Equine collagen is referred to type I collagen extracted from horse tissues that, in the last two decades, aroused great scientific and industrial interest in the field of life-science and bioengineering as alternative to bovine collagen for the manufacture of implantable medical devices. Commonly used sources of collagen are represented by bovine and swine, but their limited applications because of the zoonosis transmission risks, the immune response and the religious constrains lead to the identification of other collagen sources. In this circumstance, type I collagen isolated from horse tendon recently gained interest as an attractive alternative, so that, although bovine- and porcine-derived collagens still remain the most common ones, more and more companies started to bring to market several of equine tendon collagen-based products. Its favorable structural properties, its well-known bioactivity, its freedom from zoonosis transmission risks and the ability to not trigger immune reactions make equine collagen particularly appealing in medicine, cosmetics and pharmaceuticals.
- type I collagen
- equine
- biomaterials
- tissue engineering and regenerative medicine
- medical device
1. Introduction
Collagen is the body’s cement that keeps everything in place [1]. With its 28-members family it is the most important protein of vertebrates’ connective tissues that accounts for the 30% of the total body protein content [2]. Among collagens, fibril-forming type I subspecies is the most abundant since it accounts for the 70% of the whole family [3,4,5]. The structure of type I collagen, distributed at the level of all tissues in the organism, is known from 1938 [6,7,8]. It consists in three right-handed polyproline-II helices of about 1000 amino acids (called α strand) that by mean of interchain hydrogen bonds are held together in a left-handed triple helix [9]. As it is already known, each α strand is characterized by the repetition of the Gly-X-Y triplet, where the “X” and “Y” positions are usually occupied by proline and hydroxyproline [10]. In this neat sequence, glycine plays a key role in the three α strand packing [11], while proline and hydroxyproline cover a fundamental role in stabilizing the triple helical structure by preventing helices free rotation, thanks to the presence of pyrrolidine rings which reduce the degree of freedom of the polypeptide chain [12,13]. Moreover, the post-translational hydroxylation of the 11%–14% of proline residues by the enzyme proxyl-4-hydroxylase (PH4) (EC 1.14.11.2) is a process that gives to collagen a unique characteristic attributable only to type I collagen, important both for its recognition and quantification [14].
Collagen covers a crucial structural role for the maintenance of tissues’ architecture and shape and it dictates specialized regulatory functions, especially during development and repair [15,16,17]. Thus, collagen is not only responsible of tensile strength and elasticity [16] but also of the integrity preservation of skin, connective tissues, tendons and bones by mediating a fundamental inter- and intracellular signaling. The repetition of the Gly-X-Y sequence is indeed fundamental for collagen to properly perform its function and, to this, it remains almost unchanged during the course of evolution of the animal kingdom [18]. To this, mutations in the collagen COL1A1 gene, have been associated to more than 400 human disease [19].
Because of the important role in cell signaling, the collagen triple helical molecule is characterized by the presence of a high number of integrin binding sites (i.e., the “GxOGER” sequence, where “G” is glycine, “O” is hydroxyproline, “E” is glutamate, “R” is arginine and “x” is a hydrophobic amino acid) fundamental for cells adhesion and interaction [20,21]. Therefore, non-structural functions of collagen are of great relevance for cell communication, proliferation, differentiation besides for healing processes [4,22,23].
The prevalence of collagen in human tissues and the important roles covered in the extracellular matrix (ECM), make it a natural choice for its employment as raw material [10]. Being the main component of the ECM, collagen is intrinsically biodegradable, biocompatible and bioactive [15,24,25,26,27]. Its abundance and ubiquity make it not perceived as exogenous constituent of the body [10]. As befits the primary structural protein in the body, collagen is naturally resistant to proteolysis but susceptible to attack of matrix metalloproteinase (MMPs) (i.e., MMP-1, MMP-2, MMP- 8, MMP-13 and MMP-14) [25,28,29]. The collagen fragments resulting from the action of collagenases, are further degraded by gelatinases and non-specific proteases. The presence of an accurate and complex degradation system for the endogenous collagen makes the exogenous collagen highly biodegradable [25]. Moreover, collagen and its degradation products could also promote the tissue’ structure and function restoration [30]. Lastly, collagen can be easily processed to fabricate several kinds of substrates like sponges, hydrogels, tubes, powders and films according to the final application [31].
All these attractive and advantageous features of type I collagen make it one of the most widely used biomaterial in health-related sectors, including medical care, pharmaceutics and cosmetics [32,33,34,35,36,37]. More specifically known is its employment as biomaterial for the manufacture of Tissue Engineering Medical Products (TEMPs) for tissues healing and regeneration. Moved by the great advantages in its use, various vertebrates have been extensively employed to isolate type I collagen. In spite of several attempts of extraction from different animal species, the best collagen sources are represented by mammals, such as bovine and swine, for the high sequence homology with human collagen [19]. However, the incidence of immune responses, the risk of zoonosis transmission and some religious concerns limited their use and favored horses use as a safer collagen source. Thus, the equine tissues appear as an attractive alternative, since they are almost free from zoonosis [38] and there are no documented immune reactions [39].
2. Why Equine Collagen
Type I collagen’s use as biomaterial for the manufacture of products related to the healthcare sector, the food industries and cosmetics is very high. The industrial production of collagen is based on its purification from animal tissues rather than from recombinant production systems [40]. The inability to reproduce the full-length collagen molecule with the native post-translational modifications (i.e., hydroxylation) decreased the interest in the use of both prokaryotic and eukaryotic hosts (i.e., yeast, bacteria, mammalian cells, insects or plants) for its synthesis [41]. As regards collagen extraction from animal tissues, several sources have been investigated [36], including mammals (bovine [42], porcine [43], ovine [44], equine [45,46], rat [47]), avian (chicken [48]) and fish (jellyfish, fish, sponges) [49], with the aim of finding the optimal one in terms of biocompatibility, safety and availability.
The highly available marine collagen, that has a lower threat of transmissible diseases and no religious concerns, is limited in its use in the healthcare sector because of its low denaturation temperature and enzymatic resistance [49]. On the other side, although the evolutionary closeness to vertebrates, poultry collagen molecule has an amino acid composition different from other mammals [50]. Moreover, the avian influenza transmission risk is not a negligible aspect [51].
Definitely, mammals represent the best source for the high sequence homology with human collagen (Figure 1) [19,52,53,54]. Moreover, the abundancy of waste materials (e.g., skin, tendons, bones, fatty tissues) from meat processing favored the exploitation of low-cost by-products for the purification a biomaterial with a high added value. The use of waste products for the extraction of a highly required product, such as collagen, not only makes discards valuable resource but also reduces their disposal costs and environmental impact. However, only in the last 50 years the use of heterologous collagen as medical product spread with the development of both accurate extraction processes that allowed removing allergenics and effective sterilization procedures [55].
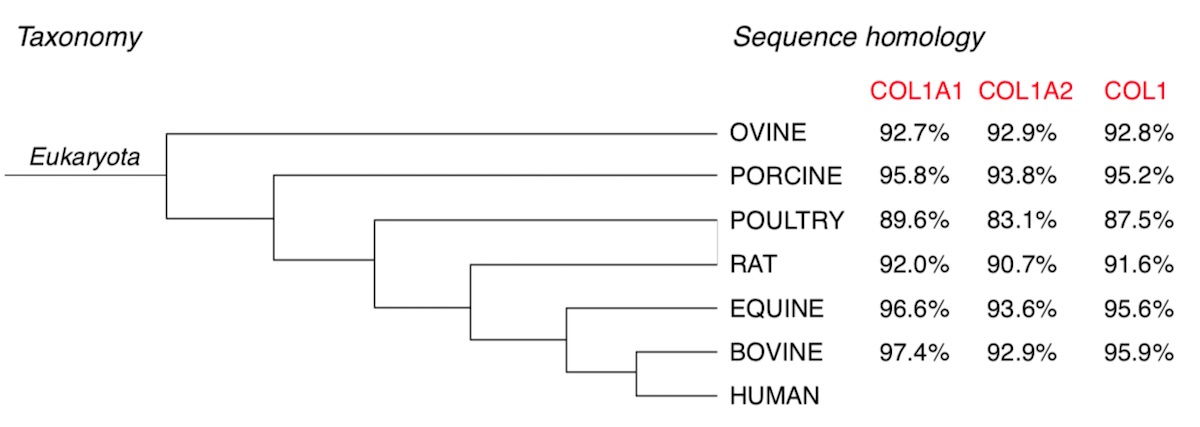
Figure 1. Taxonomy and sequence homology of selected mammalian collagen compared to human collagen. Identity percentages esteemed by collagen sequence alignment evaluation of α1 and α2 chains of equine, bovine, rodents, avian, swine and ovine in comparison with human collagen, by mean of UniProt (https://www.uniprot.org/align/) sequence alignment bioinformatic tool (last accessed on 3 April 2020).
Type I collagen could be isolated from several body districts. Among these, mammal skin and tendons are preferred due to the high protein yield [24,56]. As regards tendons, roughly 60%–85% of the dry weight is collagen [24,56] and type I collagen constitutes 90%–95% of the total collagen content [48,57,58]. To this, the lowest amount of protein contaminants is present in this district [59]. Otherwise, collagen content in mammalian dermal tissue is about 60%–70% and includes many other components such as blood vessels, lymph vessels, hair follicles and sweat glands [25,43], for which an accurate purification step is needed.
The extraction source not only influences the yield and purity of the final product but also its physical-chemical properties since the collagen structure and characteristics are deeply affected by the function it has in the belonging tissue [60]. The structure of tendon is such that the collagen fibers are aligned in the main load bearing direction and fiber diameter is larger than in skin [61,62]. The diameter and the orientation of fibers play an important role in tissue stability: a greater orientation of the fibers reflects a higher molecules compaction, resulting in a greater chemical-physical stability. Herein, collagen molecules were typically aligned and packed with a conserved stagger of 67 nm to form fibers with a medium diameter of 400 nm [46,59,63]. In skin instead, even if collagen is anisotropically distributed (along Langer’s lines) it is arranged in a loose network [60,64].
Besides, the collagen extracted from a tissue with a strict hierarchical organization, such as tendon, still retains a partial lateral packing arrangement despite the disruptive treatments of the extraction process [60,63,65]. The partial retention of the lateral arrangement of collagen molecules could also be ascribed to the well-known higher percentage of lysine and hydroxylysine in the α- helices of tendon collagen than in other tissues. These amino acids, fundamental for the intra- and intermolecular crosslinks, make tendon an extraction source of a type I collagen with superior properties over collagens derived from other tissues [56].
Thus, the highest type I collagen content and the lowest amount of protein contaminants in this body district [59], besides the appealing physical-chemical properties, make mammalian tendon as an attractive source for medical grade collagen. Moreover, mammalian tendons could be easily harvested from slaughterhouses without interfering with the meat harvesting process [56], while mammalian skins need to be appropriately separated from meat and hair.
Among mammals, bovine and swine are the most common extraction sources. The reason lies in the fact that these two species are the highest consumed mammalian meats per capita in the United States [31,66]. However, although bovine and porcine collagens cover most of the market size and tendon recover is easy, their use is limited because of immune response, zoonosis problems and religious constraints. Despite collagen is particularly poor immunogen [67] and the triple helical domains of bovine and porcine collagens are highly homologous to human collagen, immunologically relevant differences lay in the telopeptide regions [68]. Bovine collagen triggers immune reactions in about the 2%–4% of the World population [69]. However, this sensitivity has been considered generally acceptable for tissue engineered implants for human use [70]. Furthermore, the fact that up to 3% of the population manifests an inherent immunity [67,71], is enough to routinely perform allergy testing prior to material implantation [70]. To this, two consecutive negative skin tests at 6 and 2 weeks are required before any treatment [72]. Among issues, the zoonosis transferring risk (e.g., the foot and mouth disease (FMD) and the group of the bovine spongiform encephalopathies (BSE), among which the most dangerous for humans is the transmissible spongiform encephalopathy (TSE)) is the most serious. Porcine collagen causes less allergic response [36] but, just like the bovine source, the setback of zoonosis limited its use [73]. In addition, there are cultural or religious concerns associated with the use of porcine (Jewish, Islamic faith) and bovine (Sikh, Buddhism) collagen, which further restrict their applicative potential [34].
The ovine, a mammal of interest as dietary source of milk and meat, has no religious constrains but has the drawback of being susceptible to a special type of transmissible spongiform encephalopathy, namely scrapie. However, such a prion is known to not cause any diseases as the human-like variant Creutzfeldt–Jakob disease (vCJD), which is caused by BSE exposure to humans [74]. The only exploitable ovine source is the Australian one that is the sole disease and prion free in the world [75]. Holista Colltech, with a patent production process for ovine collagen, has the exclusivity to produce a zoonosis-free ovine collagen and does not have the adequate means (in terms of raw materials disposability) to sustain the World’s high demand of collagen-based products [75].
Rat tail tendon is one of the most commonly used source of type I collagen among researchers (in contrast to the industrial use), given the extensive amount of literature concerning isolation and characterization [40]. However, it is not used for the manufacture of medical products because of the unavailability of medical-grade type I collagen.
Conversely, horse-derived collagen is generally recognized as almost free from zoonosis transmission risks [38], with no reported immune reactions [39,57,76,77]. However, equine meat and thus equine collagen-based products are religiously not accepted by Jews and Muslims and are subjected to some ethical and social issues (see the article full version).
Although it is not well known, equids are also exposed to alphaviral equine encephalomyelitis (AEE), a mosquito-borne zoonotic infection that includes: (i) Eastern equine encephalomyelitis (EEE), (ii) Western equine encephalomyelitis (WEE) and (iii) Venezuelan equine encephalomyelitis (VEE) [78]. The AEE endemic life cycle involves different mosquito species (i.e., Psorophora, Ochlerotatus, Coquillettidia, Ochlerotatus, Aedes and Culex) and mammalian hosts (i.e., birds, rodents) to be spread to horses and other animals which are dead-end hosts [79]. Occasionally AEE can spill over to involve humans as dead-end hosts. In particular VEE often causes massive epizootics in horses and epidemics in human, whereas for EEE and WEE individual cases or limited outbreaks in both horses and humans were registered [78]. Some AEE cases have been reported in the past but in recent years, only few cases annually occurred and no epidemics have never been reported. However, should be noted that many cases may go unreported and undiagnosed since AEE infections usually are asymptomatic and encephalitis occurs in less than 4% of symptomatic cases [79]. Moreover, mortality rates of symptomatic cases are quite low (1%–7% for VEE and WEE, 50%–70% for EEE) [78,79]. Although the zoonosis transmission risk is not negligible, the occurrence of few encephalitis cases and the very low AEE-due mortality rate lead to the consideration of equine by-products as zoonosis-free extraction sources for medical-grade collagen.
Nevertheless, compared to collagen from other mammals, equine collagen is characterized by the highest homology sequence with human collagen, after bovine (see the article full version). The high percentage of sequence alignment is due to the taxonomical closeness of equines and bovines to humans. The low evolutionary gap and the high conservation of type I collagen amino acid composition among vertebrates make that homology up to 95%. Thus, equine collagen, that compared to bovine is equally similar to human collagen from a compositional point of view, seems to be a valid alternative to bovine collagen.
Moreover, as before mentioned, collagen extracted from a tissue with a strict hierarchical organization, such as tendon, is characterized by a higher percentage of lysine and hydroxylysine than other tissues. Interestingly, collagen from equine tendon revealed to have the highest lysine and hydroxylysine level compared to those extracted from other mammal tendons (see the article full version). The peculiar amino acid composition of equine tendon collagen and the related stronger fibers packing is the reason why devices manufactured with native horse tendon collagen are intrinsically more resistant to degradation and mechanical stress [63]. As reported by Angele et al., equine tendon collagen compared to bovine tendon collagen was found to have a higher thermal stability and a tendency to rupture under higher mechanical resistance [45].
The partial preservation of fibers packing [63,81], is a key aspect because it influences not only bioengineering parameters but also the cell-biomaterial interaction since the nanometric fibril organization is recognized by cells as guide for cell growth and migration during the remodeling phase of the healing process [26,27,60,82]. By the way, it should be noted how despite collagen extraction protocols are set up in order to preserve its native structure as possible, the application of mechanical, chemical and enzymatical treatments brings to a partial de-structuration of the strict hierarchical organization of collagen fibrils. In particular, the enzymatic treatment cuts collagen molecules at the N− and C− termini, modifying their native state and making them more susceptible to enzymatic digestion and thermal denaturation. Thus, while type I collagen fibrils are regularly packed in tendon, the isolated ones are characterized by smaller diameter and length and thus by lower mechanical properties. Moreover, it should not be neglected that collagen extraction sources are animal tissues and various factors, such as animals age, sex and inter-species variability, make collagen chemical and physical properties not punctual but within a range of values.
Thus, the structural organization of the native tissue is not completely preserved in the extracted product. About this topic, some attempts were made in order to in vitro reorganize collagen fibrils (i.e., fibrillogenesis) in fibers that could resemble the natural tendon ones. Although a partial alignment could be obtained, to date it is not possible to completely reassemble the extracted tendon collagen fibrils in vitro, in the ordered hierarchical organization naturally present in tendon.
Nevertheless, the strict hierarchical organization of equine tendon, compared to other horse tissues and tissues form other mammals, allows to better retain collagen native structure after the extraction process and the following processing [60]. However, should be noted how, to the best of our knowledge, few data were reported about the comparison of equine tendon collagen properties with collagen from tendon of other animals with the same extraction method and substrate synthesis protocol applied. Moreover, the patent-due confidentiality of the collagen isolation protocols hinders such comparison since processing variations strongly influence the final products properties. Although the lack of exhaustive, numerous and public supporting data, the advantages offered by the use of equine collagen as biomaterial are visible from its employment by several well-known Companies. Definitely, the horse tendon with its structural interesting features and the freedom from the afore-mentioned source-related issues would be considered as a valid and alternative extraction site of medical grade type I collagen.
3. Currently Approved Equine Collagen-Based Devices
The use of xenogeneic collagen as a modern biomaterial began in 1881 when Joseph Lister and his former student William Macewen independently reported on the British Medical Journal the advantages of a biodegradable suture termed “catgut” derived from the small intestine of a sheep [87]. From that moment on, the idea of exploiting xenogeneic material for human surgical practices spread to the scientific community. The high conserved compositional similarity among mammals is a strong point that could be exploited to reach a better natural-like tissue healing [53]. Citations dating to the 1940s and 1950s relates to experimental attempts of purified collagen implantation in animals [88]. Only 30 years later, the first medical use of collagen in humans was reported by Knapp with an injectable collagen gel formulation for soft tissues augmentation [89]. In 1980, one of the first mammal collagen formulations (i.e., Zyderm® by McGhan Medical Corporation, Fremont, CA, USA) started to be commercialized [90]. Over the ensuing years, countless collagen-based formulations were manufactured with the aim to restore or repair soft and hard tissues physiological function [91,92].
The history of implantable collagen-based products let us know about the high interest that turns around it. Therefore, it has always been a target not only to isolate collagen from animal tissues but also to obtain a safe xenogeneic product, which meets regulatory requirements and which can be implanted without triggering unwanted reactions. For instance, medical devices to be commercialized should meet the essential requirements defined in the Annex I of the Council Directive 93/42/EEC (which is going to be replaced by the Medical Device Regulation (MDR) 2017/745 in May 2020) [93]. The manufacturing process of a device, including all aspects going from the raw materials to the delivery of the final product, should be fully validated to ensure reproducibility and safety for human use.
Among the various aspects, the approved for human use products must above all be free from allergens or toxic compounds that could trigger immune response. Even if collagen is typically low immunogenic, other ECM proteins (i.e., DNA, RNA, cells remnants, α-gal epitope and MHC-1) are able to evoke immune response, adverse reactions and rejections [70,94]. Since immunogenicity is the primary cause of immunotoxicity, the immunogenicity evaluation is a critical but essential aspect for collagen products. A not-negligible aspect is the material contamination by bacterial endotoxins (i.e., lipopolysaccharides), that are components of the external cell membrane of Gram-negative bacteria able to stimulate the inflammatory response at very low doses (0.5 EU/mL) [95,96].
Another reason why collagen products could evoke adverse effects is the crosslinking, in particular chemical crosslinking. Physical crosslinking as the dehydrothermal treatment (DHT) instead is safer and biocompatible [97,98]. During resorption, chemical crosslinking likely affects MMPs bioactivity against native collagen, producing an imbalance in ECM turnover [70]. The delayed resorption and the substrate inertness to degradation prolongs implant presence in the tissue, exacerbating host responses to the implant. Additionally, not-natural collagen degradation fragments, bearing remnants of added synthetic chemical crosslinkers, are recognized as antigens and amplify the foreign body response [99,100]. That is why almost all commercial products are not chemically crosslinked.
Devices sterilization is the last key process to accurately set prior to the products packaging. Collagen is a temperature sensitive biomaterial that could not be autoclaved. For this, alternative sterilization processes have been investigated but until today the ideal technique has not been identified. Any known sterilization technique induces molecular alteration to collagen triple helical structure with a consequent decrease of properties such as the mechanical and the enzymatical resistance [101]. However, some methods are more permissive than others. Ethylene oxide sterilization and β-ray irradiation induce less damage than γ-ray but their applicability depends on the type of collagen-based device to be produced [91,101]. The preservation of the native collagen structure as much as possible among the whole manufacturing process is preferred since it accelerates the regeneration stage, shorts the wound healing time, reduces the extent of bacterial contamination, alleviates the pain syndrome and reduces the recurrence rate [102].
To date, numerous preparations based on equine tendon collagen received the approval of the US Food and Drug Administration (FDA) for human use and are commercially and clinically available. From 1990 onwards, the date at which the first device was registered based on horse tendon collagen for wound healing (Condress® now called Biopad® by Euroresearch), companies like Baxter, Bioteck, Euroresearch, Finceramica, Fidia Farmaceutici, Innocoll Pharmaceuticals, MLM Biologics, Nycomed, Opocrin, Resorba, Savecoll, Takeda, Vebas manufactured and commercialized devices based on equine tendon collagen with several patented techniques (Table 1).
Table 1. Marketed equine tendon collagen products sort by producer, form and application.
| Company | Product | Additives | Form | Application | Ref. |
|---|---|---|---|---|---|
| B. & B. Dental (Bologna, Italy) |
T-Barrier | - | Sheet | Hemostasis, Hard tissue | [103] |
| Baxter (Rome, Italy) |
Gentafleece | Gentamicin sulphate | Sponge | Hemostasis, Wound healing | [104,105,106] |
| TissuFoil E | - | Sheet | Wound healing | [107,108] | |
| TissuDura | - | Sheet | Wound healing | [109,110,111,112,113,114] | |
| TissueFleece | - | Sponge | Hemostasis | [115,116,117] | |
| Zimmer Biomet (Warsaw, USA) |
Septocoll | Gentamicin sulphate | Sponge | Hemostasis | [118] |
| Bioteck (Vicenza, Italy) |
Biocollagen | - | Membrane | Hard tissues | [119,120,121] |
| Bio-gen | Spongy bone | Powder | Hard tissues | [122] | |
| MeRG | Glycosaminoglycans | Membrane | Soft tissues | [123,124] | |
| Xenomatrix | - | Sheet | Soft tissues | [125,126] | |
| Euroresearch (Milano, Italy) |
Biopad | - | Sponge | Wound healing, Hard tissues | [26,127,128] |
| Bioart | - | Powder | Hard tissues | - | |
| Nithya | - | Gel | Soft tissues, Anti-aging | [39] | |
| Revamil | Honey | Sponge | Wound healing | - | |
| Versuspray | Silver | Powder | Wound healing | - | |
| EUSA Pharma (Langhorne, USA) |
Collatamp | Gentamicin sulphate | Sponge | Wound healing | [129,130] |
| Finceramica (Faenza, Italy) |
MaioRegen | Hydroxyapatite | Membrane | Soft tissue | [131,132,133,134] |
| Fidia Farmaceutici (Bologna, Italy) |
Bionect pad | Hyaluronic acid | Sponge | Wound healing | [135] |
| Innocoll (Athlone, Ireland) |
Collexa | Bovine collagen | Sponge | Wound healing | [136] |
| MLM Biologics (Gainesville, USA) |
Bio-conneKt | - | Membrane | Wound healing | [136] |
| Nycomed (Munich, Germany) |
TachoTop | - | Sponge | Hemostasis, Wound healing | [137,138] |
| TachoComb | Human fibrinogen and bovine thrombin | Sponge | Hemostasis, Wound healing | [139,140,141,142,143] | |
| TachoSil | Human fibrinogen and human thrombin | Sponge | Hemostasis, Wound healing | [143,144,145,146,147,148,149,150,151,152] | |
| Opocrin (Modena, Italy) |
Antema | - | Sheet | Hemostasis, Wound healing | [57,153] |
| Resorba Medical GmbH (Nürnberg, Germany) | Genta-coll | Gentamicin sulphate | Sponge | Hemostasis, Hard tissues | [154,155,156] |
| Kollagen | - | Sponge | Hemostasis, Hard tissues | [82,157,158,159,160,161,162,163,164,165,166] | |
| Parasorb | - | Membrane | Hemostasis, Hard tissues | [77,167,168] | |
| Salvecoll (Como, Italy) |
Salvecoll-E | - | Gel | Wound healing | [100] |
| Takeda (Tokyo, Japan) |
CollGARA | - | Sponge | Hemostasis, Wound healing | [169] |
| GABA Vebas (Roma, Italy) |
Paroguide | Chondroitin sulphate | Membrane | Wound healing | [170] |
Thanks to its intrinsic biocompatibility [26,27] and regenerative properties, equine tendon collagen-based devices have been manufactured and applied in relation to a variety of medical applications (Figure 2) such as in reconstructive surgery to speed up wounds closure, to regenerate burned skin and soft tissues as well as to guide bone and cartilage repair (see the article full version). Between the listed ones, the use as hemostat is one of the most important application.
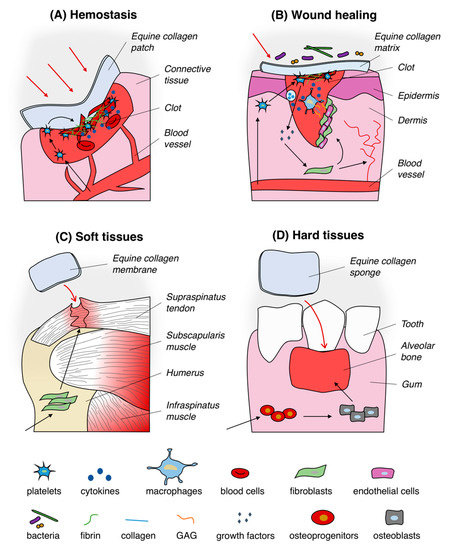
Figure 2. Typical uses of equine collagen-based products in biomedical applications. Equine collagen-based products are usually used as hemostatic agent (A), wound dressing (B), matrix for soft (C) and hard (D) tissues regeneration. Black arrows in section (A) and (B) indicate the trigger of the hemostasis process and the healing of wounds pathway by mean of equine collagen substrates, respectively. Black arrows in section (C) and (D) represent the equine collagen matrix-mediated enhancement of soft and hard tissues regeneration process, respectively.
4. Equine Collagen-Based Device Market
In the last 15 years, with the development of regenerative medicine and tissue engineering, collagen has been defined one of the best scaffolding materials, being biocompatible, biodegradable, bioactive besides easily manufactured. The remarkable advantages offered by this extraordinary and archaic natural protein means that the demand for collagen and collagen-based products never fades, rather it tends to increase with the increasing need of new effective and advanced therapies [188].
The native collagen market size was globally esteemed to be around USD 160.5 Million in 2018 [189]. Among the several application sectors in which collagen market is divided, the healthcare is the largest application area, followed by food and cosmetic. Healthcare dominates the collagen market with about 50% share of the entire market volume in 2025 [189,190]. Herein, in 2014 it has been esteemed a global addressable market of c. $16 bn at the end-market price, by counting c. $14 bn for bone graft and advanced wound healing, c. $1.2 for regenerative medicine scaffold and c. $0.2 bn for in vitro diagnostic [190]. The esteemed market should increase over years since the request of collagen for medical devices and drug delivery systems is expanding together with the trend towards minimally invasive technologies and its effectiveness in wound healing. Indeed, as regards tissue engineering products, the esteemed global market of $1.5 bn in 2014 [190], nearly doubled to c. $2.4 bn in 2017 with an expected compound annual growth rate (CAGR) from 2017 to 2025 of 10.4% [191,192].
To the best of our knowledge, even if no specific information on equine tendon collagen market are available, the interest in horse tendon collagen and derivates is clearly visible not only from the number of scientific researches but also from the number of patented manufacturing processes on equine tendon collagen and equine tendon collagen-based devices for biomedical and cosmetic application [193]. The long-time search for better strategies, the advanced manufacturing techniques used, the in-depth investigations on the properties of the products, the functionality checks (in vitro and preclinical testing), the safety assurance and the management costs gave to the healthcare products a high final cost. In general, no medical grade collagen-based products have been found worth less than $10,000 USD/kg [75]. However, even if the high final cost of all collagen-based products could be limiting, the ratio between costs and benefits should be considered. As afore mentioned, tendon collagen-based devices are able to promote natural healing processes faster and better than other biomaterials on the market [174]. Faster healing is associated with a lower risk of developing post-treatment or post-surgical complications, for which further treatments or second surgeries are needed. The decrease of healing time and complications rate reduces the needing of additional treatments, drug therapies and surgical procedures and consequently positively impacts on the patient’s psycho-physical state. Moreover, not negligible is the benefit in relation to other cost drivers such as hospital inpatient stays and personnel costs.
This entry is adapted from the peer-reviewed paper 10.3390/jfb11040079
