Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Excessive UV (ultraviolet) radiation exposure is harmful to skin cells since sunburn is accompanied by oxidative burst, leading to a rapid increase in skin cancer. However, the insufficient UV photoprotection of approved sunscreens and the negative impact of their compositions on ecosystems and human health makes the utility of sunscreen a questionable recommendation. Therefore, discovering UV filters with significant antioxidant activity and improved topical performance and photostability is an urgent need.
- sunscreen
- UVA photoprotection
- UV filter
- antioxidant
- flavonoid
- nanoparticle
- skin cancer
1. Sunscreens
The topical use of sunscreens represents the most popular strategy of skin photoprotection [1] in response to the depletion of ozone in the stratosphere and the consequent higher accumulation of UV radiation on the surface of our planet.
An ideal sunscreen should have (a) a technology to reduce the intensity of UV rays reaching the surface of the skin, and (b) a technology to prevent or reduce the oxidative burden caused by the release of free oxygen and nitrogen reactive species upon UV radiation absorption, namely, appropriate antioxidants and scavengers of free radicals, or inhibitors of their formation. This is particularly important because singlet oxygen is formed not only by endogenous photosensitizers but many commercial sunscreens containing titanium dioxide (TiO2) and zinc oxide (ZnO) are also reported to produce ROS [2] (Figure 1).
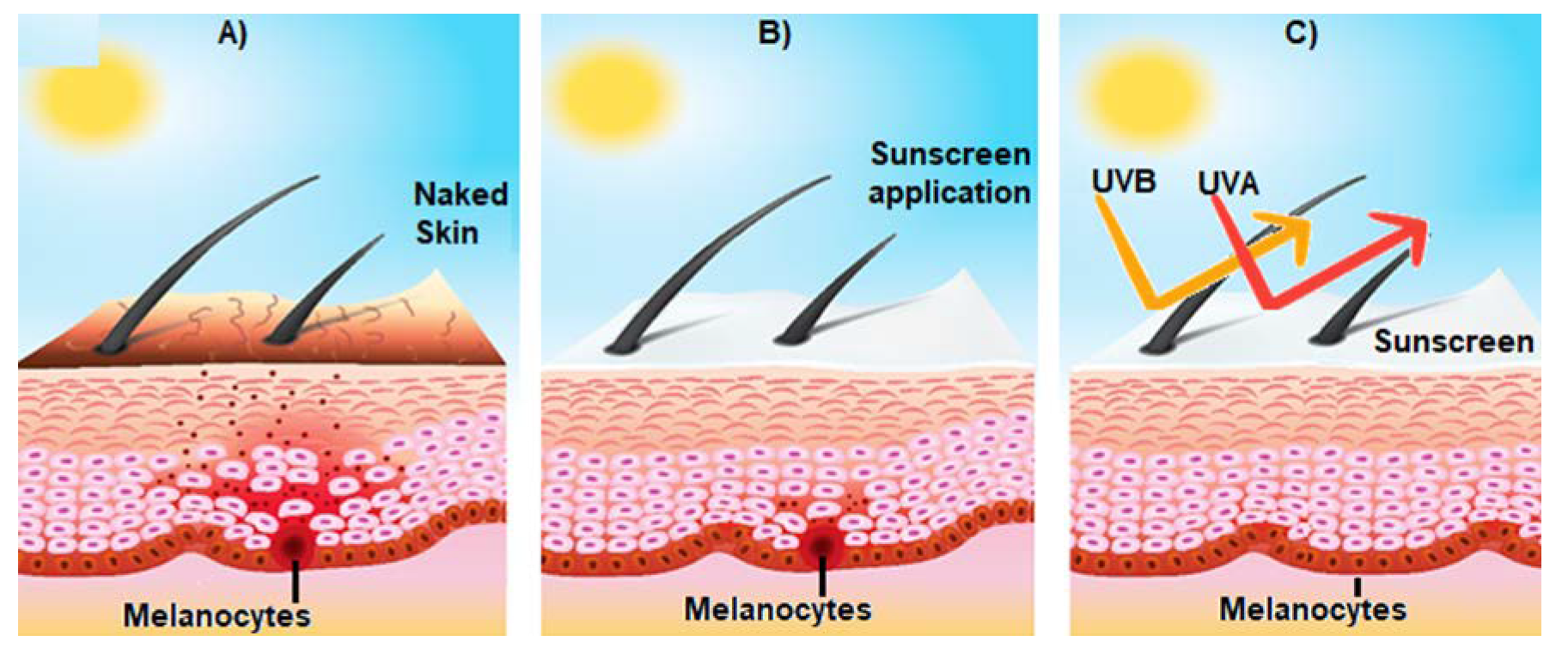
Figure 1. Effect of sunscreen on the prevention of sun-induced damage. (A) Shows the activation of epidermal melanocytes by sun UV radiation and the consequent release of melanin (brown spots) towards the surface of the skin, giving it a brown tone; (B) represents the consequences of the use of sunscreen on skin structure; and (C) broad spectrum sunscreen prevents the damage of the skin induced by UVA and UVB radiation.
Besides the mentioned requirements, an ideal sunscreen should also: (a) absorb a broad range of UV rays; (b) not be chemically broken down to prevent a decrease in efficacy or increased toxicity or irritation due to the by-products; (c) have suitable properties while formulated as a cosmetic base and penetrate the skin easily; (d) not get removed by water or perspiration; (e) avoid the need of frequent reapplication; (f) be effective, at low concentration; and (g) not cause irritation, sensitization, and toxicity to the skin [3].
Permeation of sunscreen components through the skin is a fundamental parameter to be considered when formulating the composition of a sunscreen. A sunscreen should remain at the surface of the skin and no absorption should occur so that it can perform its function without causing toxicity [4]. As referred to above, sun filters should not undergo modification when exposed to UV radiation, therefore, the photostability of the sunscreen also becomes mandatory. If the UV filters are not photostable enough, they become less absorptive and their function of protecting the skin is therefore lost [5].
The efficacy of a sunscreen is usually measured by the Sun Protection Factor (SPF) which corresponds to the number of times the amount of 2 mg/cm2 of sunscreen application (standardized amount) increases its capacity of delaying the formation of erythema due to sun exposure, compared with unprotected skin. SPF is also known as a measure of how much more sun exposure it takes to undergo sunburn. For example, a sunscreen with an SPF of 30 means that it will take 30 times more sun exposure to develop erythema when compared with the same skin without photoprotection [6]. Therefore, sunscreens are mostly classified according to their SPF which can reach up to 50 in Europe [7] and should be, at least, 30. It is assumed that this range of SPF values is adequate to provide enough daily photoprotection against UVB radiation. In theory, when applied evenly on the skin at 2 mg/cm2, SPF 50 sunscreen should filter out 98% of UVB rays and lengthen the time it takes for the skin of a person to redden in the sun [8][9]. Accordingly, SPF does not consider protection against UVA radiation, even though many current sunscreens have UV filters for both UV rays. Nevertheless, the protection against UVA rays tends to be inferior. Although there are European standards for UVA protection, which recommend that the UVA Protection Factor (PFUVA) should be, at least, one-third of the marketed SPF, many of the currently marketed sunscreens do not meet the requirement mentioned above.
Sunscreens should be applied at least 15 min before going outside. It is important to use a sun-protective lip balm, as well. For the correct use of sunscreen, it should be reapplied every 2 h and after swimming or excessive sweating, to provide sustained skin photoprotection. Many cosmetics contain UV filters, which are beneficial for the product but insufficient to provide adequate protection against both UVA and UVB radiation. Applying sunscreen after the usual cosmetics products should be a daily habit, throughout the year [10]. It is already demonstrated that sunscreens inhibit sunburn because they suppress the mechanisms that cause erythema. However, it is still unclear if they prevent the underlying biochemical processes.
A person may not get sunburned, but still, have several unwanted effects occurring on the skin. The UV filters oxybenzone, octocrylene, octinoxate, PABA (para-aminobenzoic acid), and 4–methylbenzyliden camphor have been reported to induce free radicals [11], induce caspase enzymes linked with photosensitization [12], stimulate melanoma tumor growth [13], and neurotoxicity [13][14][15][16][17]. A very damaging free radical, singlet oxygen, is formed by commercial sunscreens containing TiO2 and ZnO [2], as mentioned earlier. Therefore, it is suggested that the elimination of sunburn by sunscreen use is not free of toxic effects which can lead to the future development of skin cancers and other types of toxicities [18].
Based on published scientific data, sunscreens do not prevent skin cancers associated with intentional sun exposure. Therefore, the risks associated with intentional sun exposure are outweighed by the lack of benefits. In addition to the use of sunscreen, people who want exposure to sunlight are advised to avoid peak hours of UV radiation (10 a.m.–4 p.m.), wear protective clothing including a broad-brimmed hat with sunglasses, and/or use an oversized umbrella/cabana when at the beach or pool. The practice of all these healthy sun habits will significantly help prevent the development of skin cancers [10].
2. Conventional UV Filters
2.1. Organic Filters
Organic filters absorb specific wavelengths of UV radiation depending on their chemical structure. The ground state of low energy is converted into a high-energy level. Organic filters are divided into three types based on how they process the high levels. First, the photostable filter dissipates the absorbed energy as heat energy to the atmosphere, returning it to a low-energy level. They are efficient at reabsorbing UV energy. Photo-unstable filters, upon absorption of UV energy, undergo a change in their chemical structure or degrade completely so they cannot absorb UV energy again. Photoreactive filters constitute the third type, and they interact with molecules in the microenvironment in their high energy or excited state. Photoreactive filters can react with proteins and lipids from skin cells and other ingredients from the sunscreen and surrounding oxygen; consequently, reactive oxygen and nitrogen species are generated and may lead to unwanted biological effects [3].
Dibenzoylmethane derivatives: They have a high absorption capacity in the UVA range, but they degrade in the presence of UV radiation, decreasing the efficiency of sun protection at the time of UV exposure. Photo-fragmentation of these filters occurs, leading to the formation of free radicals, which cause skin damage. Avobenzone is the most well-known derivative from this class [19].
Benzophenone derivatives: They absorb or dissipate UV radiation, mainly UVA. It was previously reported that cytotoxic effects are caused by these filters. Oxybenzone is an example of a UV filter [20].
Para-Aminobenzoic acid and derivatives: They absorb UVB radiation and can be retained for a long time on the surface of the skin. Photoallergic reactions are common adverse reactions [21][22].
Salicylate derivatives: They are weak absorbers of UVB radiation and are used to minimize the photodegradation of other photo protectants. Homosalate belongs to this class [23].
Benzotriazoles: They can be photostable broad-spectrum filters, having an efficient sun protection ability. Due to their photostability, photoaging and photosensitization are less frequent, as well. Octrizole is a member of this class of UV filters [24].
2.2. Inorganic Filters
Inorganic filters scatter and reflect UV radiation to the external environment. They function as a physical barrier to UV radiation. These filters are broad-spectrum as they can reflect the radiations in the entire UV range. The most recognized inorganic filters are TiO2 and ZnO [25].
Although the use of sunscreens has been increasing, the risk of the development of skin cancer has also been increasing [26]. Sunscreens contain UVB filters to avoid sunburn and photoaging. Due to the insufficient ozone layer, UVA rays also reach the atmosphere and permeate the human skin epidermis into the dermis to induce an oxidative burden, which can cause carcinogenesis as the worst possible consequence, as discussed.
Interestingly, research suggests that most basal cell carcinomas may be primarily attributed to UVA irradiation [27][28]. UVA rays are absorbed by endogenous photosensitizers which subsequently cause oxidation reactions, producing reactive oxygen and nitrogen species [29][30][31][32]. Fortunately, endogenous antioxidant defense systems are present in the skin, including glutathione peroxidase, catalase, and superoxide dismutase, which protect the skin against oxidative damage [33]. However, when the production of reactive free radicals exceeds the capacity of endogenous antioxidant systems to protect the target cells, oxidative stress initiates, which has been associated with the occurrence of skin cancer [34].
UVA radiation can also penetrate window glass in buildings or cars, making sun protection a daily necessity, even in the winter season [10]. The value of the sunscreen ratio SPF/PFUVA or UVA/UVB absorbances must be analyzed, to ensure homogeneous protection in the two UV ranges. Couteau et al. determined SPF, PFUVA, and UVA/UVB ratios of O/W (oil in water) creams formulated by the authors [35][36][37] with 22 organic filters (15 UVB filters, 4 UVA/UVB filters, and 3 UVA filters) and 2 inorganic filters, TiO2 and ZnO, in diverse combinations. The study of the blocking capacity in UVA and UVB range was determined using an in vitro method [35]. Each UVA filter was associated with TiO2 or ZnO used at 10%. Three associations with TiO2 resulted in an increase in the effectiveness of both the UVA and UVB ranges. Those conducted with butylmethoxydibenzoylmethane, anisotriazine, and diethylamino hydroxybenzoyl hexyl benzoate were the most successful. None of the combinations with ZnO presented a synergistic effect in both the UVA and UVB ranges. The combinations with TiO2 reach an SPF higher than 50, whereas combinations with ZnO led to a maximum SPF of approximately 39, as suggested in the results from anisotriazine assays [38]. These results suggested that the combinations of organic filters with TiO2 are preferable.
The human health risk associated with organic UV filters can be regarded as a concern because they can enter the body through percutaneous absorption and contaminated food and water consumption. The organic UV filters can reach blood circulation and be found in body fluids such as urine, semen, and breast milk [39][40][41].
Despite the biological consequences of these substances being still undiscovered, it was reported that some marketed organic UV filters exhibit endocrine-disruption activity (Table 1) on the reproduction cycle of organisms [42][43]. In addition, the maternal transfer of organic UV filters in humans [44] and animals, such as dolphins [45] and birds [46], has been proven. Another reported effect of organic UV filters in the human organism is vitamin D deficiency, which can cause negative changes in bone metabolism and weaker immune responses [5].
Table 1. Conventional synthetic UV filters approved worldwide: type, UV range of activity, maximum amount authorized in sunscreens, FDA category, and risks associated.
| UV Filter | Type | Spectrum Activity | Maximum % in Sunscreens |
Approvals and Possible Complications |
|---|---|---|---|---|
| Avobenzone | Organic or chemical | UVA | 3% U.S. 5% EU, AUS 10% JP |
Non-GRASE III Photodegradation Photosensitization |
| Octinoxate | Organic or chemical | UVA UVB |
7.5% U.S. 10% EU, AUS 20% JP |
Non-GRASE III Photodegradation Endocrine-disruption potential Skin absorption Breast milk detection |
| Octocrylene | Organic or chemical | UVA UVB |
10%—worldwide | Non-GRASE III Photosensitization Skin absorption Breast milk detection |
| Oxybenzone | Organic or chemical | UVA UVB |
6% U.S. 10% EU, AUS 5% JP |
Non-GRASE III Possible photocarcinogen Skin absorption Breast milk detection Endocrine-disruption potential |
| Ecamsule | Organic or chemical | UVA | 3% U.S. 10% EU, AUS, JP |
No GRASE rating |
| PABA | Organic or chemical | UVB | Non-GRASE II Banned in Europe Allergen, contact dermatitis Possible photocarcinogen |
|
| Trolamine salicylate | Organic or chemical | UVB | 12% U.S., CA, AUS 2.5% EU |
Non-GRASE II Skin absorption Salicylism risk |
| Titanium dioxide |
Inorganic or physical | UVA UVB |
25% U.S., EU, JP No limit—AUS |
GRASE I |
| Zinc oxide | Inorganic or physical | UVA UVB |
25% U.S., EU, JP No limit—AUS |
GRASE I |
Abbreviations: U.S. = United States, EU = Europe, AUS = Australia, JP = Japan, and CA = Canada; GRASE = “Generally Recognized as Safe and Effective”.
Oxybenzone and other organic UV filters can induce photoallergic reactions and photocarcinogenic events, as described in Table 1, as well [10]. Concerning the inorganic UV filters, TiO2 and ZnO, the most well-known filters, they can block UV rays from coral algae and inhibit photosynthesis, subsequently, and may add to local increases in water temperatures, contributing to the devastating greenhouse effect [47].
3. Regulatory Considerations on Sunscreens
In February 2019, the U.S. FDA (Food and Drugs Administration) updated the regulatory requirements for non-prescription and over-the-counter sunscreens to ensure their safety, efficacy, and consistency in labeling. Broad-spectrum sunscreens are defined by the FDA as products that provide UVA and UVB protection at the usual ratio UVA/UVB of 1:3. They must have a minimum SPF of 30, be water-resistant, reduce the risk of development of skin cancer, decrease the incidence and severity of sunburn, and prevent photoaging [18].
UV filters can absorb, reflect, or scatter UV rays. Few UV filters used in FDA-approved sunscreens are considered generally recognized as safe and effective (GRASE). However, these products are sold under the definition of “Marketed Unapproved Drugs” as they have been in use for a long time but may lack the rigorous testing needed [10]. GRASE category I includes 22 UV filters that are routinely used in sunscreen products. TiO2 and ZnO are most used as mineral or physical UV blockers (Table 1). Other currently marketed UV filters such as avobenzone, cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octocrylene, octinoxate, octisalate, oxybenzone, padimate O, and sulisobenzone are included in the GRASE category III, as they require further studies about their safety as topical agents (Table 1).
It is important to note that concerning the general safety issues of chemical UV filters, FDA moved PABA and trolamine salicylate (organic sunscreen actives) from the GRASE category to the category “not safe for human use”.
In fact, all organic sunscreen active ingredients have limited or no data characterizing their absorption profile. Therefore, the FDA has advised the industry to conduct a variety of tests, namely, carcinogenicity and reproductive toxicity, before introducing them into the GRASE category [10].
All UV filters formulated as spray or powder must have their potential risks of inhalation and/or flammability rigorously evaluated, as there is a lack of toxicity data about them. The approval process from FDA is slower than the European process (EU Cosmetic Regulation (EC No.1223/2009) by the Scientific Committee on Consumers’ Safety). The reason is the fact that the FDA classifies new UV filters as over-the-counter drugs rather than cosmetics, as in Europe and other parts of the world. Accordingly, UV filters require extensive clinical data to be recognized as safe for use in humans [10].
This entry is adapted from the peer-reviewed paper 10.3390/biom13030493
References
- Svobodová, A.R.; Gabrielová, E.; Ulrichová, J.; Zálešák, B.; Biedermann, D.; Vostálová, J. A pilot study of the UVA-photoprotective potential of dehydrosilybin, isosilybin, silychristin, and silydianin on human dermal fibroblasts. Arch. Dermatol. Res. 2019, 311, 477–490.
- Yamamoto, Y.; Imai, N.; Mashima, R.; Konaka, R.; Inoue, M.; Dunlap, W.C. Singlet Oxygen from Irradiated Titanium Dioxide and Zinc Oxide. Methods Enzymol. 2000, 319, 29–37.
- Bhattacharjee, D.; Preethi, S.; Patil, A.B.; Jain, V. A comparison of Natural and Synthetic Sunscreen Agents: A Review. Int. J. Pharm. Res. 2021, 13, 3494–3505.
- Montenegro, L.; Puglisi, G. Evaluation of sunscreen safety by in vitro skin permeation studies: Effects of vehicle composition. Die Pharm. Int. J. Pharm. Sci. 2013, 68, 34–40.
- Cefali, L.C.; Ataide, J.A.; Fernandes, A.R.; Sousa, I.M.D.O.; Gonçalves, F.C.D.S.; Eberlin, S.; Dávila, J.L.; Jozala, A.F.; Chaud, M.V.; Sanchez-Lopez, E.; et al. Flavonoid-Enriched Plant-Extract-Loaded Emulsion: A Novel Phytocosmetic Sunscreen Formulation with Antioxidant Properties. Antioxidants 2019, 8, 443.
- Widsten, P.; Tamminen, T.; Liitiä, T. Natural Sunscreens Based on Nanoparticles of Modified Kraft Lignin (CatLignin). ACS Omega 2020, 5, 13438–13446.
- Herzinger, T. Sun protection factor 50+: Pro and contra. Hautarzt 2017, 68, 368–370.
- Diaz, J.H.; Nesbitt, L.T. Sun Exposure Behavior and Protection: Recommendations for Travelers. J. Travel Med. 2013, 20, 108–118.
- Lu, C.J.; Benner, R.; Fichot, C.G.; Fukuda, H.; Yamashita, Y.; Ogawa, H. Sources and transformations of dissolved lignin phenols and chromophoric dissolved organic matter in Otsuchi Bay, Japan. Front. Mar. Sci. 2016, 3, 85.
- Sabzevari, N.; Qiblawi, S.; Norton, S.A.; Fivenson, D. Sunscreens: UV filters to protect us: Part 1: Changing regulations and choices for optimal sun protection. Int. J. Women’s Dermatol. 2021, 7, 28–44.
- Urbach, F. The negative effects of solar radiation: A clinical overview. In Sun Protection in Man; Giacomoni, P.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 39–67.
- Chung, J.O. The effects on sunlight on the skin of Asians. In Sun Protection in Man; Giacomoni, P.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 69–90.
- Young, A.R.; Sheehan, J.R. Damage from Acute vs Chronic Solar Exposure. In Biophysical and Physiological Effects of Solar Radiation on Human Skin; The Royal Society of Chemistry: Cambridge, UK, 2007; Volume 10, pp. 3–23.
- Calzavara-Pinton, P.G.; Ortel, B. Pigmentation after solar radiation. In Biophysical and Physiological Effects of Solar Radiation on Human Skin; The Royal Society of Chemistry: Cambridge, UK, 2007; Volume 10, pp. 65–97.
- Foster, K.W.; Katiyar, S.K.; Yusuf, N.; Elmets, C.A. Inflammation after solar radiation. In Biophysical and Physiological Effects of Solar Radiation on Human Skin; The Royal Society of Chemistry: Cambridge, UK, 2007; pp. 25–63.
- Heenen, M.; Giacomoni, P.U.; Golstein, P. Erythema, a link between UV-induced DNA damage, cell death and clinical effects. In Sun Protection in Man; Elsevier: Amsterdam, The Netherlands, 2001; Volume 3, pp. 277–285.
- Young, A.R.; Sheehan, J.R. UV-induced pigmentation in human skin. In Sun Protection in Man; Giacomoni, P.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 357–375.
- van der Pols, J.C.; Williams, G.M.; Pandeya, N.; Logan, V.; Green, A.C. Prolonged Prevention of Squamous Cell Carcinoma of the Skin by Regular Sunscreen Use. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2546–2548.
- Cefali, L.C.; Ataide, J.A.; Moriel, P.; Foglio, M.A.; Mazzola, P.G. Plant-based active photoprotectants for sunscreens. Int. J. Cosmet. Sci. 2016, 38, 346–353.
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol. 2014, 23, 7–12.
- Arianto, A.; Cella, G.; Bangun, H. Preparation and Evaluation of Sunscreen Nanoemulsions with Synergistic Efficacy on SPF by Combination of Soybean Oil, Avobenzone, and Octyl Methoxycinnamate. Open Access Maced. J. Med. Sci. 2019, 7, 2751–2756.
- Gause, S.; Chauhan, A. UV-blocking potential of oils and juices. Int. J. Cosmet. Sci. 2016, 38, 354–363.
- Han, Y. Rutin has therapeutic effect on septic arthritis caused by Candida albicans. Int. Immunopharmacol. 2009, 9, 207–211.
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383.
- Bonina, F.; Lanza, M.; Montenegro, L.; Puglisi, C.; Tomaino, A.; Trombetta, D.; Castelli, F.; Saija, A. Flavonoids as potential protective agents against photo-oxidative skin damage. Int. J. Pharm. 1996, 145, 87–94.
- Merhi, S.; Salameh, P.; Kaplan, P.; Banerjee, S.; Lajnef, M.; Dumont, E.; Ezzedine, K. An Ecological Study Indicates the Importance of Ultraviolet A Protection in Sunscreens. Acta Derm. Venereol. 2021, 101, adv00480.
- Kim, H.K.; Namgoong, S.Y.; Kim, H.P. Antiinflammatory activity of flavonoids: Mouse ear edema inhibition. Arch. Pharmacal Res. 1993, 16, 18–24.
- Manca, M.L.; Castangia, I.; Caddeo, C.; Pando, D.; Escribano, E.; Valenti, D.; Lampis, S.; Zaru, M.; Fadda, A.M.; Manconi, M. Improvement of quercetin protective effect against oxidative stress skin damages by incorporation in nanovesicles. Colloids Surf. B Biointerfaces 2014, 123, 566–574.
- Wadsworth, T.L.; Koop, D.R. Effects of the wine polyphenolics quercetin and resveratrol on pro-inflammatory cytokine expression in RAW 264.7 macrophages. Biochem. Pharmacol. 1999, 57, 941–949.
- Mauludin, R.; Müller, R.H.; Keck, C.M. Kinetic solubility and dissolution velocity of rutin nanocrystals. Eur. J. Pharm. Sci. 2009, 36, 502–510.
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT-Food Sci. Technol. 2008, 41, 1060–1066.
- Jiménez-Aliaga, K.; Bermejo-Bescós, P.; Benedí, J.; Martín-Aragón, S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sci. 2011, 89, 939–945.
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 21, 589–598.
- Shindo, Y.; Witt, E.; Packer, L. Antioxidant Defense Mechanisms in Murine Epidermis and Dermis and Their Responses to Ultraviolet Light. J. Investig. Dermatol. 1993, 100, 260–265.
- Chat, O.A.; Najar, M.H.; Mir, M.A.; Rather, G.M.; Dar, A.A. Effects of surfactant micelles on solubilization and DPPH radical scavenging activity of Rutin. J. Colloid Interface Sci. 2011, 355, 140–149.
- Mauludin, R.; Müller, R.H.; Keck, C.M. Development of an oral rutin nanocrystal formulation. Int. J. Pharm. 2009, 370, 202–209.
- IV CPT. Method for the In Vitro Determination of UVA Protection Provided by Sunscreen Products. COLIPA, Belgium, 2006.
- De Flora, S.; Ferguson, L.R. Overview of mechanisms of cancer chemopreventive agents. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 591, 8–15.
- Matta, M.K.; Zusterzeel, R.; Pilli, N.R.; Patel, V.; Volpe, D.; Florian, J.; Oh, L.; Bashaw, E.; Zineh, I.; Sanabria, C.; et al. Effect of Sunscreen Application Under Maximal Use Conditions on Plasma Concentration of Sunscreen Active Ingredients. JAMA 2019, 321, 2082–2091.
- León, Z.; Chisvert, A.; Tarazona, I.; Salvador, A. Solid-phase extraction liquid chromatography–tandem mass spectrometry analytical method for the determination of 2-hydroxy-4-methoxybenzophenone and its metabolites in both human urine and semen. Anal. Bioanal. Chem. 2010, 398, 831–843.
- Molins-Delgado, D.; del Mar Olmo-Campos, M.; Valeta-Juan, G.; Pleguezuelos-Hernández, V.; Barceló, D.; Díaz-Cruz, M.S. Determination of UV filters in human breast milk using turbulent flow chromatography and babies’ daily intake estimation. Environ. Res. 2018, 161, 532–539.
- Tomazelli, L.C.; de Assis Ramos, M.M.; Sauce, R.; Cândido, T.M.; Sarruf, F.D.; de Oliveira Pintoa, C.A.S.; de Oliveira, C.A.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. SPF enhancement provided by rutin in a multifunctional sunscreen. Int. J. Pharm. 2018, 552, 401–406.
- Singh, R.P.; Agarwal, R. Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur. J. Cancer 2005, 41, 1969–1979.
- Katiyar, S.K.; Vaid, M. Molecular mechanisms of inhibition of photocarcinogenesis by silymarin, a phytochemical from milk thistle (Silybum marianum L. Gaertn). Int. J. Oncol. 2010, 36, 1053–1060.
- Rajnochová Svobodová, A.; Gabrielová, E.; Michaelides, L.; Kosina, P.; Ryšavá, A.; Ulrichová, J.; Zálešák, B.; Vostálová, J. UVAphotoprotective potential of silymarin and silybin. Arch. Dermatol. Res. 2018, 310, 413–424.
- Vostálová, J.; Tinková, E.; Biedermann, D.; Kosina, P.; Ulrichová, J.; Rajnochova Svobodová, A. Skin Protective Activity of Silymarin and its Flavonolignans. Molecules 2019, 24, 1022.
- Fivenson, D.; Sabzevari, N.; Qiblawi, S.; Blitz, J.; Norton, B.B.; Norton, S.A. Sunscreens: UV filters to protect us: Part 2-Increasing awareness of UV filters and their potential toxicities to us and our environment. Int. J. Women’s Dermatol. 2021, 7, 45–69.
This entry is offline, you can click here to edit this entry!
