse Effects of Formaldehyde Vapor on Mouse Sperm Parameters and Testicular Tissue. Int. J. Fertil. Steril. 2013, 6, 250–267.
- Zang, Z.-J.; Fang, Y.-Q.; Ji, S.-Y.; Gao, Y.; Zhu, Y.-Q.; Xia, T.-T.; Jiang, M.-H.; Zhang, Y.-N. Formaldehyde Inhibits Sexual Behavior and Expression of Steroidogenic Enzymes in the Testes of Mice. J. Sex. Med. 2017, 14, 1297–1306.
- Wang, H.-X.; Wang, X.-Y.; Zhou, D.-X.; Zheng, L.-R.; Zhang, J.; Huo, Y.-W.; Tian, H. Effects of low-dose, long-term formaldehyde exposure on the structure and functions of the ovary in rats. Toxicol. Ind. Heal. 2012, 29, 609–615.
- Wang, H.-X.; Li, H.-C.; Lv, M.-Q.; Zhou, D.-X.; Bai, L.-Z.; Du, L.-Z.; Xue, X.; Lin, P.; Qiu, S.-D. Associations between occupation exposure to Formaldehyde and semen quality, a primary study. Sci. Rep. 2015, 5, 15874.
- Balabanič, D.; Rupnik, M.S.; Klemenčič, A.K. Negative impact of endocrine-disrupting compounds on human reproductive health. Reprod. Fertil. Dev. 2011, 23, 403–416.
- Longo, V.; Forleo, A.; Ferramosca, A.; Notari, T.; Pappalardo, S.; Siciliano, P.; Capone, S.; Montano, L. Blood, urine and semen Volatile Organic Compound (VOC) pattern analysis for assessing health environmental impact in highly polluted areas in Italy. Environ. Pollut. 2021, 286, 117410.
- Yang, P.; Wang, Y.-X.; Chen, Y.-J.; Sun, L.; Li, J.; Liu, C.; Huang, Z.; Lu, W.-Q.; Zeng, Q. Urinary Polycyclic Aromatic Hydrocarbon Metabolites and Human Semen Quality in China. Environ. Sci. Technol. 2017, 51, 958–967.
- Khoudja, R.Y.; Xu, Y.; Li, T.; Zhou, C.J. Better IVF outcomes following improvements in laboratory air quality. J. Assist. Reprod. Genet. 2013, 30, 69–76.
- Hall, J.; Gilligan, A.; Schimmel, T.; Cecchi, M.; Cohen, J. The origin, effects and control of air pollution in laboratories used for human embryo culture. Hum. Reprod. 1998, 13 (Suppl. S4), 146–155.
- Chen, T.; Wu, M.; Dong, Y.; Kong, B.; Cai, Y.; Hei, C.; Wu, K.; Zhao, C.; Chang, Q. Effect of e-cigarette refill liquid on follicular development and estrogen secretion in rats. Tob. Induc. Dis. 2022, 20, 36.
- Wetendorf, M.; Randall, L.T.; Lemma, M.T.; Hurr, S.H.; Pawlak, J.B.; Tarran, R.; Doerschuk, C.M.; Caron, K.M. E-Cigarette Exposure Delays Implantation and Causes Reduced Weight Gain in Female Offspring Exposed In Utero. J. Endocr. Soc. 2019, 3, 1907–1916.
- McGrath-Morrow, S.A.; Hayashi, M.; Aherrera, A.; Lopez, A.; Malinina, A.; Collaco, J.M.; Neptune, E.; Klein, J.D.; Winickoff, J.P.; Breysse, P.; et al. The Effects of Electronic Cigarette Emissions on Systemic Cotinine Levels, Weight and Postnatal Lung Growth in Neonatal Mice. PLoS ONE 2015, 10, e0118344.
- Smith, D.; Aherrera, A.; Lopez, A.; Neptune, E.; Winickoff, J.P.; Klein, J.D.; Chen, G.; Lazarus, P.; Collaco, J.M.; McGrath-Morrow, S.A. Adult Behavior in Male Mice Exposed to E-Cigarette Nicotine Vapors during Late Prenatal and Early Postnatal Life. PLoS ONE 2015, 10, e0137953.
- Greene, R.M.; Pisano, M.M. Developmental toxicity of e-cigarette aerosols. Birth Defects Res. 2019, 111, 1294–1301.
- Firns, S.; Cruzat, V.; Keane, K.N.; Joesbury, K.A.; Lee, A.H.; Newsholme, P.; Yovich, J.L. The effect of cigarette smoking, alcohol consumption and fruit and vegetable consumption on IVF outcomes: A review and presentation of original data. Reprod. Biol. Endocrinol. 2015, 13, 134.
- Freour, T.; Masson, D.; Mirallie, S.; Jean, M.; Bach, K.; Dejoie, T.; Barriere, P. Active smoking compromises IVF outcome and affects ovarian reserve. Reprod. Biomed. Online 2008, 16, 96–102.
- Klonoff-Cohen, H. Female and male lifestyle habits and IVF: What is known and unknown. Hum. Reprod. Update 2005, 11, 179–203.
- Klonoff-Cohen, H.; Natarajan, L.; Marrs, R.; Yee, B. Effects of female and male smoking on success rates of IVF and gamete intra-Fallopian transfer. Hum. Reprod. 2001, 16, 1382–1390.
- Pisinger, C.; Døssing, M. A systematic review of health effects of electronic cigarettes. Prev. Med. 2014, 69, 248–260.
- Fuoco, F.; Buonanno, G.; Stabile, L.; Vigo, P. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ. Pollut. 2014, 184, 523–529.
- Pellegrino, R.M.; Tinghino, B.; Mangiaracina, G.; Marani, A.; Vitali, M.; Protano, C.; Osborn, J.F.; Cattaruzza, M.S. Electronic cigarettes: An evaluation of exposure to chemicals and fine particulate matter (PM). Ann. Ig. 2012, 24, 279–288.
- Williams, M.; Villarreal, A.; Bozhilov, K.; Lin, S.; Talbot, P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS ONE 2013, 8, e57987.
- Lerner, C.A.; Sundar, I.K.; Watson, R.M.; Elder, A.; Jones, R.; Done, D.; Kurtzman, R.; Ossip, D.J.; Robinson, R.; McIntosh, S.; et al. Environmental health hazards of e-cigarettes and their components: Oxidants and copper in e-cigarette aerosols. Environ. Pollut. 2015, 198, 100–107.
- McAuley, T.R.; Hopke, P.; Zhao, J.; Babaian, S. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal. Toxicol. 2012, 24, 850–857.
- Goniewicz, M.L.; Knysak, J.; Gawron, M.; Kosmider, L.; Sobczak, A.; Kurek, J.; Prokopowicz, A.; Jablonska-Czapla, M.; Rosik-Dulewska, C.; Havel, C.; et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 2014, 23, 133–139.
- St. Helen, G.; Havel, C.; Dempsey, D.A.; Jacob, P., 3rd; Benowitz, N.L. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction 2016, 111, 535–544.
