Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Integrative & Complementary Medicine
Alzheimer’s disease (AD), as a neurodegenerative disorder, usually develops slowly but gradually worsens. Transcranial photobiomodulation (PBM) has emerged as a potential clinical treatment and cognitive enhancement method for various neurodegenerative pathologies by delivering red and near-infrared light to the frontal and midbrain areas, which could also help increasing the potential of pharmacological therapies.
- bionanoformulations
- integrative nanomedicine
- laser
- light
- nanomedicines
- neurodegenerative disorders
- photobiomodulation
- cerebral photomedicine
- picosecond laser stimulation
1. Light and Lasers in Medicine—A Brief Overview
Light as a healing modality goes hand in hand with human history and has probably developed over hundreds and hundreds of years, but contemporary PBM or photodynamic therapy (PDT) have only recently advanced following the discovery of the laser and its high-tech applications, such as the latest innovations in brain disorders, cancer or degenerative conditions, including AD [241,242].
Albert Einstein brilliantly predicted the phenomenon of stimulated emission as early as 1917, through a published work on the quantum theory of radiation, thus theoretically establishing the basis for MASER (Microwave Amplification by Stimulated Emission of Radiation) and LASER, acronym for Light Amplification by Stimulated Emission of Radiation, as recognized by the American Physical Society. A year earlier, Einstein turned his attention from general relativity to the interaction of matter and radiation, so he reimagined the fundamental statistical theory of heat, embracing the quantum of energy [243].
In 1964, the Nobel Prize in Physics was awarded to Dr. Townes, Dr. Basov and Dr. Prochorov, who “have made the atoms work for us in a new and most remarkable way. These magic devices called maser and laser have opened up vast new fields for research and applications which are being exploited with increasing intensity in many laboratories all over the world.” [244].
The discovery of the laser initiated a multi-billion-dollar industry, being applied in the arms industry, clinical medicine, different branches of surgery, cosmetics, sports, all industrial fields, optical communications and optical data storage, electronic devices and in almost all relevant fields of human activity, from supermarkets to entertainment. Since its first medical applications, PBM has been used for the treatment of many inflammatory diseases and especially for tissue regeneration and rehabilitation. The intensive development of advanced laser systems, light emitting diodes (LEDs), and other light devices has led to an unprecedented expansion of a multitude of therapy options, the value of which is the absence of or very few side effects, addressing precisely the energy processes inside the cells and what is more valuable—without toxic consequences [243,245].
More than half a century after the invention of the laser, PBM has become a complementary therapeutic method increasingly used in almost all fields of medicine and especially in medical recovery. Originally applied mainly for the treatment of superficial skin lesions and pain, dermatological conditions, cosmetic medicine or dentistry, today the use of PBM includes a plethora of pathologies, from diabetes, myocardial or cerebral infarction, brain trauma, spinal cord damage, peripheral nerve regeneration, neurodegenerative or chronic diseases, to modern applications with stem cells in regenerative medicine and the photoactivation of some pharmacological products with impact on the metabolism of living cells [246,247,248,249].
PBM, formerly known as “low-level laser (or light) therapy” (LLLT) remains today disputed as a therapeutic method due three main reasons. The first would be the insufficiency, ambiguity, confusion and lack of full understanding of all PBM mechanisms starting from the molecular, cellular and tissue levels; secondly, the design of a multitude of laser parameters that are very different in diverse PBM protocols (treatment time, wavelength, power density, pulse modulation, and so on); and thirdly, the biphasic dose response of PBM, that means that by increasing power density or treatment time, an inhibitory counter-effect could occur. This means that a higher laser dose does not necessarily mean better effects! Thus, the negative results in some studies can be explained, precisely because the parameters established in those studies were not well designed or chosen and proved ineffective [250,251,252].
Advent of much cheaper LEDs, which are not strictly monochromatic and lack the high coherence characteristic of lasers, has opened the way for new and multiple applications, but also ongoing disputes. Paradoxically, many of the applied PBM successes target our most complex organ, the brain, and relate to the CNS where many particularly serious conditions or injuries can be treated non-invasively by transcranial PBM or tPBM; successes are also seen in the peripheral nervous system for nerve regeneration and pain relief [251].
The following section will focus on cellular and molecular mechanisms in neurodegenerative diseases, with a special focus on AD.
2. Photobiomodulation of the Brain and the Treatment of Alzheimer’s Disease
Today’s medicine must deal with alarming health problems, among which, after cancer, the category of neurodegenerative diseases, including AD, puts increasing pressure on the world health system. Degenerative brain diseases can evolve at a slow pace, but so far contemporary medicine has not been able to successfully treat them or stop their irreversible progression. In general, degeneration of the brain, as a particularly complex organ, requires an interdisciplinary approach so that PBM could be a complementary non-invasive solution.
For example, the neuroprotective effect of a new photobiomodulation technique against Aβ25-35 peptide-induced toxicity in mice recently suggested a new hypothesis for the therapeutic approach of AD [253].
Photobiology has many objectives, an essential one being deciphering and highlighting the effects of polychromatic light on living systems. Unlike modeling the dose-response as a linear function of incident light at each wavelength in the spectrum, the action resulting from a multi-wavelength spectrum is much more complicated and should be calculated by integrating the cross-sectional product for the response at each wavelength and spectral irradiance at that wavelength, both over wavelength and time, results that are true only if the dose-response functions are linear with respect to photon dose. There are multiple linear photochemical reactions in relevant dose ranges, but many final biological reactions, especially in the case of cell survival, no longer obey linear laws. In the living world, many coupled light-driven reactions are governed by intricate non-linear dose-response rules, from which researchers are currently increasingly trying to design dosimetry guidelines, including for AD [254,255].
PBM consists of applying electromagnetic radiation with wavelengths in the visible range (400–700 nm) and in the near-infrared (NIR) (700–1100 nm) range with a low power between 1 mW and 1 W, so without thermal or mechanical effects, as a complementary method to restore health, working towards the intended goal to biostimulate the targeted cells and tissues. The result is the initiation of some photochemical reactions in the cells, the method being called biostimulation or PBM, in order to relieve pain and inflammation, prevent cell and tissue apoptosis, support recovery from illness, and ultimately, if possible, cure different diseases or disorders. To produce a result on living mammalian cells, photons from the PBM process must be absorbed by the electronic absorption bands belonging to a molecular chromophore or photo acceptor. A chromophore is that fraction of a molecule responsible for its color, where the energy difference between two separate molecular orbitals falls within the range of the visible spectrum. When light hits the chromophore, it can certainly be absorbed by exciting an electron from its ground state to an excited state, so the chromophore will cause a conformational change in that molecule [256,257].
An example of absorption of light of different wavelengths inside living cells is a phenomenon with effects in the mitochondrial respiratory chain. It has been proven that the first complex, or complex 1 (NADH dehydrogenase) absorbs blue and ultraviolet light, the third complex or complex 3 (cytochrome c reductase) absorbs green and yellow light, and the fourth, i.e., complex 4 (cytochrome c oxidase or CCO) absorbs red and infrared light [258,259].
In the first complex or NADH dehydrogenase, the flavin prosthetic set and the iron sulfur clusters have operational prominence. The flavoproteins contain the nucleic acid derivative riboflavin found in the flavin adenine dinucleotide (FAD) and the flavin mononucleotide (FMN). Riboflavin is characterized by high fluorescence which allows light absorption and is vital to the configuration of these two major coenzymes, FMN and FAD, involved in many living modi operandi such as cellular energetic metabolism, cellular respiration, antibody production, etc. [260].
The vast instability of the redox and catalytic features of the enzymes in different organisms may be due to adjustment to the specific surroundings in which these enzymes are working [261].
In redox reactions, the flavin coenzymes assist the action of more 80 flavoenzymes in the human organism (and even hundreds more across other species, including those enciphered by an archaeal, bacterial, fungal complete haploid set of chromosomes and its associated genes). Two thirds of human flavoproteins are linked to human diseases and the flavoenzymes are essential for the biosynthesis of other coenzymes and hormones [262].
For example, results from animal and cellular models suggest that FAD-deficient forms of NAD(P)H quinone oxidoreductase 1 (NQO1) may accelerate the aggregation of Alzheimer’s Aβ peptide (Aβ1-42). Aβ1-42 alone forms rod-shaped fibril structures, whereas in the presence of NQO1 isoforms, Aβ1-42 is incorporated in the middle of larger globular protein aggregates surrounded by NQO1 molecules, indicating the potential relevance of FAD-deficient forms of NQO1 in the amyloid aggregation diseases [263].
Mitochondria are the energy generators of the eukaryotic cell, converting oxygen and nutrients through oxidative phosphorylation and the electron transport chain (ETC) into adenosine triphosphate (ATP). High-energy electrons pass through a series of transmembrane complexes, including CCO to the final electron acceptor, generating a proton gradient, the latter being used to produce ATP. Multiple in vitro experiments demonstrated an upward regulation of cellular respiration when mitochondria were exposed to various forms of lighting, simultaneously with the increase of products such as ATP, NADH, ribonucleic acid (RNA), proteins and others, but also the increase of oxygen consumption [251].
For example, one research group applied to two groups of 10 healthy participants 8 min of PBM with different lasers at 800 nm, 850 nm, and 1064 nm, as well as an LED at 810 nm on the human forearm in vivo to measure the effects on vascular hemodynamics and CCO redox activity, measured before, during, and after active or sham PBM. Pruitt et al. investigated whether different laser wavelengths could determine distinct PBM effects, and if a LED at a similar wavelength to a laser could induce similar PBM effects. A broadband NIR spectroscopy system was used to assess concentration changes in oxygenated hemoglobin Δ[HbO] and oxidized CCO, Δ[oxCCO]. The results proved that all three laser wavelengths triggered significant increases in both Δ[HbO] and Δ[oxCCO], while the 1064 nm laser supported the increases longer, and that the 810-nm LED with a medium irradiance of 135 mW/cm2 induced measurable and significant increases in both parameters, compared to placebo [264].
The impact of light on living cells was proposed by Karu and begins with the absorption of visible or near-infrared photons by CCO chromophores, followed by a change in the mitochondrial membrane potential, an increase in ATP synthesis and increased levels of ROS, NO and calcium ions, phenomena that could remodel the interaction between mitochondria and the nucleus. This will alter the ultrastructure of mitochondria with direct repercussions on the intrinsic dynamics of mitochondrial fission and fusion processes, changes in ADP production, redox potential, pH, intracellular cAMP values, which will influence the activator protein-1 and NF-kB, membrane permeability and ion fluxes. Interdependent pathways have been proposed regarding the direct regulation of some genes, all these aspects being included under the umbrella of retrograde mitochondrial signaling [265].
Analyzing the action spectra in the range 600–1100 nm for different biological cells, Karu et al. demonstrated that monochromatic radiation is able to trigger photobiological processes in the cells and that there are four “active” regions in all spectra, even if the maximum is not always the same; it was assumed that the fundamental molecular mechanisms would be the modulation of mitochondrial CCO activity [265,266,267].
In fact, the mitochondrial signaling is an information channel between the mitochondrial respiratory chain and the nucleus to transduce signals regarding the functional state of the mitochondria, where CCO, as the final enzyme in this respiratory chain, functions as both a signal generator and a signal transducer [268].
As the final enzyme of the ETC, CCO has been shown to be a photoacceptor and photosignal transducer in the red (R) and NIR regions of the electromagnetic spectrum and allows the relocation of the electrons from cytochrome c to molecular oxygen, thereby increasing MMP, ROS, cyclic adenosine monophosphate (cAMP) and ATP levels [269].
ROS rapidly modulate gene expression by activating different transcription factors and signaling pathways, for example NF-κB, a redox-sensor, which has key functions for neurons in signal transfer, adjustment of intrinsic cyclic processes, production of active enzymes, nucleic acids and new protein synthesis, etc. Oxygen metabolism triggered by PBM will increase ATP which results in a brief discharge of ROS, which in reverse activates transcription factors, upregulating different stimulating and protective genes for cell proliferation and survival, as well as cell migration, growth factors, etc. When PBM is completed, the cascade stimulated by transcription factors will not end right away. ROS action is a Janus-faced mediator, helpful in reduced concentrations or brief exposures, and noxious at high concentrations or with long-term action [257,270]. Oxygen is the last acceptor in the ETC through which 90% of oxygen absorbed by cells is consumed to produce ATP, while only an insignificant quantity of oxygen is converted into ROS. Many experimental AD studies and a few clinical trials have highlighted the significant potential of PBM for AD therapy. R and NIR light at a low dose can successfully decrease the accumulation of Aβ-plaques in the CNS. Connection dose–response directly controls the therapeutic effect in AD. Discovering the best PBM parameters for AD to eliminate Aβ plaques and ameliorate AD clinical signs is a clear challenge to improve its efficacy [253,270,271].
Karu discussed the multiple roles of CCO in mammalian cells under R and NIR irradiation and highlighted the critical role of ATP as a signaling molecule [272].
In fact, in humans, 4 electrons are remoted from 4 molecules of CCO and ceded to O2 along with 4 protons, thus resulting in 2 molecules of H2O. Simultaneously, 8 protons are relocated from the mitochondrial matrix, of which only half are translocated across the membrane, increasing the proton gradient. The whole picture of the proton pump in CCO is as yet under investigation. Peter D. Mitchell won the Nobel Prize in Chemistry in 1978 for his discovery of the chemiosmotic mechanism of ATP synthesis, i.e., the coupling by a proton gradient across the inner mitochondrial membrane between the ETC and the oxidative phosphorylation [273].
Stopping ATP synthase leads to a gradual increase in protons and, consequently, a higher proton-motive force, thus causing the reverse flow of electrons [274].
PBM enhances the operation of all complexes I, II, III, IV and V of the ETC. CCO (or complex IV) is the primary photoacceptor because the main oxygen consumption occurs at this site during the action of PBM. In addition to increasing ATP and cAMP, the level of nitric oxide (NO) will be increased by release from metal complexes in CCO; photodissociation of NO from its binding sites in CCO reverses mitochondrial inhibition of cellular respiration and facilitates ROS generation. NO suppresses mitochondrial respiration through various well-investigated processes and several nitrogen derivatives. It has been shown in cultured cells and tissues that low NO precisely and reversibly instantly inhibits CCO in competition with oxygen; meanwhile, higher NO and its derivatives can induce irreversible suppression of RCT and/or cell death. Hence, the inhibition of CCO by NO may be involved in the physiological and/or pathological adjustment of respiration rate and its affinity for oxygen, which depends on different factors such as pH, proton motive force, supply of O2 to cells and tissues, as well as the generation of reactive nitrogen species [275].
It has been proposed that both structural and functional changes of the crucial interaction in the R and NIR domains with mitochondria could lead to the complex effects of PBM. However, additional to CCO, many other biomolecules inside mitochondria or other parts of cells (proteins, nucleic acids, and so on) are sensitive to light and could undergo important changes in their biochemistry. Comprehensive elucidation of the processes of light interaction with biological systems is yet to be completed for extensive application in photomedicine or industrial applications [276].
Another model would be related to photons interacting with water interfacial layers and participating in ATP regulation. The interplay between ATP upregulation and ROS downregulation in oxidatively stressed cells could be interpreted in a new way, according to a recently published paper. Based on the abundant evidence that different R-NIR wavelengths, delivered by lasers or LEDs, are effective in up-regulating mitochondrial ATP levels through the interaction of photons with mitochondrial bound water and interfacial water layers, the author of a recent study suggests the interplay between light-induced changes in the following important physical parameters in biostimulated cells: density (expansion in volume), viscosity (decrease) and the interfacial tension (presumed reduction, but harder to interpret as it is a function of the first two parameters) between bound water and the surrounding bulk water matrix [277].
Regarding the mechanisms of PBM, researchers are looking for new answers and proposing new models [278].
A synthesis of molecular and cellular mechanisms resulting from PBM action on living cells, in neurodegenerative diseases, as well as recent advances and challenges in AD have been presented in various reviews [241,257,259,279,280].
New insights into light-matter interactions are being advanced by interdisciplinary teams, modeling light-induced forces in systems with tens of thousands of atoms and arbitrary nanostructures. Ambrosetti et al. demonstrated the existence of both types of mechanical forces (attraction and repulsion) in the interaction of light with molecules, i.e., both attractive and repulsive optical van der Waals (vdW) forces can be induced by light, so generating new frameworks for the activation and the command of the living dynamic processes by light. Surprisingly, extensive analysis of the human formaldehyde dehydrogenase protein revealed both types of mechanical deformations (localized and delocalized) that depend mainly on photon energy. So, at low energies, photons activate only local deformations, while at higher energy, photons are able to initiate large-scale motions of this protein, i.e., the absorbed light could trigger productive energy transfer and selectively activate collective molecular vibrations.
The deep understanding of light-matter interactions in living complex molecular systems is one of the ultimate challenges of science today, especially for medicine, physics, chemistry, biotechnologies and so on. In fact, Ambrosetti et al. have opened the door for novel practical and precise treatments with photon-induced mechanical vibrations in large molecules, with a huge potential to investigate a multitude of new, previously inaccessible phenomena [281].
In current research for the management of brain diseases, noninvasive procedures (which do not require the introduction of instruments into the brain, i.e., do not invade the brain) that are simply directed to a specific area, without effort, without unwanted repercussions, are increasingly used for fast tracking, in decoding the relationship between brain regions, their particular activities and connections to patient behavior and final results. One of these noninvasive brain stimulations is PBM. With a non-heating action, the photons from PBM engage chromophores in tissues or cells and trigger physical and chemical photo effects at multiple living levels. Regardless of the extensive proofs for benefits in the complementary management of many medical conditions, a comprehensive insight into the fundamental, intrinsic healing processes is not yet available, especially in brain disorders such as Alzheimer’s disease, depression and so on.
Anti-beta-amyloid and anti-tau antibody therapy, vaccines, and other methods of reducing tau and/or amyloid have not lived up to clinical expectations after all the efforts of researchers in the pharmaceutical and nanobiotech fields.
An achievement with major prospects from the scientific community seems to be the idea of using laser devices or LEDs that emit radiation in R-NIR range. PBM can activate molecules and modulate intracellular pathways, pumps, and biochemical reactions, with favorable short- and long-term consequences, both local and systemic. Transcranial or intranasal PBM for brain stimulation with R and NIR light has focused attention on positive outcomes on cognitive functions, the repair of neurodegenerative processes, control of energy metabolism, and adjustment of chronic brain inflammatory processes in AD subjects. Publications of experimental and clinical studies on tPBM in AD and other neurodegenerative disorders and ischemic brain injuries have demonstrated that this modality of therapy stimulates metabolic processes in brain tissue, increases intracellular ATP production and revives neuronal mitochondrial activity; modulates local oxidative stress; favors angiogenesis, capillary and lymphatic revascularization; restores destroyed synapses and generates new synapses; revitalizes the growth of new neural cells and acts as a neuroprotector, thus improving cognitive function and memory [282,283,284,285,286,287,288,289,290].
The outstanding effects of PBM on the CNS, regardless of the administration method, can be considered as a “green” drug in Alzheimer’s disease and dementia. The monitoring of patients for long periods of time, up to 15 years, has objectively shown positive results in all cases. For example, after many years from the transcatheter intracerebral stimulation, conclusive images proved neurogenesis inside the cerebral areas with the complete restoration of the lobes and normal blood supply to the brain due to angiogenesis, aspects clearly highlighted by CT, MRI and Magnetic Resonance Angiography (MRA); from a clinical point of view, there was rapid recovery of memory, reduction of the severity of dementia and an improvement of cognitive functions throughout the monitoring period [310].
An illustrative diagram of the remarkable effects of PBM applied in various ways and with different technologies in AD, as evidenced by results from the recent human studies, is presented in Figure 1.
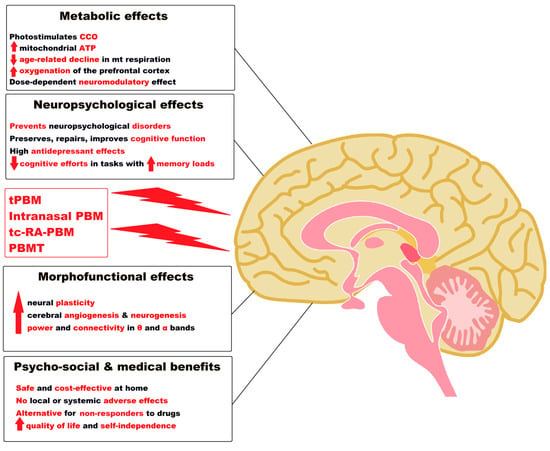
Figure 1. Notable effects of tPBM, intranasal PBM, tc-RA-PBM and PBMT as a “green” medicine in Alzheimer’s disease and dementia. Legend: ATP = Adenosine triphosphate; CCO = Cytochrome c oxidase; PBM= Photobiomodulation; mt = mitochondrial; tPBM = Transcranial PBM; tc-RA-PBM = Transcutaneous radial artery PBM; PBMT= Transcatheter Intracerebral PBM; α = alpha; θ = theta; ↑ = increase; ↓ = decrease. (Figure 1 was imagined and drawn by L.M.A. using Microsoft Paint 3D for Windows 10 and using completely free picture material (Open—Human Brain Png) from SeekPNG.com (accessed on 31 January 2023)).
Recent advances in tPBM and cutting-edge breakthroughs in AD treatment are opening previously unimagined perspectives.
Reversible modulation of the BBB by transcranial picosecond laser stimulation with molecular-targeted gold nanoparticles (AuNPs) has recently been demonstrated experimentally. Li et al. advanced a simple nanotechnology by applying picosecond-laser excitation of TJ-targeted AuNPs for BBB modulation. The researchers experimentally tested the possibility of targeting a TJ complex in vivo. They selected 50 nm spherical AuNPs with a surface plasmon resonance peak around 530 nm, which perfectly matched with the 532 nm picosecond laser used. As a result, the paracellular permeability of the BBB was reversible and gradually increased, thus allowing the systemic delivery of immunoglobulins and other particles to the brain without affecting the dynamics of blood circulation or damage to neurons, thereby opening innovative avenues for noninvasive therapeutic interventions in the CNS [312].
This latest experiment demonstrates how tPBM could be successfully used to cross the BBB together with the latest nanotechnologies, nanodrugs and DDSs in AD therapy.
A better understanding of the interaction and effects of PBM on the targeting of TJs and the molecular mechanisms involved in the modulation of BBB permeability can have a huge impact on future understanding, ranging from the pathophysiology of neurodegeneration to autoimmunity [313].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15030916
This entry is offline, you can click here to edit this entry!
