Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Involvement of glutamate and its peripheral N-Methyl-D-Aspartate Receptor (NMDAR) in migraine pathophysiology.
- glutamate
- headache
- pain
- aura
1. Introduction
Migraine is a primary headache disorder, affecting more than 15% of the global adult population in their most productive years of life with a health and economic burden of billions of dollars globally [1]. The clinical manifestation of migraine is recurrent attacks with a severe, and usually unilateral and throbbing headache, lasting 4–72 h and associated with nausea and/or light and sound sensitivity [2]. In one-third of individuals with migraine, the headache phase is preceded by transient focal neurological disturbances, the so-called migraine aura-phase [3], whose underlying mechanism is considered to be cortical spreading depression (CSD) [4]. CSD is a self-sustaining, slowly propagating wave of transient neuronal and glial depolarization [3] that silences brain and electrical activity for several minutes [5][6][7]. In the past three decades, advances made in migraine research led to the development of several acute and preventive medications. Yet, a significant proportion of patients reports an inadequate response and a lack of tolerability [8].
Glutamate is the principal excitatory neurotransmitter in the central nervous system [9], and its role in migraine pathophysiology has been under the spotlight for more than three decades [10][11][12][13]. Glutamate receptors are pharmacologically classified as ionotropic and metabotropic receptors. N-Methyl-D-Aspartate Receptor (NMDAR) is an ionotropic glutamate receptor [9] that modulates excitatory neurotransmission by conducting sodium (Na+) and calcium (Ca2+) ions into the cell and potassium (K+) ions outside the cell. Key structures related to migraine pain, including the trigeminal ganglion (TG), trigeminal nucleus caudalis (TNC), and thalamus contain high densities of NMDAR-positive neurons, pinpointing a potential connection between the NMDAR and migraine pathophysiology [9][12][14][15][16].
2. N-Methyl-D-Aspartate Receptor (NMDAR)
Glutamate receptors are pharmacologically classified as ionotropic (ligand-gated ion channels) and metabotropic (GPCRs). NMDAR along with alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor (AMPAR) and Kainate receptor (KAR) are categorized as ionotropic glutamate receptors [9]. NMDAR modulates excitatory neurotransmission by conducting Na+ and Ca2+ ions into the cell and K+ ions outside the cell (Figure 1).
The NMDAR is a heterotetrameric complex assembled from three different NMDAR subunits (NR1, NR2, and NR3) [17] (Figure 2). The NR1 subunit is essential for a functional NMDAR complex [17][18] and is represented by eight splice variants, which influence the channel properties. Four NR2 subunits (NR2A, NR2B, NR2C and NR2D) and two NR3 subunits (NR3A and NR3B) have been mapped. The NR1-NR2A heterodimer suggests a mechanism for ligand-induced ion channel opening and is the functional unit in tetrameric NMDARs [19]. All NMDAR subunits share a common membrane structure characterized by a large N-terminus domain (NTD), an agonist/ligand binding domain (ABD/LBD), a transmembrane region (TMD), and a cytoplasmic region (CTD) (Figure 2) [17]. NMDAR activation requires the simultaneous binding of glycine and glutamate, together with the removal of the endogenous channel-pore blocker, magnesium ion (Mg2+), in a voltage-dependent manner [20][21]. The particular importance of the NMDAR is due to the high permeability to Ca2+ ions that gives NMDARs a significant role in both synaptic plasticity under physiological conditions and neuronal death under excitotoxic pathological conditions [17]. A typical NMDAR complex consists of two glycine-binding NR1 subunits and two glutamate-binding NR2 subunits [18]. Incorporating either NR3A or NR3B subunits showed decreased channel activity via reduced single-channel conductance, reduced Ca2+ permeability and increased Mg2+ blockade [17][22][23][24]. Notably, the NR3 subunit can bind to glycine rather than glutamate, which is similar to the NR1 subtype. Thus, a novel type of NMDAR complex composed of NR1 and NR3 subunits would only require glycine and not glutamate for activation [25]. The entry of Ca2+ into dendritic spines through NMDARs is essential for long-term potentiation (LTP) and long-term depression (LTD) [26]. LTP is induced by a high-frequency synaptic activity that causes postsynaptic membrane depolarization, a decrease in voltage-dependent Mg2+ blockage of the NMDAR pore, and a massive entry of Ca2+ ions into dendritic spines leading to calmodulin (CaM) and CaM-dependent kinase II activation (Figure 1).
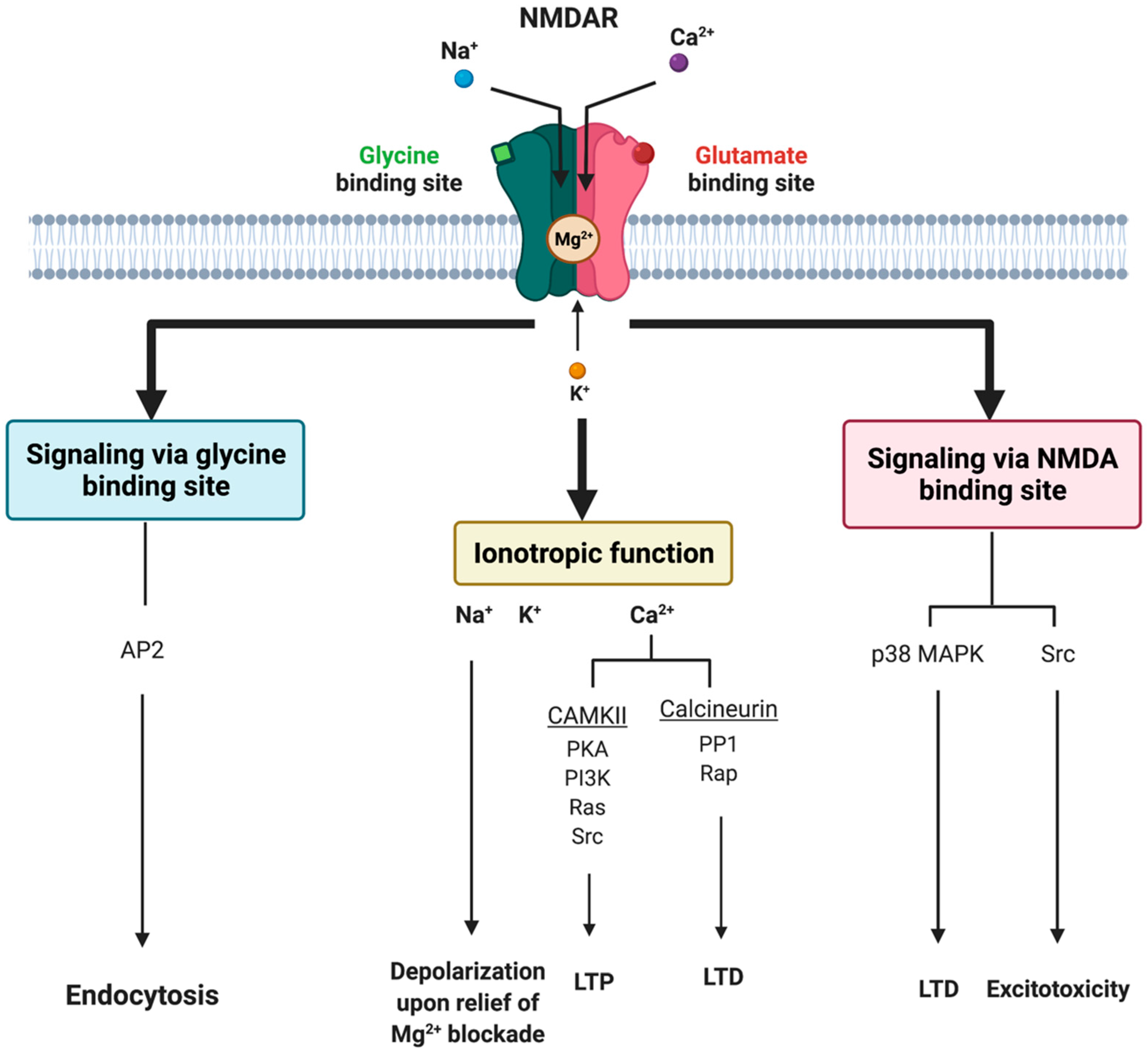
Figure 1. Tripartite signaling of N-Methyl-D-Aspartate Receptor (NMDAR). This model illustrates three parallel signaling pathways of NMDAR transduction. Binding of glutamate and glycine results in ionotropic function and membrane depolarization through efflux of K+ ions and influx of Na+ and Ca2+ ions mediating downstream Ca2+-dependent pathways. NMDAR signaling can also happen non-ionotropically when glycine or glutamate bind independently and induce conformational changes and downstream protein interactions. Glycine stimulation enhances NMDAR association with the AP2 and subsequent activation of a downstream endocytic pathway. Lastly, glutamate binding can result in LTD through p38 MAPK and excitotoxicity though an activation of Src. AP2 = endocytic adaptor protein 2, CaMKII = calcium/calmodulin kinase II, LTD = long-term depression, LTP = long-term potentiation, p38 MAPK = p38 mitogen-activated protein kinase, PP1 = protein phosphatase 1, Rap = ras-related protein, Src = thyrosin kinase family.
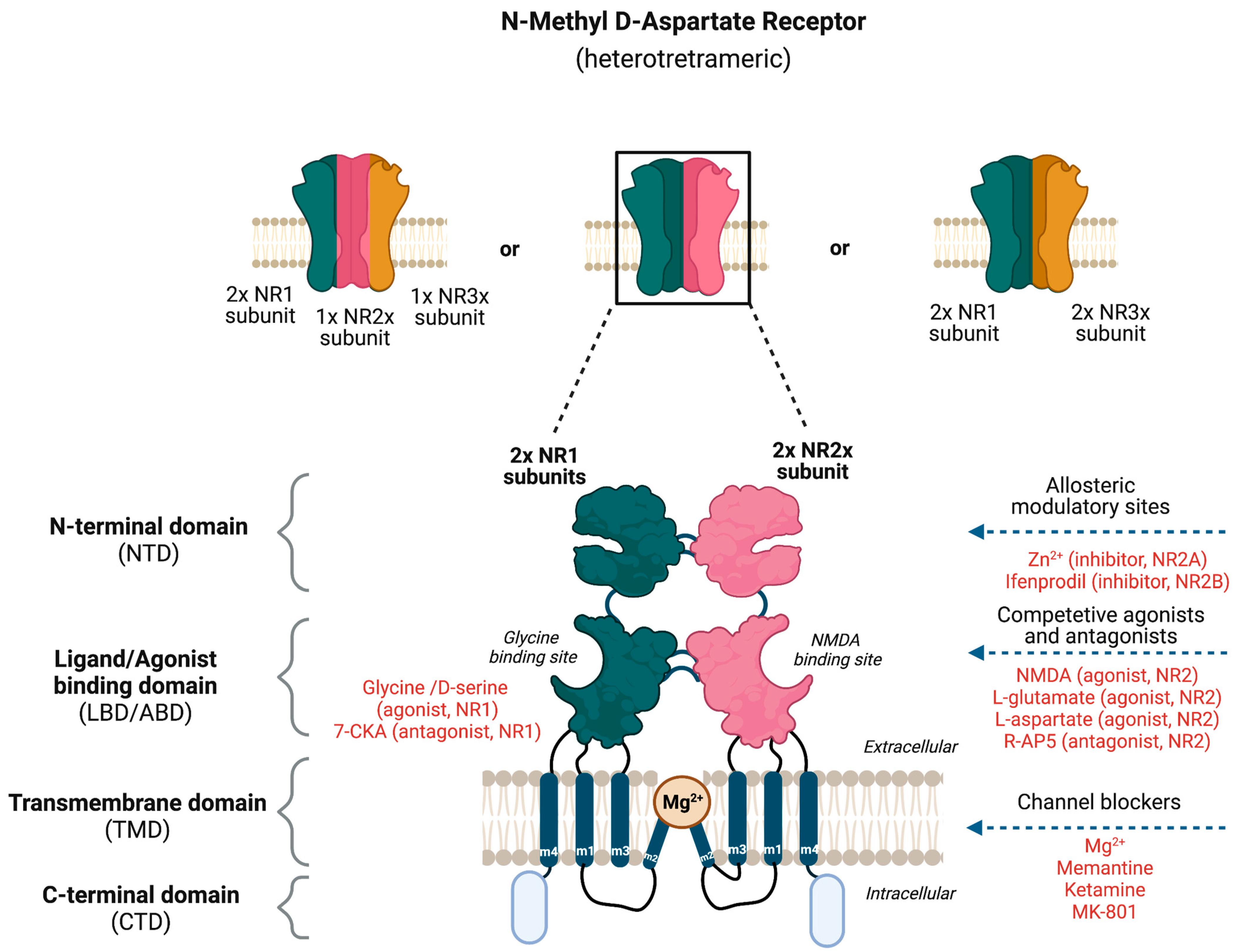
Figure 2. Potential binding sites for ligands that modulate the activity of N-Methyl-D-Aspartate Receptor (NMDAR). The NMDAR is assembled as tetramers; two NR1 and two NR2 subunits forming a dimer–dimer structure or two NR1 subunits associating with one NR2 and NR3 subunit. The channel construction with different domains gives rise to ligand binding or allosteric modulatory sites that either work as an NMDAR agonist or antagonist.
2.1. Evidence Implicating NMDAR in Migraine
NMDARs are expressed in trigeminovascular neurons (trigeminal neurons innervating dural blood vessels) [27]. Glutamate transporter (excitatory amino acid transporter-2 (EAAT-2)) was identified in dural blood vessels [28]. In rat models, the expression of NMDAR subunits NR1, NR2A, and NR2B have been demonstrated in TG [29][30][31][32]. Specifically, the NR2B subunit was expressed in nerve fibers that innervated dural blood vessels [33]. In a recent study, Guerrero-Toro and colleagues investigated the role of peripherally released glutamate in trigeminal nociception by using TG neurons from rodent models. The authors revealed (1) the presence of NR2A and NR2B subunits in TG neurons through immunolabeling, (2) the expression of other glutamate receptors in TG neurons, (3) the presence of calcitonin gene-related peptide (CGRP) enhanced the fraction of NMDAR-positive neurons, and (4) the removal of Mg2+ increased the nociceptive firing of trigeminal afferents by NMDAR in in vivo conditions [34]. Moreover, NMDAR activation mediated nociceptive transmission in the TNC [35], and the stimulation of dural structures caused a co-release of glutamate and CGRP in the TCC [36]. In rats, intravenous administration of 50 mg/kg MSG activated and sensitized the trigeminovascular neurons associated with dural vasodilation. Co-administration of a peripherally restricted NMDAR antagonist, (2R)-amino-5-phosphonovaleric (APV) attenuated the MSG-induced effects [33]. Glutamate is a negatively charged, polar amino acid. The central uptake of glutamate and other anionic excitatory amino acids from the peripheral circulation is limited by the blood–brain barrier (BBB). At physiologic plasma concentrations, glutamate flux from plasma into the brain is mediated by a high-affinity transport system at the BBB. Efflux from the brain back into plasma appears to be driven in large part by a Na+-dependent active transport system at the capillary abluminal membrane. The glutamate concentration in brain interstitial fluid is only a fraction of that of plasma and is maintained fairly independently of small fluctuations in plasma concentration [37]. Thus, glutamate-induced headache is likely due to peripheral activation of NMDAR, indicating that targeting peripheral NMDAR might be a possible strategy for the treatment of individuals with migraine to avoid side effects due to central action.
2.2. NMDAR Antagonists in Preclinical Studies
Based on the knowledge about NMDAR subunits and their distinctive domains, several potential therapeutic approaches such as allosteric modulators, and competitive and non-competitive antagonists have been proposed for anti-migraine therapy (Figure 2). The negative allosteric modulators of NMDAR include the endogenous zinc ion (Zn2+) and ifenprodil-like compounds [17][38], which both act on the NR2 NTD as non-competitive antagonists. Zn2+ has been shown to be a selective antagonist of the subunits NR2A and NR2B, whereas ifenprodil and its derivatives are selective antagonists of NR2B with more than a 100-fold higher affinity [39]. The pore domain of the NMDAR forms binding sites for non-competitive pore blockers such as the endogenous Mg2+ and exogenous compounds such as MK-801 (also known as dizocilpine), memantine, and ketamine. Memantine and Mg2+ are low-affinity NMDAR blockers with faster blocking and unblocking kinetics, which are linked to fewer and less severe side effects than substances with slower receptor kinetics such as ketamine and MK-801 [20]. The administration of dizocilpine maleate, an NDMAR antagonist, resulted in a substantial blockade of neuronal firing and the inhibition of trigeminovascular-evoked responses in the trigeminovascular complex in cats [27]. Apart from being involved in the trigeminal pain pathway, the NMDAR seems to be implicated in the initiation and propagation of CSD, which is believed to be the pathophysiological mechanism underlying migraine aura [5][35][40]. Mg2+ selectively suppressed glutamate-induced CSD [41], and MK-801 (non-specific NMDAR antagonist) and ifenprodil (NR2B selective antagonist) inhibited experimental-induced CSD [40][42][43][44] (Box 1). In 2019, Hoffmann and colleagues investigated the efficacy of Mg2+ and memantine in rat models by comparing microiontophoretic application and systemic administration to modulate trigeminovascular nociception. They observed that intravenous systemic administration of moderate concentrations of low-affinity NMDAR channel blockers such as Mg2+ and memantine does not inhibit stimulus-evoked firing significantly compared to microiontophoretic application [20].
2.3. NMDAR Antagonists in Clinical Studies
NMDAR antagonists such as Mg2+, ketamine, and memantine have been investigated for migraine treatment and prophylaxis, respectively [45]. Administration of Mg2+ has been examined in numerous clinical trials either perorally or intravenously [46]. In a prospective quasi-experimental study, Baratloo and colleagues found that intravenous magnesium sulfate might be superior to intravenous caffeine for the acute management of migraine headaches [47]. Additionally, the administration of transdermal MgCl2 solution is described as an ideal approach in acute migraine treatment due to the immediate absorption through the skin [46][48]. Collectively, Mg2+ seems to have beneficial effect for the acute and preventive treatment of migraine.
Several studies have investigated the use of ketamine or AMPA (LY293558; BGG492) antagonists as abortive therapies in migraine with aura and familial hemiplegic migraine [49][50][51]. Although most of these studies concluded that these drugs possessed limited or at best moderate efficacy in the reduction in severity of headaches and analgesic consumption, the presence of side effects owing to their effects on central glutamate receptors limited future exploration of their use in migraine. Regarding migraine prophylaxis, the efficacy of memantine has been investigated in retrospective (in 2007, [45]), open-label (in 2008, [52]), and randomized double-blinded placebo-controlled studies (in 2016, [53]). Together, these studies reported a memantine-induced significant reduction in (1) the monthly headache frequency, (2) the mean number of days with severe pain, and (3) the mean disability score.
For the treatment of migraine with severe and prolonged aura, ketamine was studied in a small open-label study (n = 11) (in 2000, [49]) and a randomized double-blinded placebo-controlled trial (in 2013 [54] and 2018 [55]). The intranasal administration of ketamine (25 mg) reduced the severity, and to a lesser extent, the duration of aura [49][54]. However, intravenous administration of a low-dose (0.2 mg/kg) of ketamine for the treatment of acute migraine did not produce a significant reduction in pain or disability-related scores [55]. In 1995, Nicolodi and colleagues investigated the administration of subcutaneous ketamine hydrochloride in individuals with migraine and stated that there was noticeable pain relief in the ketamine group compared to the placebo group [56]. In 2016, Lauritsen and colleagues reported in a retrospective study that six out of six individuals with refractory migraine achieved the target pain relief endpoint with intravenous ketamine infusion [50]. Collectively, it can be assumed that migraine with aura involves glutamate release and NMDAR activation, which could initiate migraine attacks [16].
The most common side effects reported from the above-mentioned trials with ketamine and memantine are asthenia, dizziness, nausea, psychiatric symptoms such as hallucinations, rashes, cognitive dysfunctions, somnolence, and changes in cardiovascular and respiratory stability [45][50][52][56]. So far, the American Headache Society [57][58] and European Headache Federation [59] have not recommended ketamine or memantine for the treatment of migraine. Mg2+, on the contrary, takes place as a nutraceutical in the migraine prevention treatment [57][59][60].
This entry is adapted from the peer-reviewed paper 10.3390/jcm12062156
References
- Ashina, M.; Katsarava, Z.; Do, T.P.; Buse, D.C.; Pozo-Rosich, P.; Özge, A.; Krymchantowski, A.V.; Lebedeva, E.R.; Ravishankar, K.; Yu, S.; et al. Migraine: Epidemiology and systems of care. Lancet 2021, 397, 1485–1495.
- Ashina, M. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876.
- Eikermann-Haerter, K.; Ayata, C. Cortical spreading depression and migraine. Curr. Neurol. Neurosci. Rep. 2010, 10, 167–173.
- Holland, P.R.; Akerman, S.; Goadsby, P.J. Cortical spreading depression-associated cerebral blood flow changes induced by mechanical stimulation are modulated by AMPA and GABA receptors. Cephalalgia 2010, 30, 519–527.
- Pietrobon, D. Ion channels in Migraine disorders. Curr. Opin. Physiol. 2018, 2, 98–108.
- Olesen, J.; Friberg, L.; Olsen, T.S.; Iversen, H.K.; Lassen, N.A.; Andersen, A.R.; Karle, A. Timing and topography of cerebral blood flow, aura, and headache during migraine attacks. Ann. Neurol. 1990, 28, 791–798.
- Olesen, J.; Larsen, B.; Lauritzen, M. Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann. Neurol. 1981, 9, 344–352.
- Lee, M.J.; Al-Karagholi, M.A.; Reuter, U. New migraine prophylactic drugs: Current evidence and practical suggestions for non-responders to prior therapy. Cephalalgia 2023, 43, 3331024221146315.
- Vikelis, M.; Mitsikostas, D.D. The role of glutamate and its receptors in migraine. CNS Neurol. Disord. Drug Targets 2007, 6, 251–257.
- Ferrari, M.D.; Odink, J.; Bos, K.D.; Malessy, M.; Bruyn, G.W. Neuroexcitatory plasma amino acids are elevated in migraine. Neurology 1990, 40, 1582–1586.
- Lauritzen, M. Spreading depression and migraine. Pathol. Biol. 1992, 40, 332–337.
- Tallaksen-Greene, S.J.; Young, A.B.; Penney, J.B.; Beitz, A.J. Excitatory amino acid binding sites in the trigeminal principal sensory and spinal trigeminal nuclei of the rat. Neurosci. Lett. 1992, 141, 79–83.
- Alam, Z.; Coombes, N.; Waring, R.H.; Williams, A.C.; Steventon, G.B. Plasma levels of neuroexcitatory amino acids in patients with migraine or tension headache. J. Neurol. Sci. 1998, 156, 102–106.
- Kalia, L.V.; Kalia, S.K.; Salter, M.W. NMDA receptors in clinical neurology: Excitatory times ahead. Lancet Neurol. 2008, 7, 742–755.
- Waung, M.W.; Akerman, S.; Wakefield, M.; Keywood, C.; Goadsby, P.J. Metabotropic glutamate receptor 5: A target for migraine therapy. Ann. Clin. Transl. Neurol. 2016, 3, 560–571.
- Chan, K.; MaassenVanDenBrink, A. Glutamate receptor antagonists in the management of migraine. Drugs 2014, 74, 1165–1176.
- Paoletti, P.; Neyton, J. NMDA receptor subunits: Function and pharmacology. Curr. Opin. Pharmacol. 2007, 7, 39–47.
- Ye, R.; Kong, X.; Han, J.; Zhao, G. N-methyl-D-aspartate receptor antagonists for migraine: A potential therapeutic approach. Med. Hypotheses 2009, 72, 603–605.
- Furukawa, H.; Singh, S.K.; Mancusso, R.; Gouaux, E. Subunit arrangement and function in NMDA receptors. Nature 2005, 438, 185–192.
- Hoffmann, J.; Storer, R.J.; Park, J.W.; Goadsby, P.J. N-Methyl-d-aspartate receptor open-channel blockers memantine and magnesium modulate nociceptive trigeminovascular neurotransmission in rats. Eur. J. Neurosci. 2019, 50, 2847–2859.
- Kleckner, N.W.; Dingledine, R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science 1988, 241, 835–837.
- Ciabarra, A.M.; Sullivan, J.M.; Gahn, L.G.; Pecht, G.; Heinemann, S.; Sevarino, K. Cloning and characterization of chi-1: A developmentally regulated member of a novel class of the ionotropic glutamate receptor family. J. Neurosci. 1995, 15, 6498–6508.
- Nishi, M.; Hinds, H.; Lu, H.P.; Kawata, M.; Hayashi, Y. Motoneuron-specific expression of NR3B, a novel NMDA-type glutamate receptor subunit that works in a dominant-negative manner. J. Neurosci. 2001, 21, Rc185.
- Matsuda, K.; Kamiya, Y.; Matsuda, S.; Yuzaki, M. Cloning and characterization of a novel NMDA receptor subunit NR3B: A dominant subunit that reduces calcium permeability. Brain Res. Mol. Brain Res. 2002, 100, 43–52.
- Low, C.M.; Wee, K.S. New insights into the not-so-new NR3 subunits of N-methyl-D-aspartate receptor: Localization, structure, and function. Mol. Pharmacol. 2010, 78, 1–11.
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39.
- Storer, R.J.; Goadsby, P.J. Trigeminovascular nociceptive transmission involves N-methyl-D-aspartate and non-N-methyl-D-aspartate glutamate receptors. Neuroscience 1999, 90, 1371–1376.
- Shin, H.E.; Han, S.J.; Lee, K.S.; Park, J.-W. Polymorphism of the Glutamate Transporter Protein EAAT2 and Migraine Transformation into Chronic Daily Headache. J. Clin. Neurol. 2011, 7, 143–147.
- Dong, X.D.; Mann, M.K.; Kumar, U.; Svensson, P.; Arendt-Nielsen, L.; Hu, J.; Sessle, B.; Cairns, B. Sex-related differences in NMDA-evoked rat masseter muscle afferent discharge result from estrogen-mediated modulation of peripheral NMDA receptor activity. Neuroscience 2007, 146, 822–832.
- Lee, J.; Ro, J.Y. Differential regulation of glutamate receptors in trigeminal ganglia following masseter inflammation. Neurosci. Lett. 2007, 421, 91–95.
- Ivanusic, J.J.; Beaini, D.; Hatch, R.J.; Staikopoulosl, V.; Sesslel, B.; Jenningsl, E.; Ivanusic, J.J.; Beaini, D.; Hatch, R.J.; Staikopoulos, V.; et al. Peripheral N-methyl-d-aspartate receptors contribute to mechanical hypersensitivity in a rat model of inflammatory temporomandibular joint pain. Eur. J. Pain 2011, 15, 179–185.
- Fernandez-Montoya, J.; Buendia, I.; Martin, Y.B.; Egea, J.; Negredo, P.; Avendaño, C. Sensory Input-Dependent Changes in Glutamatergic Neurotransmission- Related Genes and Proteins in the Adult Rat Trigeminal Ganglion. Front. Mol. Neurosci. 2016, 9, 132.
- O’Brien, M.; Cairns, B.E. Monosodium glutamate alters the response properties of rat trigeminovascular neurons through activation of peripheral NMDA receptors. Neuroscience 2016, 334, 236–244.
- Guerrero-Toro, C.; Koroleva, K.; Ermakova, E.; Gafurov, O.; Abushik, P.; Tavi, P.; Sitdikova, G.; Giniatullin, R. Testing the Role of Glutamate NMDA Receptors in Peripheral Trigeminal Nociception Implicated in Migraine Pain. Int. J. Mol. Sci. 2022, 23, 1529.
- Wang, X.M.; Mokha, S.S. Opioids modulate N-methyl-D-aspartic acid (NMDA)-evoked responses of trigeminothalamic neurons. J. Neurophysiol. 1996, 76, 2093–2096.
- Xiao, Y.; Richter, J.A.; Hurley, J.H. Release of glutamate and CGRP from trigeminal ganglion neurons: Role of calcium channels and 5-HT1 receptor signaling. Mol. Pain 2008, 4, 12.
- Mészáros, M.; Phan, T.H.M.; Vigh, J.P.; Porkoláb, G.; Kocsis, A.; Páli, E.K.; Polgár, T.F.; Walter, F.R.; Bolognin, S.; Schwamborn, J.C.; et al. Targeting Human Endothelial Cells with Glutathione and Alanine Increases the Crossing of a Polypeptide Nanocarrier through a Blood-Brain Barrier Model and Entry to Human Brain Organoids. Cells 2023, 12, 503.
- Paoletti, P.; Ascher, P.; Neyton, J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J. Neurosci. 1997, 17, 5711–5725.
- Faerber, L.; Drechsler, S.; Ladenburger, S.; Gschaidmeier, H.; Fischer, W. The neuronal 5-HT3 receptor network after 20 years of research—Evolving concepts in management of pain and inflammation. Eur. J. Pharmacol. 2007, 560, 1–8.
- Shatillo, A.; Salo, R.A.; Giniatullin, R.; Gröhn, O.H. Involvement of NMDA receptor subtypes in cortical spreading depression in rats assessed by fMRI. Neuropharmacology 2015, 93, 164–170.
- Lauritzen, M. Pathophysiology of the migraine aura. The spreading depression theory. Brain 1994, 117 Pt 1, 199–210.
- Lauritzen, M.; Hansen, A.J. The effect of glutamate receptor blockade on anoxic depolarization and cortical spreading depression. J. Cereb. Blood Flow Metab. 1992, 12, 223–229.
- Peeters, M.; Gunthorpe, M.J.; Strijbos, P.J.; Goldsmith, P.; Upton, N.; James, M.F. Effects of pan- and subtype-selective N-methyl-D-aspartate receptor antagonists on cortical spreading depression in the rat: Therapeutic potential for migraine. J. Pharmacol. Exp. Ther. 2007, 321, 564–572.
- Faria, L.C.; Mody, I. Protective effect of ifenprodil against spreading depression in the mouse entorhinal cortex. J. Neurophysiol. 2004, 92, 2610–2614.
- Charles, A.; Flippen, C.; Romero Reyes, M.; Brennan, K.C. Memantine for prevention of migraine: A retrospective study of 60 cases. J. Headache Pain 2007, 8, 248–250.
- Dolati, S.; Rikhtegar, R.; Mehdizadeh, A.; Yousefi, M. The Role of Magnesium in Pathophysiology and Migraine Treatment. Biol. Trace Elem. Res. 2020, 196, 375–383.
- Baratloo, A.; Mirbaha, S.; Delavar Kasmaei, H.; Payandemehr, P.; Elmaraezy, A.; Negida, A. Intravenous caffeine citrate vs. magnesium sulfate for reducing pain in patients with acute migraine headache; a prospective quasi-experimental study. Korean J. Pain 2017, 30, 176–182.
- Shin, H.J.; Na, H.S.; Do, S.H. Magnesium and Pain. Nutrients 2020, 12, 2184.
- Kaube, H.; Herzog, J.; Kaufer, T.; Dichgans, M.; Diener, H.C. Aura in some patients with familial hemiplegic migraine can be stopped by intranasal ketamine. Neurology 2000, 55, 139–141.
- Lauritsen, C.; Mazuera, S.; Lipton, R.B.; Ashina, S. Intravenous ketamine for subacute treatment of refractory chronic migraine: A case series. J. Headache Pain 2016, 17, 106.
- Gomez-Mancilla, B.; Brand, R.; Jurgens, T.P.; Göbel, H.; Sommer, C.; Straube, A.; Evers, S.; Sommer, M.; Campos, V.; O Kalkman, H.; et al. Randomized, multicenter trial to assess the efficacy, safety and tolerability of a single dose of a novel AMPA receptor antagonist BGG492 for the treatment of acute migraine attacks. Cephalalgia 2014, 34, 103–113.
- Bigal, M.; Rapoport, A.; Sheftell, F.; Tepper, D.; Tepper, S. Memantine in the preventive treatment of refractory migraine. Headache 2008, 48, 1337–1342.
- Noruzzadeh, R.; Modabbernia, A.; Aghamollaii, V.; Ghaffarpour, M.; Harirchian, M.H.; Salahi, S.; Nikbakht, N.; Noruzi, N.; Tafakhori, A. Memantine for Prophylactic Treatment of Migraine Without Aura: A Randomized Double-Blind Placebo-Controlled Study. Headache 2016, 56, 95–103.
- Afridi, S.K.; Giffin, N.J.; Kaube, H.; Goadsby, P. A randomized controlled trial of intranasal ketamine in migraine with prolonged aura. Neurology 2013, 80, 642–647.
- Etchison, A.R.; Bos, L.; Ray, M.; McAllister, K.B.; Mohammed, M.; Park, B.; Phan, A.V.; Heitz, C. Low-dose Ketamine Does Not Improve Migraine in the Emergency Department: A Randomized Placebo-controlled Trial. West J. Emerg. Med. 2018, 19, 952–960.
- Nicolodi, M.; Sicuteri, F. Exploration of NMDA receptors in migraine: Therapeutic and theoretic implications. Int. J. Clin. Pharmacol. Res. 1995, 15, 181–189.
- Oskoui, M.; Pringsheim, T.; Holler-Managan, Y.; Potrebic, S.; Billinghurst, L.; Gloss, D.; Hershey, A.D.; Licking, N.; Sowell, M.; Victorio, M.C.; et al. Practice guideline update summary: Acute treatment of migraine in children and adolescents: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2019, 93, 487–499.
- Ailani, J.; Burch, R.C.; Robbins, M.S. The American Headache Society Consensus Statement: Update on integrating new migraine treatments into clinical practice. Headache J. Head Face Pain 2021, 61, 1021–1039.
- Antonaci, F.; Dumitrache, C.; De Cillis, I.; Allena, M. A review of current European treatment guidelines for migraine. J. Headache Pain 2010, 11, 13–19.
- AHS-First-Contact-PreventativeTreatment; American Headache Society: Mount Royal, NJ, USA, 2021.
This entry is offline, you can click here to edit this entry!
