Biosurveillance defines the process of gathering, integrating, interpreting, and communicating essential information related to all-hazards threats or disease activity affecting human, animal, or plant health to achieve early detection and warning, contribute to overall situational awareness of the health aspects of an incident, and to enable better decision making for action at all levels. Animal health surveillance is an important component within biosurveillance systems comprising a continuum of activities from detecting biological threats, to analyzing relevant data, to managing identified threats, and embracing a One Health concept. The animal health community can strengthen biosurveillance by adopting various developments such as increasing the alignment, engagement, and participation of stakeholders in surveillance systems, exploring new data streams, improving integration and analysis of data streams for decision-making, enhancing research and application of social sciences and behavioral methods in animal health surveillance, and performing timely evaluation of surveillance systems.
- biosurveillance
- one health
- animal health
- zoonosis
- disease surveillance
- epidemiology
1. Biosurveillance and One Health
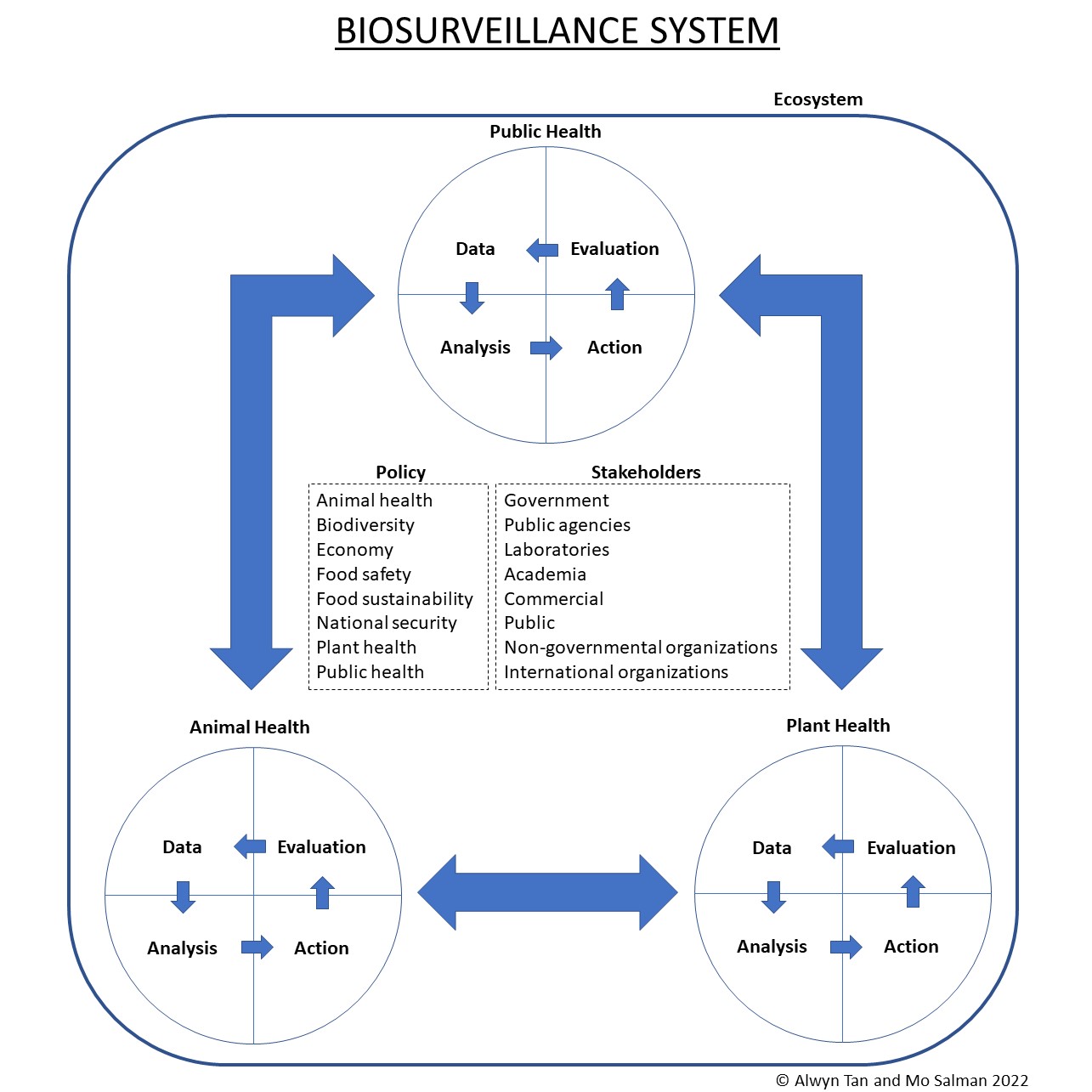
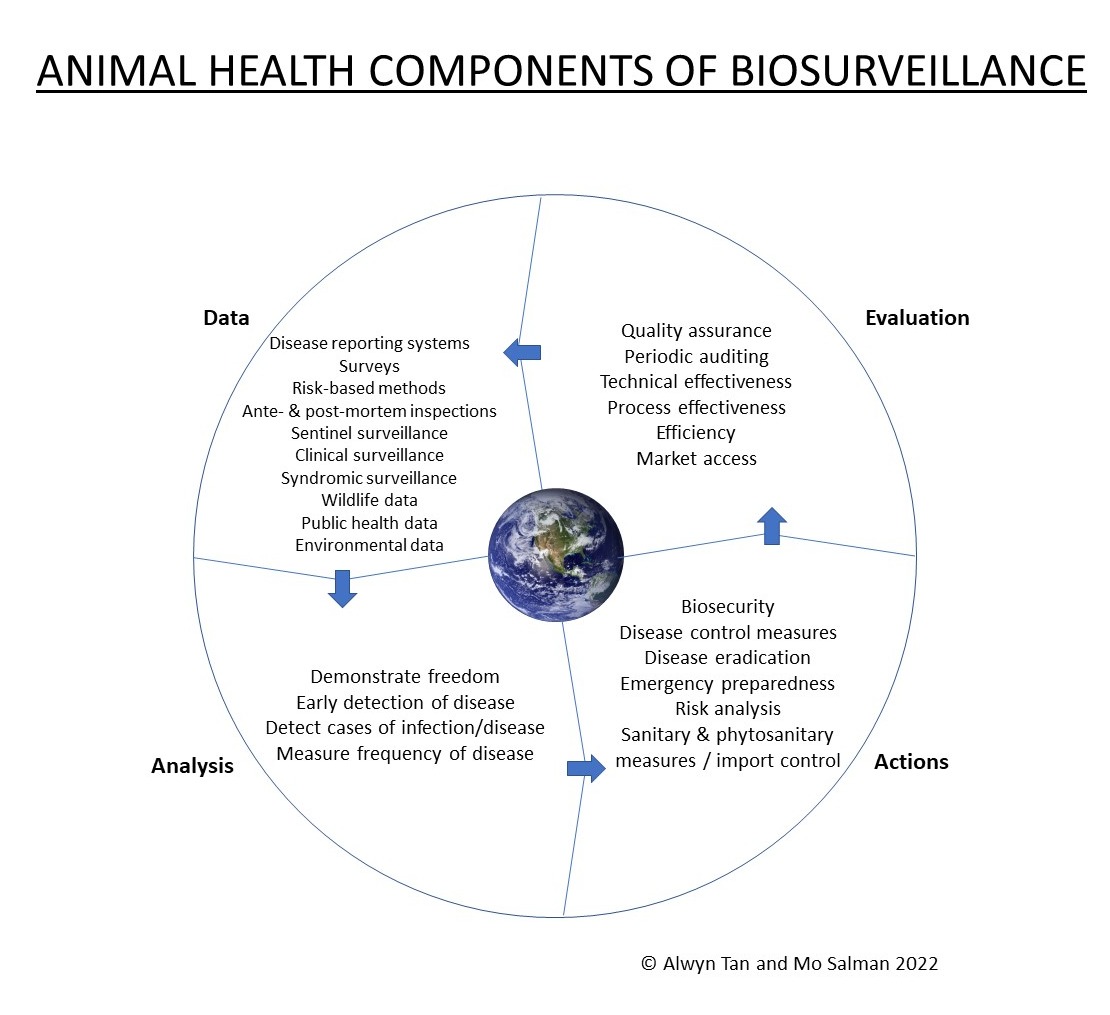
2. Animal Health Surveillance
3. Policy
4. Stakeholders
5. Data Streams
6. Data Stream Integration, Processing, Analysis, and Access by Decision Makers
7. Surveillance Evaluation
This entry is adapted from the peer-reviewed paper 10.3390/agriculture13020457
References
- Wagner, M.M. Introduction. In Handbook of Biosurveillance; Wagner, M.M., Moore, A.W., Aryel, R.M., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2006; pp. 3–12.
- Huff, A.G.; Allen, T.; Whiting, K.; Williams, F.; Hunter, L.; Gold, Z.; Madoff, L.C.; Karesh, W.B. Biosurveillance: A systematic review of global infectious disease surveillance systems from 1900 to 2016. Sci. Tech. Rev. 2017, 36, 513–524.
- Kim, A.J.; Tak, S. Implementation System of a Biosurveillance System in the Republic of Korea and Its Legal Ramifications. Health Secur. 2019, 17, 462–467.
- World Health Organization. One health. Available online: https://www.who.int/health-topics/one-health (accessed on 21 September 2022).
- World Organisation for Animal Health. One Health. Available online: https://www.woah.org/en/what-we-do/global-initiatives/one-health/ (accessed on 14 September 2022).
- Salman, M.D. Animal Disease Surveillance and Survey Systems: Methods and Applications, 1st ed.; Iowa State Press: Ames, IA, USA, 2003.
- World Organisation for Animal Health. Terrestrial Animal Health Code; Animal Health Surveillance; World Organisation for Animal Health: Paris, France, 2021.
- Food and Agriculture Organization of the United Nations; United Nations Environment Programme; World Health Organization; World Organisation for Animal Health. One Health Joint Plan of Action (2022–2026): Working Together for the Health of Humans, Animals, Plants and the Environment; Food and Agriculture Organization of the United Nations; United Nations Environment Programme; World Health Organization; World Organisation for Animal Health: Rome, Italy, 2022.
- World Health Organization; Food and Agriculture Organization of the United Nations; World Organisation for Animal Health; United Nations Environment Programme. Strategic Framework for Collaboration on Antimicrobial Resistance—Together for One Health; World Health Organization, Food and Agriculture Organization of the United Nations and World Organization for Animal Health: Geneva, Switzerland, 2022.
- The White House. National Biodefense Strategy and Implementation Plan; The White House: Washington, DC, USA, 2022.
- Hobbs, E.C.; Reid, T.J. Animals and SARS-CoV-2: Species susceptibility and viral transmission in experimental and natural conditions, and the potential implications for community transmission. Transbound. Emerg. Dis. 2021, 68, 1850–1867.
- World Organisation for Animal Health. OIE Technical Factsheet: Infection with SARS-CoV-2 in Animals. Available online: https://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/COV-19/A_Factsheet_SARS-CoV-2.pdf (accessed on 19 October 2022).
- Food and Agriculture Organization; World Organisation for Animal Health; World Health Organization. Joint Statement on the Prioritization of Monitoring SARS-CoV-2 Infection in Wildlife and Preventing the Formation of Animal Reservoirs; World Health Organization: Geneva, Switzerland, 2022.
- World Health Organization; Food and Agriculture Organization; World Organisation for Animal Health; Global Alliance for Rabies Control. ZERO BY 30 The Global Strategic Plan to End Human Deaths from Dog-Mediated Rabies by 2030; World Health Organization; Food and Agriculture Organization; World Organisation for Animal Health; Global Alliance for Rabies Control: Geneva, Switzerland, 2018.
- Administration for Strategic Preparedness & Response. About ASPR. Available online: https://aspr.hhs.gov/AboutASPR/Pages/default.aspx (accessed on 25 September 2022).
- Schneeberger, P.M.; Wintenberger, C.; van der Hoek, W.; Stahl, J.P. Q fever in the Netherlands—2007–2010: What we learned from the largest outbreak ever. Méd. Mal. Infect. 2014, 44, 339–353.
- Peters, B.G. American Public Policy: Promise and Performance, 11th ed.; SAGE Publications: Los Angeles, CA, USA, 2018.
- Howlett, M.; Ramesh, M.; Perl, A. Studying Public Policy: Principles and Processes, 4th ed.; Oxford University Press: Toronto, ON, Canada, 2020.
- The White House. National Strategy For Biosurveillance; The White House: Washington, DC, USA, 2012.
- Australia Department of Agriculture Fisheries and Forestry. Agricultural Competitiveness White Paper—Biosecurity Surveillance and Analysis. Available online: https://www.agriculture.gov.au/biosecurity-trade/policy/agwhitepaper-bio-surveillance-analysis (accessed on 14 September 2022).
- Salman, M.; Silano, V.; Heim, D.; Kreysa, J. Geographical BSE risk assessment and its impact on disease detection and dissemination. Prev. Vet. Med. 2012, 105, 255–264.
- Wagner, M.M.; Hogan, W.R. The Healthcare System. In Handbook of Biosurveillance; Wagner, M.M., Moore, A.W., Aryel, R.M., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2006; pp. 89–109.
- Royal Veterinary College. About VetCompass. Available online: https://www.rvc.ac.uk/vetcompass/about/overview (accessed on 22 September 2022).
- O’Neill, D. VetCompass clinical data points the way forward. Vet. Irel. J. 2012, 2, 353–356.
- Alders, R.G.; Ali, S.N.; Ameri, A.A.; Bagnol, B.; Cooper, T.L.; Gozali, A.; Hidayat, M.M.; Rukambile, E.; Wong, J.T.; Catley, A. Participatory Epidemiology: Principles, Practice, Utility, and Lessons Learnt. Front. Vet. Sci. 2020, 7, 532763.
- Bordier, M.; Goutard, F.L.; Antoine-Moussiaux, N.; Pham-Duc, P.; Lailler, R.; Binot, A. Engaging Stakeholders in the Design of One Health Surveillance Systems: A Participatory Approach. Front. Vet. Sci. 2021, 8, 646458.
- Swine Health Information Center. Plan of Work. Available online: https://www.swinehealth.org/plan-of-work/ (accessed on 3 February 2023).
- Betlach, C.; Baldry, H.; Homann, T.; VanderWaal, K.; Kanankege, K.; Morrison, B. Swine Health Monitoring Project serving as swine disease surveillance system. Available online: https://www.nationalhogfarmer.com/health/swine-health-monitoring-project-serving-swine-disease-surveillance-system (accessed on 3 February 2023).
- Extension Disaster Education Network. Structure. Available online: https://extensiondisaster.net/about/structure/ (accessed on 3 February 2023).
- U.S. Department of Agriculture. About NAHLN. Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/lab-info-services/nahln/ (accessed on 3 February 2023).
- U.S. Department of Agriculture. National Plant Diagnostic Network. Available online: https://www.nifa.usda.gov/national-plant-diagnostic-network (accessed on 3 February 2023).
- Rosenbaum, L. Escaping Catch-22—Overcoming Covid Vaccine Hesitancy. N. Engl. J. Med. 2021, 384, 1367–1371.
- World Health Organization; United Nations Children’s Fund. Data for Action: Achieving High Uptake of COVID-19 Vaccines: Gathering and Using Data on the Behavioural and Social Drivers of Vaccination: A Guidebook for Immunization Programmes and Implementing Partners: Interim Guidance, 3 February 2021; World Health Organization & United Nations Children’s Fund: Geneva, Switzerland, 2021.
- Piltch-Loeb, R.; DiClemente, R. The Vaccine Uptake Continuum: Applying Social Science Theory to Shift Vaccine Hesitancy. Vaccines 2020, 8, 76.
- Paquette, C.; Schemann, K.; Ward, M. Knowledge and attitudes of Australian livestock producers concerning biosecurity practices. Aust. Vet. J. 2020, 98, 533–545.
- Gates, M.C.; Earl, L.; Enticott, G. Factors influencing the performance of voluntary farmer disease reporting in passive surveillance systems: A scoping review. Prev. Vet. Med. 2021, 196, 11.
- Margevicius, K.J.; Generous, N.; Taylor-McCabe, K.J.; Brown, M.; Daniel, W.B.; Castro, L.; Hengartner, A.; Deshpande, A. Advancing a framework to enable characterization and evaluation of data streams useful for biosurveillance. PLoS ONE 2014, 9, e83730.
- Velikina, R.; Dato, V.; Wagner, M. Governmental Public Health. In Handbook of Biosurveillance; Wagner, M.M., Moore, A.W., Aryel, R.M., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2006; pp. 67–196.
- Shmueli, G.; Burkom, H. Statistical Challenges Facing Early Outbreak Detection in Biosurveillance. Technometrics 2010, 52, 39–51.
- Wagner, M.M. Methods of Evaluating Surveillance Data. In Handbook of Biosurveillance; Wagner, M.M., Moore, A.W., Aryel, R.M., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2006; pp. 313–319.
- Velsko, S.; Bates, T. A Conceptual Architecture for National Biosurveillance: Moving Beyond Situational Awareness to Enable Digital Detection of Emerging Threats. Health Secur. 2016, 14, 189–201.
- U.S. Department of Homeland Security. Detecting Bioterrorist Attacks. Available online: https://www.dhs.gov/biowatch-program (accessed on 26 September 2022).
- Wagner, M.M.; Pavlin, J.; Cox, K.L.; Cirino, N.M. Other Organizations That Conduct Biosurveillance. In Handbook of Biosurveillance; Wagner, M.M., Moore, A.W., Aryel, R.M., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2006; pp. 183–196.
- Hietala, S.K.; Hullinger, P.J.; Crossley, B.M.; Kinde, H.; Ardans, A.A. Environmental air sampling to detect exotic Newcastle disease virus in two California commercial poultry flocks. J. Vet. Diagn. Investig. 2005, 17, 198–200.
- Torremorell, M.; Alonso, C.; Davies, P.R.; Raynor, P.C.; Patnayak, D.; Torchetti, M.; McCluskey, B. Investigation into the Airborne Dissemination of H5N2 Highly Pathogenic Avian Influenza Virus During the 2015 Spring Outbreaks in the Midwestern United States. Avian Dis. 2016, 60, 637–643.
- BROADN. BROADN: Aerobiome Research. Available online: https://broadn.colostate.edu/research/ (accessed on 29 September 2022).
- Global Atmospheric Microbiome Project. The Global Atmospheric Microbiome Project. Available online: http://atmospheric-microbiome.com/about/about_gamp.html (accessed on 26 September 2022).
- Mainelis, G. Bioaerosol sampling: Classical approaches, advances, and perspectives. Aerosol Sci. Technol. 2020, 54, 496–519.
- Šantl-Temkiv, T.; Sikoparija, B.; Maki, T.; Carotenuto, F.; Amato, P.; Yao, M.; Morris, C.E.; Schnell, R.; Jaenicke, R.; Pöhlker, C.; et al. Bioaerosol field measurements: Challenges and perspectives in outdoor studies. Aerosol Sci. Technol. 2020, 54, 520–546.
- Champion, H.J.; Gloster, J.; Mason, I.S.; Brown, R.J.; Donaldson, A.I.; Ryall, D.B.; Garland, A.J.M. Investigation of the possible spread of foot-and-mouth disease virus by the burning of animal carcases on open pyres. Vet. Rec. 2002, 151, 593–600.
- Moore, R.A.; Bomar, C.; Kobziar, L.N.; Christner, B.C. Wildland fire as an atmospheric source of viable microbial aerosols and biological ice nucleating particles. ISME J. 2021, 15, 461–472.
- Dórea, F.C.; Revie, C.W. Data-Driven Surveillance: Effective Collection, Integration, and Interpretation of Data to Support Decision Making. Front. Vet. Sci. 2021, 8, 633977.
- Gates, M.C.; Holmstrom, L.K.; Biggers, K.E.; Beckham, T.R. Integrating novel data streams to support biosurveillance in commercial livestock production systems in developed countries: Challenges and opportunities. Front. Public Health 2015, 3, 74.
- Council for Agricultural Science and Technology. Zoonotic Diseases in Animal Agriculture and Beyond: A One Health Perspective; Council for Agricultural Science and Technology: Ames, IA, USA, 2022; p. 72.
- American Association of Veterinary Laboratory Diagnosticians; United States Animal Health Association. eCVI Data Exchange Standard. Available online: https://github.com/AAVLD-USAHA-ITStandards/eCVI (accessed on 7 December 2022).
- Estberg, L.; Luxton, J.; Spiegel, K.; Pelzel-McCluskey, A.; Gomez, B.L.; Vanden Eng, J.L. Business-centric data solutions for safeguarding American animal agriculture. World Organ. Anim. Health Sci. Tech. Rev. 2022, 41, preprint.
- Palantir Technologies. Foundry for Population Health. Available online: https://www.palantir.com/assets/xrfr7uokpv1b/7EzTCn3cz13pTAx8u3U5WM/29ae2623771441b61b2f7267b6f47789/Foundry_for_Population_Health.pdf (accessed on 7 December 2022).
- Dórea, F.C.; Revie, C.W.; McEwen, B.J.; McNab, W.B.; Kelton, D.; Sanchez, J. Retrospective time series analysis of veterinary laboratory data: Preparing a historical baseline for cluster detection in syndromic surveillance. Prev. Vet. Med. 2013, 109, 219–227.
- Dórea, F.C.; Widgren, S.; Lindberg, A. Vetsyn: An R package for veterinary syndromic surveillance. Prev. Vet. Med. 2015, 122, 21–32.
- Odoi, A.; Carter, C.N.; Riley, J.W.; Smith, J.L.; Dwyer, R.M. Application of an automated surveillance-data-analysis system in a laboratory-based early-warning system for detection of an abortion outbreak in mares. Am. J. Vet. Res. 2009, 70, 247–256.
- U.S. Department of Agriculture. 2022 Detections of Highly Pathogenic Avian Influenza in Wild Birds. Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-information/avian/avian-influenza/hpai-2022/2022-hpai-wild-birds (accessed on 12 December 2022).
- Parker, K.; Horowitz, J.M. Majority of Workers Who Quit a Job in 2021 Cite Low Pay, No Opportunities for Advancement, Feeling Disrespected. Available online: https://www.pewresearch.org/fact-tank/2022/03/09/majority-of-workers-who-quit-a-job-in-2021-cite-low-pay-no-opportunities-for-advancement-feeling-disrespected/ (accessed on 27 September 2022).
- Woolston, C. ‘Does Anyone Have Any of These?’: Lab-Supply Shortages Strike Amid Global Pandemic. Available online: https://www.nature.com/articles/d41586-021-00613-y (accessed on 27 September 2022).
- Peyre, M.; Goutard, F.; Roger, F. Why Do We Need to Evaluate Health Surveillance Systems? In Principles for Evaluation of One Health Surveillance: The EVA Book; Peyre, M., Roger, F., Goutard, F., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2022; pp. 3–24.
- World Health Organization. Joint External Evaluation Tool: International Health Regulations (2005); WHO: Geneva, Switzerland, 2022.
- World Organisation for Animal Health. OIE Tool for the Evaluation of Performance of Veterinary Services; World Organisation for Animal Health: Paris, France, 2019.
- Drewe, J.A.; Hoinville, L.J.; Cook, A.J.C.; Floyd, T.; Stärk, K.D.C. Evaluation of animal and public health surveillance systems: A systematic review. Epidemiol. Infect. 2012, 140, 575–590.
- German, R.R.; Lee, L.M.; Horan, J.M.; Milstein, R.L.; Pertowski, C.A.; Waller, M.N. Updated guidelines for evaluating public health surveillance systems: Recommendations from the Guidelines Working Group. MMWR Recomm. Rep. 2001, 50, 1–35.
- Groseclose, S.L.; Buckeridge, D.L. Public Health Surveillance Systems: Recent Advances in Their Use and Evaluation. Annu. Rev. Public Health 2017, 38, 57–79.
- Peyre, M.; Salman, M.; Steneroden, K. Frameworks and Tools for Evaluating Health Surveillance Systems. In Principles for Evaluation of One Health Surveillance: The EVA Book; Peyre, M., Roger, F., Goutard, F., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2022; pp. 43–60.
- Peyre, M.; Schulz, K.; Pham, T.T.H.; Häsler, B. The EVA Survtool: An Integrated Framework to Plan Health Surveillance Evaluation. In Principles for Evaluation of One Health Surveillance: The EVA Book; Peyre, M., Roger, F., Goutard, F., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2022; pp. 61–92.
