Common vetch (Vicia sativa L.) is a grain legume used in animal feed. It is rich in protein, fatty acid and minerals content, therefore is a very suitable component for feed enrichment. Furthermore, important pharmacological properties in humans have been described. Like other legumes, common vetch has the ability to fix atmospheric nitrogen, an important characteristic in sustainable agricultural systems. These characteristics enhance the usage of vetch as a cover crop and its use in intercropping systems. In addition, several studies have highlighted the potential of vetch in the phytoremediation of polluted soils. These features make common vetch an appropriate crop to address for various potential improvements. Comparative analyzes have allowed the identification of varieties with different flowering time, shattering resistance, yield, nutrient content and composition, drought response, rhizobacteria associations, nitrogen fixation capacity, and other agronomically relevant traits.
- Vicia sativa
- common vetch
- breeding
1. Introduction
2. Taxonomy
3. A Historical Crop
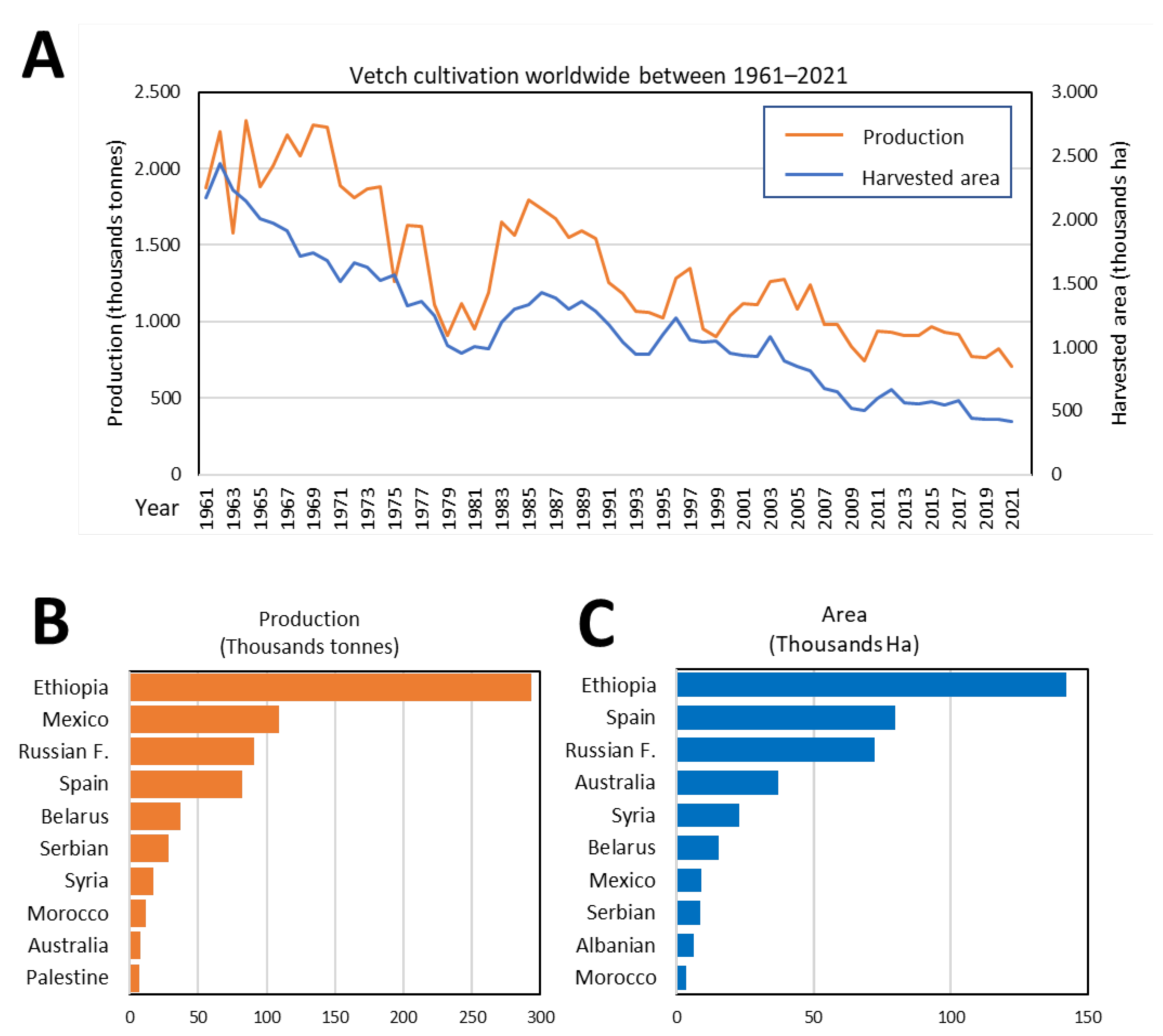
4. Worldwide Vetch Cultivation
5. Nutritional and Pharmacological Properties
6. Environmental Benefits
| Pollutant | Developed Assay | References |
|---|---|---|
| Cd | Cd tolerance. Oxidative damage accumulation. | [95] |
| Cd and Zn | Zn and Cd accumulation in different tissues | [94] |
| Zn | Zn tolerance | [97] |
| Cu | Cu tolerance | [90][91] |
| Salt | Tolerance to salt. Na and K accumulation | [83] |
| Hg | Hg accumulation in different tissues | [93][99] |
| Ni | Ni accumulation. Oxidative damage accumulation. | [96] |
| Sulfosulfuron herbicide | Tolerance to sulfosulfuron | [84] |
| Diesel fuel | Tolerance to diesel | [89] |
| Phenol derivatives | Polychlorinated biphenyl (PCB) dissipation | [86] |
| Phenolics | Tolerance to phenolics. Effects on biomass, nodulation and nitrogen fixation activity | [88] |
| Mepiquat | Tolerance to mepiquat | [87] |
This entry is adapted from the peer-reviewed paper 10.3390/plants12061275
References
- Parissi, Z.; Irakli, M.; Tigka, E.; Papastylianou, P.; Dordas, C.; Tani, E.; Abraham, E.M.; Theodoropoulos, A.; Kargiotidou, A.; Kougiteas, L.; et al. Analysis of Genotypic and Environmental Effects on Biomass Yield, Nutritional and Antinutritional Factors in Common Vetch. Agronomy 2022, 12, 1678.
- Huang, Y.F.; Gao, X.L.; Nan, Z.B.; Zhang, Z.X. Potential value of the common vetch (Vicia sativa L.) as an animal feedstuff: A review. J. Anim. Physiol. Anim. Nutr. 2017, 101, 807–823.
- Lithourgidis, A.; Dordas, C.; Damalas, C.A.; Vlachostergios, D.N. Annual intercrops: An alternative pathway for sustainable agriculture. Aust. J. Crop Sci. 2011, 5, 396–410.
- Dalias, P.; Neocleous, D. Comparative Analysis of the Nitrogen Effect of Common Agricultural Practices and Rotation Systems in a Rainfed Mediterranean Environment. Plants 2017, 6, 61.
- Maxted, N. An ecogeographical study of Vicia subgenus Vicia. In Systematic and Ecogeographic Studies on Crop Genepools; IPGRI: Rome, Italy, 1995; Volume 8, p. 184.
- Maxted, N. A phenetic investigation of Vicia L. subgenus Vicia (Leguminosae, Vicieae). Bot. J. Linn. Soc. 1993, 111, 155–182.
- Hanelt, P.; Mettin, D. Biosystematics of the Genus Vicia L. (Leguminosae). Annu. Rev. Ecol. Syst. 1989, 20, 199–223.
- Kupicha, F.K. The infrageneric structure of Vicia. Notes R. Bot. Gard. Edinb. 1976, 34, 287–326.
- Leht, M. Phylogenetics of Vicia (Fabaceae) based on morphological data. Feddes Repert. 2009, 120, 379–393.
- Tate, M.; Ennerking, D. Vetches: From feed to food? Grain Legumes 2006, 47, 12–13.
- Potokina, E. Vicia sativa L. aggregate (Fabaceae) in the flora of former USSR. Genet. Resour. Crop Evol. 1997, 44, 199–209.
- Jaaska, V. Isoenzyme diversity and phylogenetic affinities in Vicia subgenus Vicia (Fabaceae). Genet. Resour. Crop Evol. 1997, 44, 557–574.
- Jaaska, V. Isozyme Variation and Phylogenetic Relationships in Vicia subgenus Cracca (Fabaceae). Ann. Bot. 2005, 96, 1085–1096.
- van de Wouw, M.; Maxted, N.; Chabane, K.; Ford-Lloyd, B.V. Molecular taxonomy of Vicia ser. Vicia based on Amplified Fragment Length Polymorphisms. Plant Syst. Evol. 2001, 229, 91–105.
- Yeater, K.M.; Bollero, G.; Bullock, D.; Rayburn, A.L. Flow cytometric analysis for ploidy level differentiation of 45 hairy vetch accessions. Ann. Appl. Biol. 2004, 145, 123–127.
- van de Wouw, M.; Maxted, N.I.; Ford-Lloyd, B.V. A multivariate and cladistic study of Vicia L. ser. Vicia (Fabaceae) based on analysis of morphological characters. Plant Syst. Evol. 2003, 237, 19–39.
- Ennerking, D.; Tate, M. Global vetch production. Grain Legumes 2006, 47, 14–15.
- Zohary, D.; Hopf, M. Domestication of Plants in the Old World: The Origin and Spread of Cultivated Plants in West Asia, Europe and the Nile Valley (No. Ed. 3); Oxford University Press: Oxford, UK, 2000.
- Maxted, N.; Bennett, S. Plant Genetic Resources of Legumes in the Mediterranean; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2001; Volume 39.
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World: The Origin and Spread of Domesticated Plants in Southwest Asia, Europe, and the Mediterranean Basin; Oxford University Press: Oxford, UK, 2012.
- Varron, M. Rerum Rusticarum: Libri III; Cubero-Salmeron, J.I., Translator; Consejería de Agricultura y Pesca, Junta de Andalucía: Seville, Spain, 2010; 1st century BC.
- Holgado-Redondo, A.; Columela, L.J.M. (1st Century)-Translation; “De re Rustica”: De los Trabajos de Campo; Holgado-Redondo, A., Translator; Ministerio de Agricultura: Madrid, Spain, 1988; 339p, ISBN 84-323-0622-3.
- Hernandez, L.; Huerta, J. Secundus Plinius The Elder (1st Century) Historia Natural; UNAM-Universidad Nacional Autónoma de Mexico: Mexico City, Mexico, 1976; Hernandez, L. (books 1–25), Huerta, J. (books 26–37).
- Oroz-Reta, J.; Marcos-Casquero., M.A. Isidore of Seville (6–7th Century)-Translation “Etymologiae” Etimologias; Oroz-Reta, J.; Marcos-Casquero, M.A., Translators; BAC: Madrid, Spain, 1982; Volume 15.
- Cubero, J.I. Historia General de la Agricultura; Guadalmazán: Córdoba, Spain, 2018; ISBN 9788494155239. p. 840.
- Carabaza-Bravo, J.M.; L-Jayr, A.; Al-Filāḥa, K. Tratado de Agricultura; (11st–12nd Century) Translation; Carabaza-Bravo, J.M., Ed.; Instituto de Cooperación con el Mundo Arabe: Madrid, Spain, 1991.
- Jarava, J. “Historia de las Yerbas y Plantas” (from Dioscoride Anazarbeo); Gorda, L.G., Ed.; Heirs of A. Byrcman: Antwerp, Belgium, 1557.
- Weber, L.H.; Schifino-Wittmann, M.T. The Vicia sativa L. aggregate (Fabaceae) in southern Brazil: Karyotypes, phenology and qualitative morphology. Genet. Resour. Crop Evol. 1999, 46, 207–211.
- Gomez-Ortega, C. Continuacion de la Flora Española, ó Historia de las Plantas de España, Que Escribía Don Joseph Quer. Ibarra, J.: Madrid, Spain, 1784; Volume V–VI.
- Faostat. 2023. Available online: https://www.fao.org (accessed on 15 February 2023).
- Nguyen, V.; Riley, S.; Nagel, S.; Fisk, I.; Searle, I.R. Common Vetch: A Drought Tolerant, High Protein Neglected Leguminous Crop With Potential as a Sustainable Food Source. Front. Plant Sci. 2020, 11, 818.
- Grela, E.R.; Samolinska, W.; Rybinski, W.; Kiczorowska, B.; Kowalczuk-Vasilev, E.; Matras, J.; Wesolowska, S. Nutritional and Anti-Nutritional Factors in Vicia sativa L. Seeds and the Variability of Phenotypic and Morphological Characteristics of Some Vetch Accessions Cultivated in European Countries. Animals 2021, 11, 44.
- Larbi, A.; El-Moneim, A.M.A.; Nakkoul, H.; Jammal, B.; Hassan, S. Intra-species variations in yield and quality determinants in Vicia species: 3. Common vetch (Vicia sativa ssp. sativa L.). Anim. Feed Sci. Technol. 2011, 164, 241–251.
- Huang, Y.F.; Matthew, C.; Li, F.; Nan, Z.B. Common vetch varietal differences in hay nutritive value, ruminal fermentation, nutrient digestibility and performance of fattening lambs. Animal 2021, 15, 100244.
- Salehi, B.; Abu-Reidah, I.M.; Sharopov, F.; Karazhan, N.; Sharifi-Rad, J.; Akram, M.; Daniyal, M.; Khan, F.S.; Abbaass, W.; Zainab, R.; et al. Vicia plants—A comprehensive review on chemical composition and phytopharmacology. Phytother. Res. 2021, 35, 790–809.
- Abbasi, A.M.; Shah, M.H.; Li, T.; Fu, X.; Guo, X.; Liu, R.H. Ethnomedicinal values, phenolic contents and antioxidant properties of wild culinary vegetables. J. Ethnopharmacol. 2015, 162, 333–345.
- Shinwari, M.I.; Khan, M.A. Folk use of medicinal herbs of Margalla Hills National Park, Islamabad. J. Ethnopharmacol. 2000, 69, 45–56.
- Prabhu, S.; Vijayakumar, S.; Yabesh, J.E.; Ravichandran, K.; Sakthivel, B. Documentation and quantitative analysis of the local knowledge on medicinal plants in Kalrayan hills of Villupuram district, Tamil Nadu, India. J. Ethnopharmacol. 2014, 157, 7–20.
- Marc, E.; Nellya, A.; Annick, D.D.; Frederic, D. Plants used as remedies antirheumatic and antineuralgic in the traditional medicine of Lebanon. J. Ethnopharmacol. 2008, 120, 315–334.
- Nelson, K.; Lyles, J.T.; Li, T.; Saitta, A.; Addie-Noye, E.; Tyler, P.; Quave, C.L. Anti-Acne Activity of Italian Medicinal Plants Used for Skin Infection. Front. Pharmacol. 2016, 7, 425.
- Saleem, M.; Karim, M.; Qadir, M.; Ahmed, B.; Rafiq, M.; Ahmad, B. In vitro antibacterial activity and phytochemical analysis of hexane extract of Vicia sativa. Bangladesh J. Pharmacol. 2014, 9, 189–193.
- Megías, C.; Pastor-Cavada, E.; Torres-Fuentes, C.; Girón-Calle, J.; Barragán, M.A.; Juan, R.; Pastor, J.E.; Vioque, J. Chelating, antioxidant and antiproliferative activity of Vicia sativa polyphenol extracts. Eur. Food Res. Technol. 2009, 230, 353–359.
- Ford, R. Vetch pod rupture associated with unrelated streak-inducing viruses of peas. Phytopathology 1965, 55, 935.
- Mao, Z.; Fu, H.; Nan, Z.; Wan, C. Fatty acid, amino acid, and mineral composition of four common vetch seeds on Qinghai-Tibetan plateau. Food Chem. 2015, 171, 13–18.
- Fırıncıoğlu, H.K.; Ünal, S.; Erbektaş, E.; Doğruyol, L. Relationships between seed yield and yield components in common vetch (Vicia sativa ssp. sativa) populations sown in spring and autumn in central Turkey. Field Crops Res. 2010, 116, 30–37.
- Firincioğlu, H.K.; Tate, M.; Ünal, S.; Doğruyol, L.; Özcan, İ. A Selection Strategy for Low Toxin Vetches (Vicia sativa spp.). Turk. J. Agric. For. 2007, 31, 303–311.
- Matić, R.; Nagel, S.; Robertson, S.; Young, I.; Mihailović, V.; Mikić, A.; Kirby, G. Vetch (Vicia spp) expansion and use in Australia. Biotechnol. Anim. Husb. 2005, 21, 203–207.
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global Synthesis of Drought Effects on Food Legume Production. PLoS ONE 2015, 10, e0127401.
- Koumas, A.; Economides, S. Replacement of Soybean Meal by Broad Bean or Common Vetch Seed in Lamb and Kid Fattening Diets. Tech. Bull. 1987, 88, 1–5.
- Delaere, I. The Chemistry of Vivia sativa L. Selection; University of Adelaide, Department of Plant Science: Adelaide, Australia, 1996.
- Rathjen, J.M. The Potential for Vicia sativa L. as a Grain Legume for South Australia/Thesis Jane Mary Rathjen. Ph.D. Thesis, The University of Adelaide, Adelaide, Australia, 1997.
- Li, M.; Zhao, L.; Zhou, Q.; Fang, L.; Luo, D.; Liu, W.; Searle, I.R.; Liu, Z. Transcriptome and Coexpression Network Analyses Provide In-Sights into the Molecular Mechanisms of Hydrogen Cyanide Synthesis during Seed Development in Common Vetch (Vicia sativa L.). Int. J. Mol. Sci. 2022, 23, 2275.
- Akande, K.E.; Fabiyi, E.F. Effect of Processing Methods on Some Antinutritional Factors in Legume Seeds for Poultry Feeding. Int. J. Poult. Sci. 2010, 9, 996–1001.
- Lambein, F.; Kuo, Y.H.; Ikegami, F.; Kusama-Eguchi, K.; Enneking, D. Grain legumes and human health. In Food Legumes for Nutritional Security and Sustainable Agriculture, Proceedings of the 4th International Food Legumes Research Conference, New Delhi, India, 18–22 October 2005; Indian Society of Genetics and Plant Breeding: New Delhi, India, 2009; pp. 422–432.
- Ampomah, O.; Huss-Danell, K. Genetic diversity of rhizobia nodulating native Vicia spp. in Sweden. Syst. Appl. Microbiol. 2016, 39, 203–210.
- Daramola, D.A.; Hatzell, M.C. Energy Demand of Nitrogen and Phosphorus Based Fertilizers and Approaches to Circularity. ACS Energy Lett. 2003, 8, 1493–1501.
- Lei, X.; Wang, E.T.; Chen, W.F.; Sui, X.H.; Chen, W.X. Diverse bacteria isolated from root nodules of wild Vicia species grown in temperate region of China. Arch. Microbiol. 2008, 190, 657–671.
- Jorrin, B.; Imperial, J. Population Genomics Analysis of Legume Host Preference for Specific Rhizobial Genotypes in the Rhizobium leguminosarum bv. Viciae Symbioses. Mol. Plant Microbe Interact. 2015, 28, 310–318.
- Alvarez-Martinez, E.R.; Valverde, A.; Ramirez-Bahena, M.H.; Garcia-Fraile, P.; Tejedor, C.; Mateos, P.F.; Santillana, N.; Zuniga, D.; Peix, A.; Velazquez, E. The analysis of core and symbiotic genes of rhizobia nodulating Vicia from different continents reveals their common phylogenetic origin and suggests the distribution of Rhizobium leguminosarum strains together with Vicia seeds. Arch. Microbiol. 2009, 191, 659–668.
- Laus, M.C.; van Brussel, A.A.; Kijne, J.W. Exopolysaccharide structure is not a determinant of host-plant specificity in nodulation of Vicia sativa roots. Mol. Plant Microbe Interact. 2005, 18, 1123–1129.
- Laus, M.C.; van Brussel, A.A.; Kijne, J.W. Role of cellulose fibrils and exopolysaccharides of Rhizobium leguminosarum in attachment to and infection of Vicia sativa root hairs. Mol. Plant Microbe Interact. 2005, 18, 533–538.
- Muszynski, A.; Laus, M.; Kijne, J.W.; Carlson, R.W. Structures of the lipopolysaccharides from Rhizobium leguminosarum RBL5523 and its UDP-glucose dehydrogenase mutant (exo5). Glycobiology 2011, 21, 55–68.
- Tak, T.; van Spronsen, P.C.; Kijne, J.W.; van Brussel, A.A.; Boot, K.J. Accumulation of lipochitin oligosaccharides and NodD-activating compounds in an efficient plant--Rhizobium nodulation assay. Mol. Plant Microbe Interact. 2004, 17, 816–823.
- Recourt, K.; Schripsema, J.; Kijne, J.W.; van Brussel, A.A.; Lugtenberg, B.J. Inoculation of Vicia sativa subsp. nigra roots with Rhizobium leguminosarum biovar Viciae results in release of nod gene activating flavanones and chalcones. Plant Mol. Biol. 1991, 16, 841–852.
- Göttfert, M. Regulation and function of rhizobial nodulation genes. FEMS Microbiol. Rev. 1993, 10, 39–63.
- Álvarez-Aragón, R.; Manuel Palacios, J.M.; Ramirez-Parra, E. Rhizobial symbiosis promotes drought tolerance in Vicia sativa and Pisum sativum. Environ. Exp. Bot. 2023, 208, 105268.
- Zhang, E.; Li, L.; Huang, G.; Huang, P.; Chai, Q. Regulation of fertilizer application on yield and root growth of spring wheat-faba bean intercropping system. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2002, 13, 939–942.
- Allende-Montalbán, R.; Martín-Lammerding, D.; del Mar Delgado, M.; Porcel, M.A.; Gabriel, J.L. Nitrate Leaching in Maize (Zea mays L.) and Wheat (Triticum aestivum L.) Irrigated Cropping Systems under Nitrification Inhibitor and/or Intercropping Effects. Agriculture 2022, 12, 478.
- Garduno-Castro, Y.; Espinoza-Ortega, A.; Gonzalez-Esquivel, C.E.; Mateo-Salazar, B.; Arriaga-Jordan, C.M. Intercropped oats (Avena sativa)—common vetch (Vicia sativa) silage in the dry season for small-scale dairy systems in the highlands of central Mexico. Trop. Anim. Health Prod. 2009, 41, 827–834.
- Keba, W.; Tolemariam, T.; Mohammed, A. Straw dry matter yield and quality of finger millet intercropped with selected vetch species at different seeding ratios in western Oromia, Ethiopia. Heliyon 2022, 8, e10433.
- Hontoria, C.; Garcia-Gonzalez, I.; Quemada, M.; Roldan, A.; Alguacil, M.M. The cover crop determines the AMF community composition in soil and in roots of maize after a ten-year continuous crop rotation. Sci. Total Environ. 2019, 660, 913–922.
- Genard, T.; Etienne, P.; Laine, P.; Yvin, J.C.; Diquelou, S. Nitrogen transfer from Lupinus albus L., Trifolium incarnatum L. and Vicia sativa L. contribute differently to rapeseed (Brassica napus L.) nitrogen nutrition. Heliyon 2016, 2, e00150.
- Wang, Q.; Zhang, C.; Li, J.; Wu, X.; Long, Y.; Su, Y. Intercropping Vicia sativa L. Improves the Moisture, Microbial Community, Enzyme Activity and Nutrient in Rhizosphere Soils of Young Kiwifruit Plants and Enhances Plant Growth. Horticulturae 2021, 7, 335.
- Ogilvie, C.M.; Ashiq, W.; Vasava, H.B.; Biswas, A. Quantifying Root-Soil Interactions in Cover Crop Systems: A Review. Agriculture 2021, 11, 218.
- Baldwin, K.; Creamer, N. Cover Crops for Organic Farms; Center for Enviromental Farming Systems, NCSU-NCA&TSU-NCDA&CS; North Carolina Cooperative Extension Service: Raleigh, NC, USA, 2006; pp. 1–22.
- Trenton, R.; Carrie, O.; Kelsey, H.; Hannah, W.; Tyler, D. Understanding cover crops. Agric. Nat. Resour. 2018, FS2156, 1–8.
- Wiesmeier, M.; Lungu, M.; Hübner, R.; Cerbari, V. Remediation of degraded arable steppe soils in Moldova using vetch as green manure. Solid Earth 2015, 6, 609–620.
- Rodrigo-Comino, J.; Terol, E.; Mora, G.; Giménez-Morera, A.; Cerdà, A. Vicia sativa Roth. Can Reduce Soil and Water Losses in Recently Planted Vineyards (Vitis vinifera L.). Earth Syst. Environ. 2020, 4, 827–842.
- Renzi, J. Efecto de la estructura de cultivo y grado de madurez a cosecha sobre el rendimiento y la calidad de semillas de Vicia sativa L. y Vicia. villosa Roth., bajo riego. MSc. Thesis, Universidad nacional del Sur, Bahia Blanca, Argentina, 2009.
- Alonso-Ayuso, M.; Gabriel, J.L.; Pancorbo, J.L.; Quemada, M. Interseeding cover crops into maize: Characterization of species performance under Mediterranean conditions. Field Crops Res. 2020, 249, 107762.
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals-Concepts and applications. Chemosphere 2013, 91, 869–881.
- Ibañez, S.; Medina, M.I.; Agostini, E. Vicia: A green bridge to clean up polluted environments. Appl. Microbiol. Biotechnol. 2020, 104, 13–21.
- Lastiri-Hernández, M.A.; Alvarez-Bernal, D.; Bermúdez-Torres, K.; Cárdenas, G.C.; Ceja-Torres, L.F. Phytodesalination of a moderately saline soil combined with two inorganic amendments. Bragantia 2019, 78, 579–586.
- Alonso-Prados, J.L.; Hernández-Sevillano, E.; Llanos, S.; Villarroya, M.; García-Baudín, J.M. Effects of sulfosulfuron soil residues on barley (Hordeum vulgare), sunflower (Helianthus annuus) and common vetch (Vicia sativa). Crop Prot. 2002, 21, 1061–1066.
- Ibanez, S.G.; Sosa Alderete, L.G.; Medina, M.I.; Agostini, E. Phytoremediation of phenol using Vicia sativa L. plants and its antioxidative response. Environ. Sci. Pollut. Res. 2012, 19, 1555–1562.
- Halfadji, A.; Portet-Koltalo, F.; Touabet, A.; Le Derf, F.; Morin, C.; Merlet-Machour, N. Phytoremediation of PCB: Contaminated Algerian soils using native agronomics plants. Environ. Geochem. Health 2022, 44, 117–132.
- Tan, M.; Temel, S. Effect of mepiquat chloride, a growth retardant, on seed yield and yield components in common vetch (Vicia sativa). Indian J. Agric. Sci. 2005, 75, 160–161.
- Machrafi, Y.; Prévost, D.; Beauchamp, C.J. Toxicity of phenolic compounds extracted from bark residues of different ages. J. Chem. Ecol. 2006, 32, 2595–2615.
- Adam, G.; Duncan, H. The effect of diesel fuel on common vetch (Vicia sativa L.) plants. Environ. Geochem. Health 2003, 25, 123–130.
- Muccifora, S.; Bellani, L.M. Effects of copper on germination and reserve mobilization in Vicia sativa L. seeds. Environ. Pollut. 2013, 179, 68–74.
- Bellani, L.M.; Muccifora, S.; Giorgetti, L. Response to copper bromide exposure in Vicia sativa L. seeds: Analysis of genotoxicity, nucleolar activity and mineral profile. Ecotoxicol. Environ. Saf. 2014, 107, 245–250.
- Ibañez, S.G.; Villasuso, A.L.; Racagni, G.E.; Agostini, E.; Medina, M.I. Phenol modulates lipid kinase activities in Vicia sativa plants. Environ. Exp. Bot. 2016, 122, 109–114.
- Sierra, M.J.; Millán, R.; Esteban, E.; Cardona, A.I.; Schmid, T. Evaluation of mercury uptake and distribution in Vicia sativa L. applying two different study scales: Greenhouse conditions and lysimeter experiments. J. Geochem. Explor. 2008, 96, 203–209.
- Bogatu, C.; Masu, S.; Lazarovici, M. Metals extraction from polluted soils by using of pillared zeolite and Vicia sativa. In Proceedings of the 14th Symposium on Analytical and Environmental Problems, Szeged, Hungary, 24 September 2007.
- Rui, H.; Zhang, X.; Shinwari, K.I.; Zheng, L.; Shen, Z. Comparative transcriptomic analysis of two Vicia sativa L. varieties with contrasting responses to cadmium stress reveals the important role of metal transporters in cadmium tolerance. Plant Soil 2018, 423, 241–255.
- Ivanishchev, V.V.; Abramova, E.A. Accumulation of nickel ions in seedlings of Vicia sativa L. and manifestations of oxidative stress. Environ. Sci. Pollut. Res. 2015, 22, 7897–7905.
- Masu, S.; Lixandru, B.; Bogatu, C. Zinc extraction from polluted soils by using zeolite and Vicia sativa plant. In Proceedings of the 3rd International Conference on Life Cycle Management, Zurich, Switzerland, 27–29 August 2007.
- Zhang, F.; Zhang, H.; Wang, G.; Xu, L.; Shen, Z. Cadmium-induced accumulation of hydrogen peroxide in the leaf apoplast of Phaseolus aureus and Vicia sativa and the roles of different antioxidant enzymes. J. Hazard. Mater. 2009, 168, 76–84.
- Zhang, X.; Zhang, L.; Chen, L.; Lu, Y.; An, Y. Ectopic expression gamma-glutamylcysteine synthetase of Vicia sativa increased cadmium tolerance in Arabidopsis. Gene 2022, 823, 146358.
- Zhang, X.; Rui, H.; Zhang, F.; Hu, Z.; Xia, Y.; Shen, Z. Overexpression of a Functional Vicia sativa PCS1 Homolog Increases Cadmium Tolerance and Phytochelatins Synthesis in Arabidopsis. Front. Plant Sci. 2018, 9, 107.
- Ibañez, S.G.; Merini, L.J.; Barros, G.G.; Medina, M.I.; Agostini, E. Vicia sativa-Rhizospheric bacteria interactions to improve phenol remediation. Int. J. Environ. Sci. Technol. 2014, 11, 1679–1690.
- Anderson, T.A.; Guthrie, E.A.; Walton, B.T. Bioremediation in the rhizosphere. Environ. Sci. Technol. 1993, 27, 2630–2636.
- Ibañéz, S.G.; Oller, A.L.W.; Paisio, C.E.; Alderete, L.G.S.; González, P.S.; Medina, M.I.; Agostini, E. The challenges of remediating metals using phytotechnologies. In Heavy Metals in the Environment: Microorganisms and Bioremediation; CRC Press: Boca Raton, FL, USA, 2018; pp. 173–191.
