Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Layered lithium transition metal (TM) oxides LiTMO2 (TM = Ni, Co, Mn, Al, etc.) are the most promising cathode materials for lithium-ion batteries because of their high energy density, good rate capability and moderate cost. However, the safety issue arising from the intrinsic thermal instability of nickel-based cathode materials is still a critical challenge for further applications in electric vehicles and energy storage power stations.
- lithium-ion batteries
- layered lithium transition metal oxides
- safety
1. Composition
Layered lithium transition-metal oxides have a common chemical formula LiTMO2 (TM = Ni, Co, Mn, Al, etc.), in which TM representatives one or more of the transition metals. LiCoO2 is the most widely used cathode material in electronic devices because of its easy synthesis process, stable electrochemical performance, and highest compaction density [20,21]. However, the high cost and toxicity of cobalt make it unsuitable for electric vehicles and other large applications. LiNiO2 is another promising layered cathode material with a similar theoretical specific capacity of 274 mAh g−1 vs. LiCoO2 (275 mAh g−1), while the practical specific capacity of LiNiO2 (>220 mAh g−1) is much higher than that of LiCoO2 (~160 mAh g−1) below 4.3 V [22]. Unfortunately, it’s very difficult to synthesize a pure LiNiO2 since lithium vacancies, cation disordering, and spinel phase are easier to form in the sintering process [23].
As mentioned before, single transition metal oxide (such as LiCoO2 and LiNiO2) cannot meet the increasing requirements of the battery market due to various deficiencies. Ternary cathode materials, especially LiNixCoyMnzO2 (x + y + z = 1), have attracted great attention since 1999 when Liu first proposed a co-substitution of Ni by Co and Mn in LiNiO2 [24]. Nickel, Cobalt, and Manganese are randomly and uniformly distributed in the transition metal layer with performing their own functions, i.e., Ni for capacity, Co for layered characteristics, and Mn for stability. The synergistical effect of the three elements significantly improves the electrochemical performance of NCM and makes it a very successful commercial cathode material. In recent years, nickel fraction in commercial NCM materials has exceeded 60% in order to pursue higher energy density, which is called Ni-rich materials, such as NCA, NCM811 and LiNi0.89Co0.05Mn0.05Al0.01O2 (NCMA) [15,25,26]. Besides, the rapidly rising cost of cobalt also promotes the development of cobalt-free Ni-rich cathode materials (LiNi0.883Mn0.056Al0.061O2 for example).
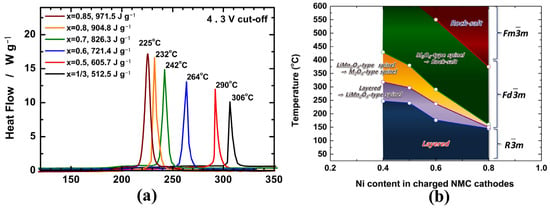
However, the biggest challenge for Ni-rich materials is how to balance the contradiction between high energy density and reduced thermal stability. It’s well known that the thermal stability of layered LiTMO2 materials strongly depends on their composition, especially the Ni content, i.e., the higher the Ni content, the lower the stability. As shown in Figure 1a, Noh [27] compared the thermal stability of delithiated NCM materials with different nickel contents and found that the exothermic peak temperature drastically decreased from 306 °C to 225 °C for Li0.37Ni1/3Co1/3Mn1/3O2 and Li0.21Ni0.85Co0.075Mn0.075O2 respectively, while the heat generation significantly increased from 512.5 J g−1 to 971.5 J g−1. These differences can be attributed to the varied valence with different Ni content. According to the results of in situ time-resolved X-ray diffraction and mass spectroscopy (TR-XRD/MS), Bak et al. schematically depicted the phase stability map of the charged NMC cathode materials during heating (Figure 1b), which also clearly showed that the thermal stability of the charged NCM samples decreases with increasing Ni content [28]. Further study shows that the average valence of Ni increases from +2 to +3 with the increasing Ni content, while Ni3+-TM bond strengths decrease and Ni3+-O bonds become increasingly covalent, leading to the intrinsic thermodynamical instability of the layered structure [29].
2. Structure and Morphology
All of the layered LiTMO2 compounds correspond to a hexagonal α-NaFeO2 structure with the space group. Specifically, oxygen atoms form a cubic close-packed lattice with rhombohedral distortion along the c-direction, resulting in layers formed by edge-sharing octahedra sites which are occupied by transition metals (3a) and lithium (3b) alternately [30]. But there is a common problem in layered nickel-based cathode materials named Li+/Ni2+ anti-site defects or cation disordering which mainly result from their similar ionic radii (Li+: 0.072 nm, Ni2+: 0.069 nm) [31]. These disordering occupations hinder the Li+ diffusion in the two-dimensional channels and greatly affect the electrochemical performance. Besides that [32], the distribution of TM ions inside the layer is no longer uniform, but the minority of TM ions form clusters at the atomic level when Ni content exceed 80%. The clusters make Mn4+ reduce to Mn3+, leading to aggravating Jahn–Teller distortions. These atomic-level structure deficiencies weaken the TM-TM interactions and TM-O bonds, thus significantly affecting the thermal stability of nickel-based materials.
In the delithiation process, the structure phase converts from hexagonal (H1) over monoclinic (M) to hexagonal (H2 and H3) phases, and the phase transitions reverse in the discharging process [33,34,35]. With the increase of lithium vacancy in the charging process, the distortion of TM-O octahedra gradually increases along the c-axis direction. As shown in Figure 2, when the amount of lithium removal exceeds the threshold (depending on the nickel content and charging cutoff voltage), parts of the region (H3 phase) cannot remain the layered characteristic anymore and convert to spinel Ni3O4 with active oxygen release [35]. The H2 to H3 phase transition usually occurs at a high delithiated state and results in oxygen evolution, which is also one of the main reasons for thermal runaway caused by overcharge [18]. With regard to the thermal decomposition under abusive conditions, the delithiated layered LiTMO2 materials go through phase transitions from layered structure to the disordered spinel structure, and finally to the rock-salt structure, accompanied by a large amount of oxygen gas release and CO2 formation [35,36]. Noted that the oxygen gas release occurs around 200 °C, at which the electrolyte has been decomposed or oxidized into combustible gas. Oxygen is the most common combustion promoter among the three elements of combustion. Therefore, batteries using layered LiTMO2 materials always catch fire or even explode when thermal runaway happens. Similarly, layered oxides like LiCoO2 and Mn-based Li-rich cathode materials also have the common problem of oxygen release. Samsung’s Galaxy Note 7 explosion strongly indicates that LiCoO2 battery with design defects may cause serious accidents. In contrast, the dilithiated LiFePO4 remains quite stable even above 350 °C and with little oxygen release, thus the weaknesses of the LiFePO4 battery are the separator and electrolyte rather than the cathode material.
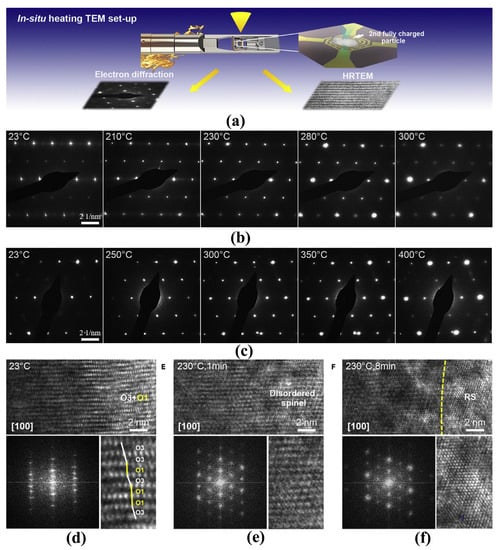
Figure 2. In situ structural evolution of delithiated LNO and doped LNO induced by oxygen loss. (a) Schematic illustration of the in-situ heating TEM experimental setup. (b,c) Time-resolved EDPs of LNO (b) and doped LNO (c) during in situ heating at different temperatures. (d–f) Time-resolved HRTEM images showing structural transformations of delithiated LNO from layered structure (O3) with O1 phases (d) to disordered spinel (e) and then to rock-salt structure (f) induced by oxygen loss during in situ heating. The lower panels show corresponding fast Fourier transforms and enlarged images of local regions. O3 and O1 phases are indicated by white and yellow lines, respectively [35].
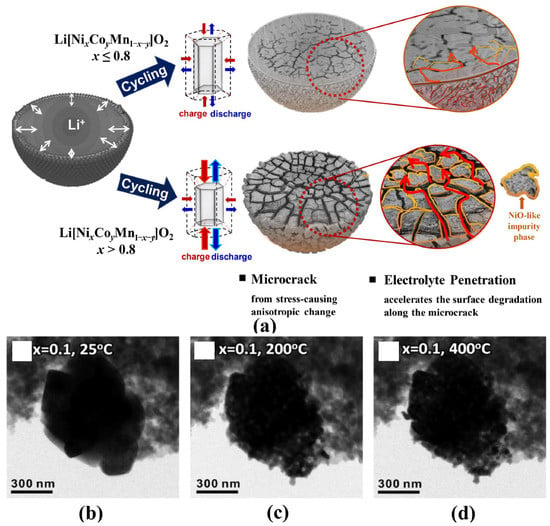
The particle microstructures and morphologies also play an important role in influencing the electrochemical properties and thermal stability of electrode materials. For commercial Ni-based materials, the spherical precursors are firstly agglomerated by nano-sized primary crystals with random orientations and a large number of defects in the coprecipitation process. Then the subsequent high-temperature treatment makes nanocrystals grow up and poly-crystallization, which means many crystal defects, such as grain boundaries, still exist inside the particle [23]. As shown in Figure 3a, the resulting unbalanced mechanical stress inside the particles during the charge-discharge process can cause microcracks that weaken the structural stability of particles and aggravate the undesired cathode-electrolyte side reactions [37,38,39,40]. In particular, the particles in at highly delithiated state may smash at high temperatures and accelerate side reactions and oxygen release (Figure 3b–d) [36].
3. Chemical Activity
The chemical activity of electrode materials is directly related to the gas production inside the battery. Because of the different out shell electron configurations for Ni and Co, the Ni2+/Ni3+/Ni4+ redox contributes to most of the capacity of Ni-based materials below 4.3 V (vs. Li/Li+) while Co3+/Co4+ redox occurs at around 4.3 V in the charging process [41]. However, the highly active Ni4+ on the particle surface can easily be reactive with liquid electrolytes accompanied by the gas generation (mainly including CO2 and CO) [42,43]. Secondly, the lithium residues (such as Li2CO3 and LiOH) introduced in the synthesis process or by-products of the slow reduction of Ni3+ to Ni2+ for Ni-rich materials when exposed to moisture, can also induce gas generation inside the battery [44,45]. Finally, transition metals (especially Mn) can slowly dissolve into the electrolyte, then migrate and incorporate into solid electrolyte interphase (SEI) film of the graphite-based negative electrode, resulting in the damage of SEI and continues alkane gas evolution [46,47]. Actually, the gas evolution related to the chemical activity of electrode materials is a common phenomenon of battery failure, which makes the battery bulge and leakage, and causes safety problems.
In summary, the thermal instability of layered LiTMO2 materials attributes to: (1) compositions, specifically, the higher the Ni content, the worse the thermal stability; (2) oxygen release from delithiated materials due to the phase transitions under overcharge or heated conditions; (3) gas evolution caused by the highly oxidative Ni4+, lithium residues, and transition metals dissolution. Nevertheless, the core reason is the oxygen release due to the astable layered structure of delithiated LiTMO2 materials under abusive conditions.
This entry is adapted from the peer-reviewed paper 10.3390/batteries9030156
This entry is offline, you can click here to edit this entry!
