Killer yeast for the Biological Control of Postharvest Fungal Crop Diseases
- killer yeast, fruits, postharvest, preharvest
1. Introduction
Postharvest fungal diseases of fruit and vegetables cause major crop losses ranging from 25% in industrialized up to 50% in developing countries [1]. Counteractive measures include chemical, physical, and biological approaches. Fungicides most frequently serve to combat fungal infestations of field crops as such chemically synthesized compounds are rather inexpensive, can be stored for long periods of time, and they are sufficiently effective. However, concern is continuously rising over their routine use because, besides threatening human health [2,3,4] and the environment [5], resistant fungal pathogenic biotypes concomitantly arose [6,7,8]. It is, thus, not surprising that a number of countries, such as the USA and those that form the European Union, promote a project called “Integrated Pest Management (IPM)” which aims at reducing or, whenever possible, completely replacing chemical pesticides [9,10,11,12,13,14].
2. Yeasts as Biocontrol Agents
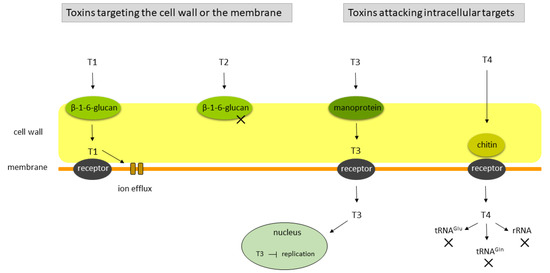
2.1. The Yeast Killer Phenotype
2.2. Genetics of the Killer Phenotype
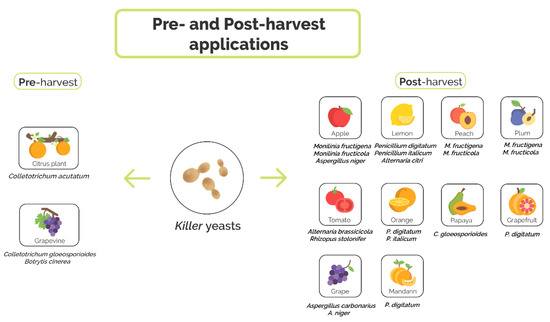
3. Application of Killer Yeasts as Biocontrol Agents
Fruit and vegetables seem to provide a favorable environment for yeasts in general and for killer yeasts in particular since approximately a quarter of the strains isolated from such source display the phenotype [42,45]. Killer yeasts have a great potential to act as biocontrol agents, producing toxins which do not harm humans or the fruit [74]. Moreover, killer yeasts display fruit-protective qualities in both pre- and postharvest applications.
3.1. Preharvest Application of Killer Yeast
3.2. Postharvest Application of Killer Yeasts
In spite of the rather promising results obtained in preharvest biocontrol attempts, postharvest use of killer yeasts is most common [80].
In citrus, the main fungal postharvest affliction is the “green mold disease” produced by P. digitatum. Control efforts routinely include chemical fungicides such as imazalil or thiabendazole [81]. Seeking for safer alternatives, we isolated more than 400 native yeasts originating from citrus, 8.5% of which showed killer activities [82]. In in vivo assays, two strains of Pichia sp. (strains 27 and 28), and Wickerhamomyces sp. strain 56 conferred significant shelter from the decay caused by P. digitatum. Efficiencies reached 93.6%, 82.5%, and 72.5%, respectively. Furthermore, the native killer yeast Clavispora lusitaniae 146 displayed, again in in vivo tests, strong antagonistic activities against the fungal pathogen (Figure 3), conferring consistent protection during the entire harvesting period [39]. The biocontrol potential of C. lusitaniae 146 was seen to be stronger than that of a commercial product containing C. oleophila. Moreover, the strain was able to grow with agriculturally applied concentrations of fungicides, which allows for the combined use of the yeast along with—then minor—doses of the fungicides. The strain’s commercial potential unequivocally became evident when we studied action mechanisms, such as wound colonization and the inhibition of P. digitatum spore germination, as C. lusitaniae 146 was seen to efficiently control even a fungicide-resistant P. digitatum strain. In addition, strain 146 recently demonstrated a broad range of protective activity in various citrus varieties besides lemons, such as mandarins, oranges, and grapefruits [83,84,85].
Several killer species are able to control the green mold disease of the Tarocco orange (Citrus sinensis), a predominant cultivar in Eastern Sicily/Italy [86]. S. cerevisiae (strains BS46 and BCA61) and Wickerhamomyces anomalus (strains BCU24, BS91, BS92, and BCA15) isolated from fermented olives were tested in vivo and in vitro for their capability to restrain P. digitatum. Only W. anomalus gave positive results in vitro; the in vivo antagonism assays eventually identified W. anomalus BS91, BS92 and BCA15 to be most effective with respect to the reduction of the disease incidence (1, 4 and 44%, respectively). The deleterious and vice versa the positive effect on the pathogen and the cultivar, respectively, lasted up to 10 storage days counted from the beginning of the artificially provoked infection. In vitro, W. anomalus BS91 had an effect on mycelial structures of P. digitatum: the hyphae became wilted, folded, and coiled with a grainy appearance, an effect presumably due to the production of hydrolytic enzymes by BS91 (β-1, 3-glucanase) as previously reported by Muccilli et al. [70,87].
Only rather recently yeasts capable of antagonizing Penicillium italicum in “Valencia” sweet oranges ((Citrus sinensis (L.) Osbeck (rutaceae)) were isolated [88]. The fungus can infect injured fruit peels, generating the symptomatology known as “blue mold disease”. Sapping all parts of the fruit the fungus makes infected oranges uneatable. From 14 citrus producing areas of the São Paulo State/Brazil, ninety-seven yeast strains were isolated from leaves, flowers, fruits, and soils. Screening for protective representatives was performed in vivo and in vitro. Candida stellimalicola strains ACBL-04 and ACBL-05, and S. cerevisiae ACBL-11 and ACBL-10 were most successful in preventive treatments, whereas ACBL-08 additionally displayed curative effects. With respect to the control mechanism, the killer trait appeared to be crucial for the biocontrol of P. italicum.
Monilinia fructigena and Monilinia fructicola are major pathogens of stone fruit (peaches and plums). They cause a panoply of symptoms including blossom blighting, woody cankers, and preharvest fruit rotting; however, the most serious damage happens after harvesting during transport and storage. Since currently there is no efficient treatment to control Monilinia spp. infections, biocontrol agents, among them killer yeasts, were considered to presumably meet the challenge [89]. Debaryomyces hansenii MI1a and D. hansenii K12a, isolated from blue-veined Rokpol cheese, and W. anomalus BS91, previously shown control P. digitatum in oranges (see above and [86]), were selected due to their killer phenotype and antagonistic activity against other fungal pathogens. Exclusively W. anomalus BS91 was able to inhibit both of the Monilinia pathogens by producing volatile antifungal compounds in an in vitro dual culture method. In vivo antagonism tests identified D. hansenii MI1A as totally M. fructicola ineffective in both plum and peach, and there was only a weak impact on M. fructigena. D. hansenii K12a and W. anomalus BS91 conferred clear fruit protection. When peaches were treated 24 h prior to inoculation with M. fructigena, the disease severity was mitigated to 33 and 25%, respectively. For the plums, a similar effect against M. fructigena was seen. The effect observed for peaches was even more promising as both, D. hansenii K12a and W. anomalus BS91 lessened disease severity caused by M. fructicola to 55%, when applied 24 h before the fungus was added. For plums, the best results were obtained with W. anomalus BS91 that reduced disease severity to 30%.
Cells and killer toxins from D. hansenii strains (TEM8 and TEM7), previously isolated from a Turkish-style homemade dairy product [90], were tested for their biocontrol efficiency in tomato against Alternaria brassicicola and Rhizopus stolonifer [91]. A. brassicicola is a necrotrophic fungus that causes the black spot disease in a broad range of host plants [92] and R. stolonifer is an important soft rot-causing fungus affecting tomatoes during postharvest stages [93]. The efficiency of the toxin was compared with the action of viable yeast cells. When the fruit was inoculated with a killer yeast or treated with killer toxins prior to applying fungal spore suspensions, the lesion sizes were smaller than for the control, and there were even samples with no damages at all. Furthermore, the killer proteins and the cells also cropped up to be effective against Alternaria citri and A. niger in lemons and apples, indicating a wider application spectrum than solely for tomatoes.
In the north of Brazil where papaya crops agriculturally predominate, C. gleosporoides, the causative agent of anthracnosis, commonly produces severe postharvest losses. Additionally, the pathogen effectuates small reddish-brown surface patches unattractively modifying the fruit´s physical appearance. Two killer yeast species isolated from street markets and producer farms in the Ceará State/Brazil, W. anomalus 422 and Meyerozyma guilliermondii 443 [94] were able to reduce the diameter of disease lesions by 31.4 and 41.17%, respectively, when applied 24 h prior to the application of the pathogen. Such killer yeasts displayed protective rather than curative effects; they were able to reduce both the incidence and pathogenicity of C. gleosporoides presumably due to multiple factors such as competition for space and nutrients, mycoparasitism and secretion of β-1, 3-glucanases, the latter probably attacking the phytopathogen´s cell wall [95].
In apples, M. fructigena is the causative agent of the brown rot. The fungus infects wounds and subsequently–and rapidly–develops the rather firm rot. The respective areas are arranged as small cottony masses forming pathogen characteristic concentric circles [96]. Five killer yeasts, isolated from the peel of apples, were tested in vivo and in vitro for their potential to tame the pathogen: Schwanniomyces vanrijiae, Galactomyces geotrichum, Pichia kudriavzevii, D. hansenii and Rhodotorula glutinis [97]. In vivo tests included three different approaches: (1) simultaneous application of both the antagonist and the pathogen, (2) placing the yeast 24 h prior to and (3) 24 h after infection. Disease incidence decreases of 84.02–89.5%, 80.1–86.9% and 56.3–86.9%, respectively, were obtained. In addition, it was shown that hydrolytic enzymes such as chitinases, pectinases, β-1, 3-glucanases, and proteases were produced.
More recently, W. anomalus BS91, D. hansenii K12a and D. hansenii AII4b were used to protect apples against M. fructicola [98]. All of the above strains were additionally and successfully tested against Monilinia spp. in stone fruits as well [89]. W. anomalus BS91 and D. hansenii K12a inhibited mycelial growth in vitro by 69 and 66%, respectively. D. hansenii AII4b was less effective (56%). Nevertheless, in vivo BS91 and K12a reduced the M. fructicola disease by 92 and 85%, respectively, while AII4b was less effective (70% disease reduction). Compared to untreated fruit, rot severity was reduced by approximately 52 up to 69%. Stress induction assays by measuring peroxidase and catalase levels in the peel of fruit indicated highest levels of peroxidase (POD) when wounds were treated with W. anomalus BS91. Lowest catalase (CAT) values were obtained with D. hansenii AII4b. The above findings led to the conclusion that the yeasts were able to modulate the activities of CAT and POD in apple tissues, leading the authors to suggest the yeasts promising candidates for developing effective biofungicides against M. fructicola.
Grapes are almost routinely infected by the rot-causing A. carbonarius and A. niger, both responsible for the accumulation of ochratoxin A, a potent nephrotoxic and carcinogenic compound that can even be found in secondary grape products [99,100].
Epiphytic yeasts isolated from grapes in Southern Italy, after molecular identification and inspection of the killer phenotype, were tested in vivo and in vitro to identify those representatives which are able to conquer the co-inoculated pathogenic fungi in their common ecological niches. Issatchenkia orientalis 17C2 and 16C2 efficiently reduced disease incidences of A. carbonarius and A. niger on grapes (100% growth reduction), due to the production of secondary antifungal metabolites and competition for essential nutrients [101].
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms8111680
