Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Hexavalent chromium (Cr(VI)) is a toxic, mutagenic, teratogenic, and carcinogenic species. Its origin is in industrial activities. Therefore, its effective control is realized on a source basis. Although chemical methods proved effective in removing Cr(VI) from wastewaters, more economic solutions with a minimum sludge production have been sought. Among them, the use of electrochemical processes has emerged as a viable solution to the problem. Much research was conducted in this area.
- chemical reduction
- electrocoagulation
- hexavalent chromium
1. Introduction
Chromium is a transient metal that is relatively abundant in the earth’s crust. Oxidation states of chromium vary from +6 to +2. The most stable and, therefore, dominant forms are Cr(VI) and Cr(III) [1][2]. Cr(III) is common in the soil as a mineral, in ultramafic igneous and metamorphic rocks. Cr(III) is stable and has a strong affinity for particle surfaces. Cr(III) hydroxide is a sparingly soluble salt; therefore, the mobility of Cr(III) is limited. Cr(III) oxidation can only be realized in the soil by manganese oxides. Cr(III) is much less toxic than Cr(VI) and it is a vital element for life, being used for lipid, amino acid, and glycose digestion [2][3][4][5][6]. The majority of Cr(VI) in the environment is from anthropogenic sources. A wide spectrum of industrial activities involves the use of chromium, among them, are electroplating, leather tanning, mineral processing, metallurgical processes, mining, paints, pigment, and glass [2][3][6][7][8]. Cr(VI) is an oxidant, it is reactive and mobile since it is not adsorbed in most sediment, particularly after pH 7. Cr(VI) is highly toxic, mutagenic teratogenic, and carcinogenic. It causes many health effects on humans such as renal impairment, neutral cell injury, liver dysfunction, and stomach ulcers [7][9][10]. Chromium chemistry in soils is well studied. Cr(VI) has an affinity to water, is not sorbed in most sediments, and migrates above neutral pH. Organic matters, particularly those having sulfhydryl groups and humic and fulvic acids, reduce Cr(VI). It is also reduced by ferrous iron (Fe2+) and S2− [1][6][7][9][10].
The above information indicates the need for control and treatment of chromium–laden wastewaters at the source. There are various techniques used for the treatment of chromium in wastewaters. Among them are ion exchange, adsorption using a wide spectrum of natural and waste material, membrane processes such as electrodialysis, electro-ionization used separately or coupled with ion exchange, and biological treatment using bacteria, fungi, yeast, or algae, all suffering from the production of high amounts of contaminated waste material, sludge, or concentrate [4][7][11]. There are also less common methods such as photocatalysis and solvent extraction [12][13]. On the other hand, the chemical reduction of Cr(VI) followed by the chemical precipitation of Cr(III) has some drawbacks such as the requirement of higher doses of chemicals for diluted wastewaters and excess sludge production, which are still being used as a reliable method [14]. The alternative came from the idea of producing the reductant mainly Fe(II) in situ by electrolysis. Electrochemical methods have begun to be used or tested for several decades for chromium removal from wastewaters and proved to be efficient and flexible methods. Therefore, these methods are considered the most promising technology on which substantive research has been made.
Electrochemical methods named electroreduction and electrocoagulation, or shorthand notation as EC methods, combine chromium reduction and precipitation in a single step. The process is based on electrolysis. While solubilized anode material such as Fe2+ or Al3+ carries out chromium reduction, increasing pH through the conversion of hydrogen ions to hydrogen gas at the cathode precipitate Cr(III) as Cr(OH)3. The studies on the subject are reflected by scientific publications as well as review papers indicating increasing improvements and new applications in the field [15][16]. The material of electrodes in the EC process has been the subject of a number of studies. While metallic electrodes such as iron, aluminium, and copper were widely used, non-sacrificial electrodes made of carbon, gold, and conducting polymers were also tested for the performance [15][17][18]. Carbon electrodes as carbon felt and graphite felt have been utilized as cathode [16]. Electrode shape and area, reactor types such as the use of column reactor, and the use of direct and pulse current were tested as potential applications [3][19][20]. The optimization of basic operation parameters of EC such as initial pH, electrode distancing, current density, electrolyte concentration, and reaction time was the subject of a significant number of studies where iron electrodes were preferentially used [8][20][21][22][23][24]. Several studies dealt with the kinetics of the EC process as well as pH adjustment using real-time control [8][20][25]. There are a few studies that attempted to make an economic analysis of the EC process [20][26][27].
2. Operating Parameters
Initial pH and solution pH attained at the end of the electrocoagulation operation play a determining role on the effluent quality and sludge characteristics as well as chromium removal efficiency. Applied current or current density, initial Cr(VI) concentration, type and dose of electrolyte, and material of anode and cathode also have a great influence on the process performance as well as operation cost and sludge production. The effect of all mentioned operating parameters will be discussed in the following subsections for each electrode material.
2.1. Iron-Based Electrodes
2.1.1. Initial pH
Initial pH is a key parameter determining both the rate of the reduction of Cr(VI) to Cr(III) and total chromium ((Cr(VI) + Cr(III)) removal efficiency. The reduction reaction strictly depends on the initial pH as well as the solution pH reached at any time due to continuous hydroxyl ion production (Equation (2) [28]. Acidic pH conditions accelerate the production of Fe2+ acting as a reducing agent by electrolytic oxidation of the anode [25][28][29][30]. Extremely acidic initial pH conditions may lead to effluents of acidic character at which both Cr3+ and Fe3+ remain in the effluent without precipitating as seen in Equations (7) and (8) [23][25][31]. Such high solubilities were explained by the formation of their positively charged soluble hydroxo-complex species. For instance, CrOH2+ is the dominant chromium species pH from 3.8 to 6.4 [32], and FeOH2+ and Fe(OH)+2
are the dominant species at pH values below 5 [33]. In alkali pH values, negatively charged soluble hydroxo-complex species become dominant, resulting in high remaining Cr(III) and Fe3+ concentrations [31]. Moreover, high concentrations of hydroxyl ions trigger the formation of small and dense Fe(OH)3 flocs, which are difficult to be attached by the gas bubbles. This difficulty creates sludge floatation and a separation problem [31]. Considering that residual chromium and iron concentrations are extremely high at pH values below 3 and over 10, some researchers reported that neutral initial pH values such as 4–8 [34], 6–8 [31], and 6 [3][28] as optimum pH or pH range. Conversely, in some studies, optimum pH was determined as extremely acidic values such as 1 [19][35], 2 [30], 3 [36], 4 [37][38], 4.5 [39], and 4.9 [40]. These data suggest that solution pH at any time and at the end of electrocoagulation rather than the initial pH is of importance. If enough hydroxyl ions are produced according to Equation (2), the buffer capacity of the solution will be exceeded, and the initial acidic pH shifts towards an alkaline region where precipitate and/or co-precipitation of Cr(III)/Fe3+ are possible. Such an electrocoagulation application makes it possible to achieve the maximum chromium removal performance.
pH evolution during electrocoagulation is affected by some operation parameters. Xu et al. [25] investigated the effect of initial Cr(VI) concentration (10, 52, and 94 mg/L) and initial pH (pHi = 2, 3, and 4)) on the pH evolution and found a relation between p[Cr(VI)] and initial pH. In the case of p[Cr(VI)] = pHi, the final pH values were alkaline; when p[Cr(VI)] was smaller or greater than pHi, it reached alkali and acidic values, respectively. Based on the rate of Cr(VI) removal, four stages were described, namely (i) rapid removal, (ii) constant removal, (iii) decelerating removal, and (iv) complete removal [25]. When p[Cr(VI)] = pHi, in the first stage, the combined effect of reductions of chemical, cathodic, and zero-valent iron yielded a rapid Cr(VI) removal. In the second stage, ferrous iron dissolution was found to be a rate-limiting step with a constant Cr(VI) reduction rate. During the third stage, a reduction in DO in the reaction solution and a significant increase in the pH dramatically decelerated the Cr(VI) removal rate. In the last stage, the solution pH remained constant at around pH 7 during the simultaneous oxidation of ferrous iron and the formation of Fe(OH)3. When p[Cr(VI)] < pHi, the third stage occurred before the second stage as a result of a rapid Fe2+ oxidation with DO with an increasing pH to 7.0 at the end of the first stage. In the case of p[Cr(VI)] > pHi, the stages of II and III disappeared due to faster Cr(VI) reduction in acidic conditions, and high residual chromium concentration was measured at the end of electrocoagulation (final pH < 4.5) [25].
2.1.2. Initial Cr(VI) Concentration
High remaining Cr(VI) concentrations in effluents were also obtained at extremely high initial chromium concentrations. Scientific data indicated that elevating initial chromium concentrations resulted in an increase in remaining chromium concentrations for a constant current density [3][19][20][30][37][39][41]. In the study of Das and Nandi [37], the residual chromium concentration increased from 0.0046 to 44.205 mg/L with an increase in initial Cr(VI) concentration from 10 to 100 mg/L at the end of a 60 min electrocoagulation operation at 43.03 A/m2. Similarly, El-Taweel et al. [19] reported that an increase in the initial Cr(VI) concentration from 40 to 200 mg/L caused an increase in the residual Cr(VI) concentration from 0 to 107 mg/L. In all studies, this behaviour was explained by a constant amount of Fe2+ being released due to electro-dissolution of the anode at a constant current density which was insufficient to reduce all of Cr(VI) ions [3][19][30][37][41]. Therefore, a proportional increase either in current density or in electrode surface area are recommended for enough Fe2+ production to minimize residual Cr(VI) in the effluent [33]. Such unsatisfactory removal efficiencies were also attributed to the increase in the amount of Cr(VI) adsorbed onto the anode surface resulting in the anode passivation [34].
2.1.3. Current Density or Current
It is well known that an increase in current density improves the metal removal efficiency [19][22][30][34][36][37][38][42][43][44]. Current density determines the rate of electrochemical metal dosing to the water, the rate and size of electrolytic bubble production, and the flocs growth [38][45][46]. Bubble density increases while its size decreases with increasing current density, bringing about a greater upwards flux and a faster removal of pollutants. Current density directly affects the electrical energy requirement and electrode material consumption, and, consequently, the operating cost of the electrocoagulation process [28]. In the study of Lu et al. [28], increasing current density from 0.42 to 0.94 mA/cm2 enhanced the Cr(VI) removal efficiency from 74.35 to 100% while increasing the energy consumption from 0.24 to 0.94 kWh/m3. The complete chromium removal was attained at the highest energy consumption for an initial Cr(VI) concentration of 106 mg/L, at an initial pH of 6, and for an electrolysis time of 50 min. El-Taweel et al. [19] also reported that increasing the current from 0.2 to 1 A caused an increase in energy consumption from 0.002 to 0.009 kWh per gram of Cr(VI) removed, and in iron consumption from 0.02 to 0.37 g iron per gram of Cr(VI) removed for an initial Cr(VI) concentration of 140 mg/L, initial pH of 4.66, an NaCl concentration of 1 g/L, and an electrolysis time of 14 min. Similarly, an extension in electrolysis time led to an increase in energy consumption together with an improvement in Cr(VI) removal efficiency [20][47].
According to Heidmann and Calmano [48], two different Cr(VI) removal mechanisms were possible at high currents (1.0–3.0 A) and at low currents (0.05–1.0 A) for an initial Cr(VI) concentration of 20 mg/L and initial pH 5–6. Their data obtained at high currents indicated that Cr(VI) removal efficiency lines were nearly congruent, an increase in current did not accelerate the process, and the process seemed to be mainly controlled by the chromium concentration. Therefore, they concluded that Cr(VI) was directly reduced at the cathode. At low currents, an increasing current accelerated the chromium removal due to an increase in the production of both Fe2+ and OH−. Based on this improvement, the chemical reduction by Fe2+ dissolved from electrodes was assumed to be the dominant removal mechanism.
2.1.4. Supporting Electrolyte
The energy consumption of the electrochemical process is reduced by the addition of an electrolyte. The addition of a supporting electrolyte improves the Cr(VI) removal efficiency since the conductivity of the reaction solution increases and the anode passivation is prevented [23]. Scientific data on this subject confirmed higher or almost complete Cr(VI) removal efficiencies within a shorter electrolysis time attained by the addition of the proper amount of electrolyte. Salts of monovalent ions are reported as the best supporting electrolytes [49][50]. Among them, NaCl is the most common electrolyte used in electrocoagulation applications. In addition to NaCl [19][23][35][37][39][51], NaNO3 [35][37][51], Na2SO4 [19][35][37][51], and H2SO4 [23][43][52] were also tested as supporting electrolytes in these studies. Das and Nandi [53] found that NaCl was superior to NaNO3 and Na2SO4 in a dosage range of 0.33–0.83 g/L, for electrocoagulation performed at an initial pH of 4.0, a current density of 43.103 A/m2, and an initial Cr(VI) concentration of 40 mg/L for 60 min electrolysis time. At the maximum electrolyte dose (0.83 g/L), Cr(VI) removal efficiencies were determined as 99.99% for NaCl, 99.94% for NaNO3, and 99.18% for Na2SO4. They concluded that chloride ions improved the electro-dissolution of the anode by destroying the iron oxide film formed on the anode, while nitrate and sulphate anions interfered with its dissolution, resulting in a lower production of iron flocs in the solution. In their study, the lowest energy consumption was obtained by the addition of 0.83 g/L NaCl corresponding to the highest dose tested. Mouedhen et al. [42] also compared the effect of NaCl (0.5 g/L) and Na2SO4 (1 g/L) on solution pH change, residual chromium, and iron concentrations. Electrocoagulation operating conditions were the initial Cr(VI) concentration of 45 mg/L, current density of 1 A/dm2, and initial pH of 7.4 and 7.2 for Na2SO4 and NaCl, respectively. pH changes were practically the same for both supporting electrolytes, and the pH rose from an initial value to reach a steady state at 11. Almost complete chromium removal was achieved after 15 min and dissolved iron did not exceed 0.1 mg/L during the electrocoagulation operation for both electrolytes. These data, reported for Na2SO4 and NaNO3 as supporting electrolytes, did not conform to those of Aber et al. [35] and Lakshmipathiraj [51] whose studies indicated that extremely low (<20%) reduction efficiencies were obtained in the presence of both electrolytes.
H2SO4 was used together with NaCl to create an acidic medium [23]. The experimental study results proved that (i) chloride ions enhanced the anode dissolution by pitting corrosion and favoured the reduction of Cr(VI) to Cr(III) and the subsequent precipitation of Fe3+/Cr3+ hydroxides, particularly at low concentrations of H2SO4 (0.001 and 0.01 M); (ii) when higher pH values were attained in some operation conditions such as the higher NaCl concentration (1 g/L) and the lower H2SO4 concentration (0.001 M), the residual iron concentrations were low. Zewail and Yousef [39] investigated the effect of NaCl concentration (0.5; 1.0 and 2.0 g/L) on the removal efficiency of Cr(VI) and Cr(III) for an initial chromium concentration of 150 mg/L at 10.02 A/m2. Electrocoagulation was operated at an initial pH of 3.4 and 4.5 and for a time of 10 and 20 min for Cr(III) and Cr(VI), respectively. As expected, Cr(VI) removal efficiency improved with increasing NaCl concentration. However, Cr(III) removal efficiency decreased beyond 1 g/L NaCl concentration since the solubility of Cr(OH)3 increased with increasing ionic strength. A similar negative effect was also reported for NaCl concentrations greater than 0.7 g/L [23] and 1.5 g/L [19].
It should be noted that while an overdose of electrolytes induces overconsumption of the anode due to a pitting corrosion [45], an insufficient dose does not prevent passivation. Therefore, electrolyte addition needs to be optimized. Xu et al. [54] proposed an approach based on galvanostatic measurements to optimize chloride ions for depassivation and to determine an optimum chloride concentration for the pitting dissolution of iron electrodes during electrocoagulation. This optimum chloride concentration was described as a concentration preventing passivation during the evolution of pH and Cr(VI) reduction in the process.
2.2. Aluminium Electrodes
In the literature, only a few studies have focused on the Cr(VI) removal by electrocoagulation utilizing aluminium electrodes as both anode and cathode. The published data on operating parameters affecting the process performance will be summarized as follows.
The effect of the initial pH on the Cr(VI) removal efficiencies was investigated in some studies [55][56][57][58][59][60][61]. Rezaee et al. [59] explored the simultaneous removal of Cr(VI) and nitrate ions from an aqueous solution containing both pollutants. The results of electrocoagulation initialized at pH 4, 6, and 8 indicated that Cr(VI) removal efficiency decreased with increasing initial pH, and pH 4 yielded the highest Cr(VI) removal. Similar conclusions were also made by the other authors. Yu et al. [60] reported that the best Cr(VI) removal ratio was obtained at pH 5 as 99.92%. El-Ashtoukty et al. [58] achieved the highest Cr(VI) removal efficiencies within the pH range of 4.5–5.5. Golder et al. [61] performed electrocoagulation at initial pH values of 2, 4.87, 7, and 10, and obtained the highest Cr(VI) removal (42.3%) at pH 4.87. In the study of Kumar and Basu [55], the initial Cr(VI) concentration remained unchanged in the pH range of 4–5; Cr(VI) removal efficiency enhanced with increasing initial pH between 4 and 5 and decreased beyond pH 5.
The above-mentioned data have revealed that the highest Cr(VI) removal efficiencies can be obtained at an initial pH range of 4–5.5 depending on the operating conditions. In fact, the process performance is directly related to the interaction of Al3+ with water to form hydrolytic species at any time during the electrocoagulation [61][62]. The formation of these species can be represented as follows [62]:
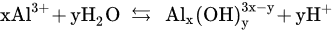
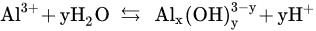
Al(H2O)3+6; Al(H2O)5(OH)2+;Al(H2O)4(OH)+2 pH < 4Al6(OH)3+15Al8(OH)4+20 pH 4–5
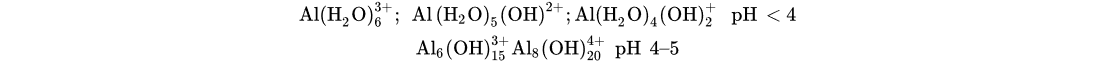
The number of positively charged surface sites decreases while pH shifts to pHzpc of Al(OH)3 (8.4). In addition to a decrease in active sites to be attached, dissolution of Al(OH)3 takes place to form Al(OH)−4, having poor coagulative properties at pH > 7 [55][60][61]. Within the pH range of 4–5, polymeric hydroxo complex species with 3 and 4 positive charges are formed; these species attract the negatively charged Cr(VI) anions (i.e., CrO−4) and capture them more efficiently than the less charged or negatively charged hydroxo complex species.
Similar to iron electrodes, when aluminium electrodes were used as both anode and cathode, Cr(VI) removal efficiency increased with increasing current density and extending electrolysis time [57][58][59][60][61]. In a study, increasing current density from 10 to 25 mA/cm2 resulted in an increase in Cr(VI) removal efficiency from 52.74 to 91.48 % for 60 min, and an extension in electrolysis time from 5 to 60 min brought about a remarkable increase in Cr(VI) removal efficiency from 6.52 to 91.48% [60]. In another study, the highest Cr(VI) removal efficiency (99.99%) was achieved at the highest current density (8.6 A) [57]. This improvement with both current density and electrolysis time was ascribed to (i) increasing the amount of the dissolved Al3+ at the anode according to Faraday’s law and (ii) enhancement in mixing conditions with increasing the discharge rate of H2 bubbles at the cathode [55][58]. On the other hand, an increase in both current density and electrolysis time caused higher sludge production and specific electrical energy consumption (SEEC) [55][58][59][61][65].
As expected, increased initial Cr(VI) concentration had a negative effect on the Cr(VI) removal efficiency. Cr(VI) removal efficiency drastically reduced at a constant current density and at high initial Cr(VI) concentrations [55][56][60]. This was attributed to the limited in-situ generation of the coagulant which was quickly exhausted from the system and led to a higher residual Cr(VI) in the effluent [37][55][56].
A direct comparison of the systems using iron and aluminium electrodes can hardly be made since there is no work specifically targeted to this purpose. It is even difficult to find experiments conducted at similar conditions, and almost all experiments are bench-scale. Bench-scale experiments do not clearly demonstrate the operation problems nor are they aimed to. While both electrodes can work with high efficiencies, a more precise control of the reduction process seems to be required for aluminium electrodes. The produced aluminium hydroxide flocs serve only to adsorb and entrap chromium. If Cr(VI) cannot be effectively reduced, this may only mean that the Cr(VI) problem is transferred from the water to sludge phase. Aluminium hydroxide sludge is voluminous, and its separation may not be easy. The economic aspects of the systems need to account for sludge handling and disposal. This point will be also emphasized in the applications for industrial effluents section.
3. Optimum Operating Conditions and Interactions of Process Variables
Response surface methodology (RSM) is a combination of mathematical and statistical techniques used for developing, improving, and optimizing the processes and used to evaluate the relative significance of several affecting factors even in the presence of complex interactions [66]. This methodology has been employed to evaluate the effects of various operating conditions on the Cr(VI) removal by electrocoagulation with sacrificed electrodes. The process has been optimized using RSM in a combination with the Box-Behnken design (BBD) [24][67][68], Taguchi method [69], and central composite design (CCD) [8][20][21][22][27][52][65] for several process variables such as current density, initial Cr(VI) concentration, initial pH, electrolyte concentration, electrical energy consumption, settled sludge volume, operation cost, reaction temperature, or H2SO4 dose.
Khan et al. [20] employed a four-factor CCD together with RSM to evaluate the effects of the process parameters on response variables: Cr(VI) removal efficiency and energy consumed per gram removal of chromium for electrocoagulation with a mild steel rod anode and a hollow cylindrical iron mesh cathode. In their study, the optimum conditions were determined as a pH of 3.0, an applied current of 1.48 A, an initial Cr(VI) concentration of 49.96 mg/L, and an electrolysis time of 21.47 min for complete Cr (VI) removal from K2Cr2O7 aqueous solution. For these conditions, energy consumption was found as 12.97 W hour per gram removal of Cr (VI). The average operational cost per gram removal was calculated as 0.116 and 0.084 Indian rupees for electrode material and electricity, respectively. In a study, electrocoagulation with an iron electrode was optimized using CCD with RSM to maximize Cr(VI) removal from forward osmosis reject water and to minimize operating cost, electrical energy consumption, and settled sludge volume [22]. Under optimized conditions (an electrolysis time of 59.7 min and a current of 1.24 A (J = 6.32 mA/cm2)), operating costs of 0.014 USD/m3, the electrical energy consumption of 0.005 kWh/m3, and settled sludge volume of 445 mL/L were obtained for 90.0% chromium removal. Another RSM with CCD modelling study was employed to evaluate the effects and interactions of process variables: applied electric current, electrolyte concentration, and application time on the Cr(VI) removal from a hard chromium coating process effluent (Cr(VI): 1470 mg/L) [21]. For the electrocoagulation with stainless steel, the optimum conditions for complete (100%) Cr(VI) removal were established as 7.4 A applied electric current, 33.6 mM electrolyte (NaCl) concentration, and 70 min application time. Gilhotra et al. [27] applied RSM-based CCD to optimize independent process variables viz. pH, current density, and treatment time. In their optimization study, Cr(VI) removal efficiency and energy were selected as response variables. Under optimized process conditions (a pH of 5, a current density of 68 A/m2, and a treatment time of 17 min) 97.5% chromium removal efficiency was achieved by electrocoagulation with SS. Bhatti et al. [65] used RSM with CCD to achieve an energy-efficient removal of Cr(VI) from electrocoagulation with aluminium electrodes. Their data, obtained from predictive models using ANOVA and multiple response optimization, indicated that optimal Cr(VI) removal efficiency (50%) was attained at 11 V and 18.6 min treatment time with a consumption of 15.46 KWh/m3 energy.
Patel and Parikh [8] made an optimization using RSM in combination with CDD to investigate the effect of initial Cr(VI) concentration, pH, electrode distance, current density, and supporting electrolyte (NaCl) concentration on Cr(VI) removal from K2Cr2O7 aqueous solution. For the electrocoagulation using copper electrodes, current density of 41.32 A/m2, an electrode distance of 1.4 cm, an initial pH of 5.65, an electrolysis time of 40 min, and an initial conductivity of 0.21 ms were determined as optimum operating conditions to achieve 93.33% chromium removal efficiency. RSM with CCD was also employed to evaluate the effect of the H2SO4 dosage, current intensity, reaction time, and reaction temperature on the chromium removal from K2Cr2O7 aqueous solution by electrocoagulation with a steel electrode [52]. The optimization data indicated that the effect of single factor on Cr(VI) removal efficiency followed the order H2SO4 dosage > reaction time > reaction temperature > current intensity. Kumar and Basu [55] optimized the removal of Cr(VI) from K2Cr2O7 aqueous solution (with 1 g/L NaCl) by electrocoagulation with vertically rotating cylindrical aluminium electrodes using RSM with CCD. Considering that excess input energy would bring about a marginal improvement in the Cr(VI) removal efficiency, the process that was optimized for maximum Cr(VI) removal corresponded to minimum energy input. Under the optimum conditions (Cr(VI) 34.99 mg/L, 2.189 A, pHo 4.5, and a rotational speed of 72.46 rpm) the Cr(VI) removal efficiency, SEEC, and operating cost were found as 89.808%, 0.14 kW·h/g Cr(VI) removed, and $0.728/m3, respectively.
BBD is another optimization technique used to evaluate the effects and interactions of process variables. Shen et al. [24] employed this technique for Cr(VI) removal from K2Cr2O7 aqueous solution by electrocoagulation with an iron electrode. Under the optimum conditions (pHo: 5.48; electrode distance: 2.51 cm, J: 87.55 mA/cm2, and t: 25.6 min for Cr(VI) of 50 mg/L), 99.34% Cr(VI) removal efficiency was achieved. Yadav and Khandegar [67] also applied BBD to investigate the effects of voltage (5, 10 and 15 V), electrolysis time (20, 30, and 40 min) and pH (3, 5, and 7) and to optimize these parameters for Cr(VI) removal from K2Cr2O7 aqueous solution by electrocoagulation using either an iron or an aluminium electrode. Their predicted values of responses obtained using the model fitted well with their experimental data. Singh et al. [68] also employed RSM with BBD for Cr(VI) removal from K2Cr2O7 aqueous solution by electrocoagulation with an aluminium anode and an aluminium or graphite cathode. Their results indicated that the usage of a graphite cathode (0.194 kWh/m3) instead of an aluminium cathode (0.425 kWh/m3) significantly reduced the power consumption under the optimized conditions (2.38 mA/cm2, pH of 7.29, 23 min, and electrode distance 3.5 cm) for maximum Cr(VI) removal (74.07% for Al-Al and 70.83% for Al-graphite).
Kumar and Basu [69] adopted the Taguchi method (L9 orthogonal array (OA) with 3 factors in 3 levels) for the optimization of current density, initial Cr(VI) concentration, and initial pH to maximize Cr(VI) removal by electrocoagulation with aluminium electrodes. They determined the optimum working conditions as an initial Cr(VI) concentration of 15 mg/L; a current density of 49.3 mA/cm2; and an initial pH of 5. The experimental Cr(VI) removal efficiency (96.6%) was in excellent agreement with that of the predicted efficiency (98.4%). The operating cost corresponding to a maximum Cr(VI) removal efficiency at the optimum conditions was calculated as $10.77/m3.
Artificial neural network (ANN) was another technique used for the modelling of the experimental study results obtained from an electrocoagulation application performed with an iron anode and a steel cathode [35]. This model was developed using a 3-layer feed-forward backpropagation network with 4, 10, and 1 neurons in the first, second, and third layers, respectively. Since a comparison model results with experimental data gave a high correlation coefficient (R2 = 0.976), the usage of the model was proposed for the prediction of the residual Cr(VI) concentration in the effluent.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28052411
References
- Gorny, J.; Billon, G.; Noiriel, C.; Dumoulin, D.; Lesven, L.; Madé, B. Chromium behavior in aquatic environments: A review. Environ. Rev. 2016, 24, 503–516.
- Tumolo, M.; Ancona, V.; De Paola, D.; Losacco, D.; Campanale, C.; Massarelli, C.; Uricchio, V.F. Chromium Pollution in European Water, Sources, Health Risk, and Remediation Strategies: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 5438.
- Zhou, R.; Liu, F.; Wei, N.; Yang, C.; Yang, J.; Wu, Y.; Li, Y.; Xu, K.; Chen, X.; Zhang, C. Comparison of Cr (VI) removal by direct and pulse current electrocoagulation: Implications for energy consumption optimization, sludge reduction and floc magnetism. J. Water Process Eng. 2020, 37, 101387.
- Pavithra, K.G.; Jaikumar, V.; Kumar, P.S.; SundarRajan, P. A review on cleaner strategies for chromium industrial wastewater: Present research and future perspective. J. Clean. Prod. 2019, 228, 580–593.
- Sedlak, D.L.; Chan, P.G. Reduction of hexavalent chromium by ferrous iron. Geochim. Cosmochim. Acta 1997, 61, 2185–2192.
- Zhou, B.; Huang, D.; Wu, J.; Zhu, Q.; Zhu, H. Horizontal and Vertical Distributions of Chromium in a Chromate Production District of South Central China. Int. J. Environ. Res. Public Health 2018, 15, 571.
- Kerur, S.; Bandekar, S.; Hanagadakar, M.S.; Nandi, S.S.; Ratnamala, G.; Hegde, P.G. Removal of hexavalent Chromium-Industry treated water and Wastewater: A review. Mater. Today Proc. 2021, 42, 1112–1121.
- Patel, S.R.; Parikh, S.P. Statistical optimizing of electrocoagulation process for the removal of Cr(VI) using response surface methodology and kinetic study. Arab. J. Chem. 2020, 13, 7032–7044.
- Palmer, C.D.; Puls, R.W. Natural Attenuation of Hexavalent Chromium in Groundwater and Soils: Ground water issue. Environ. Prot. Agency 1994.
- Shanker, A.; Venkateswarlu, B. Chromium: Environmental Pollution, Health Effects and Mode of Action. Encycl. Environ. Health 2011, 650–659.
- Evliyaogullari, N.E.; Oden, M.K.; Kucukcongar, S. The removal of chromium from aqueous solutions using an industrial waste material. Int. J. Ecosyst. Ecol. Sci.-Ijees 2017, 7, 671–676.
- Singh, P.; Itankar, N.; Patil, Y. Biomanagement of hexavalent chromium: Current trends and promising perspectives. J. Environ. Manag. 2020, 279, 111547.
- Barrera-Díaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012, 223–224, 1–12.
- Tünay, O.; Kabdaşlı, I.; Hung, Y.-T. Treatment of metal finishing wastes. In Handbook of Industrial Hazardous Waste Treatment; Wang, L.K., Hung, Y.-T., Lo, H.H., Yapijakis, C., Eds.; Marcel Dekker Inc.: Boca Raton, FL, USA, 2007; pp. 289–359.
- Jin, W.; Du, H.; Zheng, S.; Zhang, Y. Electrochemical processes for the environmental remediation of toxic Cr(VI): A review. Electrochim. Acta 2016, 191, 1044–1055.
- Zhao, Y.X.; Kang, D.J.; Chen, Z.; Zhan, J.J.; Wu, X.Q. Removal of Chromium Using Electrochemical Approaches: A Review. Int. J. Electrochem. Sci. 2018, 13, 1250–1259.
- Marghaki, N.S.; Jonoush, Z.A.; Rezaee, A. Improving the performance of Cr (VI) removal by electrochemical process using microbial cellulose/magnetic nanoparticles electrode. J. Clean. Prod. 2020, 277, 123195.
- Shaker, O.A.; Matta, M.E.; Safwat, S.M. Nickel and chromium removal by electrocoagulation using copper electrodes. DESALINATION Water Treat. 2021, 213, 371–380.
- El-Taweel, Y.A.; Nassef, E.M.; Elkheriany, I.; Sayed, D. Removal of Cr(VI) ions from waste water by electrocoagulation using iron electrode. Egypt. J. Pet. 2015, 24, 183–192.
- Khan, S.U.; Islam, D.T.; Farooqi, I.H.; Ayub, S.; Basheer, F. Hexavalent chromium removal in an electrocoagulation column reactor: Process optimization using CCD, adsorption kinetics and pH modulated sludge formation. Process. Saf. Environ. Prot. 2018, 122, 118–130.
- Ölmez, T. The optimization of Cr(VI) reduction and removal by electrocoagulation using response surface methodology. J. Hazard. Mater. 2009, 162, 1371–1378.
- Mousazadeh, M.; Naghdali, Z.; Kabdaşlı, I.; Sandoval, M.A.; Titchou, F.E.; Malekdar, F.; Nasr, M.; Martínez-Huitle, C.A.; Lichtfouse, E.; Emamjomeh, M.M. Reclamation of forward osmosis reject water containing hexavalent chromium via coupled electrochemical-physical processes. Environ. Technol. 2022, 1–14.
- Arroyo, M.; Pérez-Herranz, V.; Montañés, M.; Garcia-Anton, J.; Guiñón, J. Effect of pH and chloride concentration on the removal of hexavalent chromium in a batch electrocoagulation reactor. J. Hazard. Mater. 2009, 169, 1127–1133.
- Shen, Y.; Wang, Y.; Shi, J.; Tan, D.; Jing, X.; Xu, Q. Modeling and optimization of the electric flocculation of wastewater containing Cr6+ using response surface methodology. Sep. Sci. Technol. 2017, 19, 749–762.
- Xu, H.-Y.; Yang, Z.-H.; Zeng, G.-M.; Luo, Y.-L.; Huang, J.; Wang, L.-K.; Song, P.-P.; Mo, X. Investigation of pH evolution with Cr(VI) removal in electrocoagulation process: Proposing a real-time control strategy. Chem. Eng. J. 2013, 239, 132–140.
- Krystynik, P.; Masin, P.; Krusinova, Z.; Kluson, P. Application of electrocoagulation for removal of toxic metals from industrial effluents. Int. J. Environ. Sci. Technol. 2018, 16, 4167–4172.
- Gilhotra, V.; Yadav, R.; Sugha, A.; Das, L.; Vashisht, A.; Bhatti, R.; Bhatti, M.S. Electrochemical treatment of high strength chrome bathwater: A comparative study for best-operating conditions. Clean. Eng. Technol. 2021, 2, 100093.
- Lu, J.; Wang, Z.-R.; Liu, Y.-L.; Tang, Q. Removal of Cr ions from aqueous solution using batch electrocoagulation: Cr removal mechanism and utilization rate of in situ generated metal ions. Process. Saf. Environ. Prot. 2016, 104, 436–443.
- Barrera-Díaz, C.; Lugo-Lugo, V.; Roa-Morales, G.; Natividad, R.; Martínez-Delgadillo, S. Enhancing the electrochemical Cr(VI) reduction in aqueous solution. J. Hazard. Mater. 2011, 185, 1362–1368.
- Thella, K.; Verma, B.; Srivastava, V.C.; Srivastava, K.K. Electrocoagulation study for the removal of arsenic and chromium from aqueous solution. J. Environ. Sci. Health Part A 2008, 43, 554–562.
- Gao, P.; Chen, X.; Shen, F.; Chen, G. Removal of chromium(VI) from wastewater by combined electrocoagulation–electroflotation without a filter. Sep. Purif. Technol. 2005, 43, 117–123.
- Rai, D.; Sass, B.M.; Moore, D.A. Chromium(III) hydrolysis constants and solubility of chromium(III) hydroxide. Inorg. Chem. 1987, 26, 345–349.
- Duan, J.; Gregory, J. Coagulation by hydrolysing metal salts. Adv. Colloid Interface Sci. 2003, 100–102, 475–502.
- Dermentzis, K.; Christoforidis, A.; Valsamidou, E.; Lazaridou, A.; Kokkinos, N. Removal of hexavalent chromium from electroplating wastewater by electrocoagulation with iron electrodes. Glob. Nest J. 2011, 13, 412–418.
- Aber, S.; Amani-Ghadim, A.; Mirzajani, V. Removal of Cr(VI) from polluted solutions by electrocoagulation: Modeling of experimental results using artificial neural network. J. Hazard. Mater. 2009, 171, 484–490.
- Cheballah, K.; Sahmoune, A.; Messaoudi, K.; Drouiche, N.; Lounici, H. Simultaneous removal of hexavalent chromium and COD from industrial wastewater by bipolar electrocoagulation. Chem. Eng. Process. Process. Intensif. 2015, 96, 94–99.
- Das, D.; Nandi, B.K. Removal of Hexavalent Chromium from Wastewater by Electrocoagulation (EC): Parametric Evaluation, Kinetic Study and Operating Cost. Trans. Indian Inst. Met. 2020, 73, 2053–2060.
- Verma, S.K.; Khandegar, V.; Saroha, A.K. Removal of Chromium from Electroplating Industry Effluent Using Electrocoagulation. J. Hazard. Toxic Radioact. Waste 2013, 17, 146–152.
- Zewail, T.; Yousef, N. Chromium ions (Cr6+ & Cr3+) removal from synthetic wastewater by electrocoagulation using vertical expanded Fe anode. J. Electroanal. Chem. 2014, 735, 123–128.
- Golder, A.K.; Chanda, A.K.; Samanta, A.N.; Ray, S. Removal of hexavalent chromium by electrochemical reduction–precipitation: Investigation of process performance and reaction stoichiometry. Sep. Purif. Technol. 2011, 76, 345–350.
- Khalaf, A.M.; Mubarak, A.A.; Nosier, S.A. Removal of Cr(VI) by Electrocoagulation Using Vertical and Horizontal Rough Cylinder Anodes. Int. J. Electrochem. Sci. 2016, 11, 1601–1610.
- Mouedhen, G.; Feki, M.; De Petris-Wery, M.; Ayedi, H. Electrochemical removal of Cr(VI) from aqueous media using iron and aluminum as electrode materials: Towards a better understanding of the involved phenomena. J. Hazard. Mater. 2009, 168, 983–991.
- Peng, H.; Leng, Y.; Guo, J. Electrochemical Removal of Chromium (VI) from Wastewater. Appl. Sci. 2019, 9, 1156.
- Kabdaşlı, I.; Arslan, T.; Arslan-Alaton, I.; Ölmez-Hancı, T.; Tünay, O. Organic matter and heavy metal removals from complexed metal plating effluent by the combined electrocoagulation/Fenton process. Water Sci. Technol. 2010, 61, 2617–2624.
- Kabdaşlı, I.; Arslan-Alaton, I.; Ölmez-Hancı, T.; Tünay, O. Electrocoagulation applications for industrial wastewaters: A critical review. Environ. Technol. Rev. 2012, 1, 2–45.
- Kabdaşlı, I.; Arslan, T.; Olmez-Hanci, T.; Arslan-Alaton, I.; Tünay, O. Complexing agent and heavy metal removals from metal plating effluent by electrocoagulation with stainless steel electrodes. J. Hazard. Mater. 2009, 165, 838–845.
- Lu, D.; Huang, Z.; Luo, J.; Zhang, X.; Sha, S. Primary concentration—The critical step in implementing the wastewater based epidemiology for the COVID-19 pandemic: A mini-review. Sci. Total. Environ. 2020, 747, 141245.
- Heidmann, I.; Calmano, W. Removal of Cr(VI) from model wastewaters by electrocoagulation with Fe electrodes. Sep. Purif. Technol. 2008, 61, 15–21.
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41.
- Arslan-Alaton, I.; Kabdaşlı, I.; Vardar, B.; Tünay, O. Electrocoagulation of simulated reactive dyebath effluent with aluminum and stainless steel electrodes. J. Hazard. Mater. 2009, 164, 1586–1594.
- Lakshmipathiraj, P.; Raju, G.B.; Basariya, M.R.; Parvathy, S.; Prabhakar, S. Removal of Cr (VI) by electrochemical reduction. Sep. Purif. Technol. 2008, 60, 96–102.
- Peng, H.; Leng, Y.; Cheng, Q.; Shang, Q.; Shu, J.; Guo, J. Efficient Removal of Hexavalent Chromium from Wastewater with Electro-Reduction. Processes 2019, 7, 41.
- Cao, V.; Alyoussef, G.; Gatcha-Bandjun, N.; Gwenzi, W.; Noubactep, C. Characterizing the impact of MnO2 addition on the efficiency of Fe0/H2O systems. Sci. Rep. 2021, 11, 1–12.
- Xu, H.-Y.; Yang, Z.-H.; Luo, Y.-L.; Zeng, G.-M.; Huang, J.; Wang, L.-K.; Song, P.-P.; Yang, X. A novel approach to sustain Fe 0 -electrocoagulation for Cr(VI) removal by optimizing chloride ions. Sep. Purif. Technol. 2015, 156, 200–206.
- Kumar, A.; Basu, D. Optimization of Removal of Cr(VI) from Wastewater by Electrocoagulation Process Using Response Surface Methodology. J. Hazardous, Toxic, Radioact. Waste 2023, 27, 04022038.
- Sarkhosh, M.; Atafar, Z.; Ahmadi, E.; Nazari, S.; Fakhri, Y.; Rezaei, S.; Mohseni, S.M.; Saghi, M.H.; Torkashvand, M. Treatment of Electroplating Cr(VI) for Reduction Cr(VI) by Electrocoagulation in Continuous Operation. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 615–625.
- Soukaina, N.M.; Chaimaa, M.; Kabriti, M.; Abdelmotalib, N.; Naamane, A.; Mohamed, C.; Nadia, I. Treatment of Surface Treatment Effluents by Electrocoagulation Process Using Aluminium Electrodes. J. Ecol. Eng. 2022, 23, 91–99.
- El-Ashtoukhy, E.-S.Z.; Abdel-Aziz, M.H.; Sedahmed, G.H. Simultaneous Removal of Greases and Hexavalent Chromium from Electroplating and Chromate Conversion Coating Waste Solution by Electrocoagulation. Water Air Soil Pollut. 2018, 229, 325.
- Rezaee, A.; Hossini, H.; Masoumbeigi, H.; Soltani, R.D.C. Simultaneous Removal of Hexavalent Chromium and Nitrate from Wastewater using Electrocoagulation Method. Int. J. Environ. Sci. Dev. 2011, 2, 294–298.
- Yu, Y.; Zhong, Y.; Sun, W.; Xie, J.; Wang, M.; Guo, Z. A novel electrocoagulation process with centrifugal electrodes for wastewater treatment: Electrochemical behavior of anode and kinetics of heavy metal removal. Chemosphere 2023, 310, 136862.
- Golder, A.; Chanda, A.K.; Samanta, A.N.; Ray, S. Removal of Cr(VI) from Aqueous Solution: Electrocoagulation vs Chemical Coagulation. Sep. Sci. Technol. 2007, 42, 2177–2193.
- Benezeth, P.; Palmer, D.A.; Wesolowski, D.J. The aqueous chemistry of aluminum. A new approach to high-temperature solubility measurements. Geothermics 1997, 26, 465–481.
- Scully, J.C. The Fundamentals of Corrosion, 2nd ed.; Pergamon Press: New York, NY, USA, 1975.
- Rebhun, M.; Lurie, M. Control of Organic Matter by Coagulation and Floc Separation. Water Sci. Technol. 1993, 27, 1–20.
- Bhatti, M.S.; Reddy, A.S.; Kalia, R.K.; Thukral, A.K. Modeling and optimization of voltage and treatment time for electrocoagulation removal of hexavalent chromium. Desalination 2011, 269, 157–162.
- Kabdaşlı, I.; Olmez-Hanci, T.; Tünay, O.; Gülhan, D.; Ecer, C. Application of response surface methodology for dimethyl phthalate treatment via H2O2/UV-C process. Desalination Water Treat. 2016, 57, 26165–26173.
- Yadav, A.; Khandegar, V. Dataset on statistical reduction of highly water-soluble Cr (VI) into Cr (III) using RSM. Data Brief 2018, 22, 1074–1080.
- Singh, H.; Sonal, S.; Mishra, B.K. Hexavalent chromium removal by monopolar electrodes based electrocoagulation system: Optimization through Box–Behnken design. J. Water Supply Res. Technol. 2017, 67, 147–161.
- Kumar, A.; Basu, D. Economic and performance evaluation of electrocoagulation unit for the treatment of hexavalent chromium using Taguchi method. Int. J. Environ. Sci. Technol. 2022, 1–10.
This entry is offline, you can click here to edit this entry!
