Cortisol is a steroid hormone that is involved in a broad range of physiological processes in human/animal organisms. Cortisol levels in biological samples is a valuable biomarker -e.g., of stress and stress-related diseases; thus, cortisol determination in biological fluids, such as serum, saliva, and urine, is of great clinical value. Although cortisol analysis can be performed with chromatography-based analytical techniques, such as liquid chromatography – tandem mass spectrometry (LC-MS/MS), conventional immunoassays (radioimmunoassays (RIAs), enzyme-linked immunosorbent assays (ELISAs), etc.) are considered the “gold standard” analytical methodology for cortisol, due to their high sensitivity along with a series of practical advantages, such as low-cost instrumentation, fast and easy to perform assay-protocol, and high sample throughput. Especially in the last decades, research efforts have pointed at the replacement of conventional immunoassays by cortisol immunosensors, which may offer further improvements in the field, such as real-time analysis at the point-of-care (e.g., continuous cortisol monitoring in sweat through wearable electrochemical sensors).
- biological samples
- blood plasma/serum
- cortisol
- electrochemical immunosensors
- biomarkers
- optical immunosensors
- saliva
- sweat
1. Electrochemical Cortisol Immunosensors
The first cortisol immunosensors, reported in the mid-1990s [1,2] as already mentioned, were electrochemical. In the meantime, many more electrochemical immunosensors for cortisol have been described in the literature, while some recent review articles have provided accumulative information on cortisol electrochemical sensors [3,4].
During the last decade, various wearable electrochemical sensing platforms, which in general are considered ideal for analyzing sweat samples [5], have been developed and applied to the immunodetection of cortisol in sweat, as critically presented in previous review papers [6,7]. Moreover, several electrochemical cortisol immunosensors for sweat analysis have been described in the literature during the last couple of years (2020-2022) [8][9][10][11][12][13]. Tear analysis by means of electrochemical cortisol immunosensing has also been reported [14].
In Table 1, we present most of the articles published in the last decades regarding the development of electrochemical immunosensors for the detection of cortisol in a variety of samples, ranging from plain buffer solutions [1][15][16], to complex biological specimens. As shown (Table 1), electrochemical cortisol immunosensors have been mainly applied to the analysis of saliva samples [17][18][19][20][21][22][23][24][25][26][27][28][29][30][31], and, to a less extent, blood plasma or serum [32][33][34][35][36][37][38], as well as interstitial fluid [31][39], buffer solutions of rat adrenal gland acute slices [26] and zebra-fish whole body [19]. Early developed electrochemical immunosensors applied to detect cortisol in vivo, in dialysates of the extracellular fluid of animal brain (amygdala region) or in dialysates of circulating blood in conscious animals, are also listed in Table 1 [2][40]. Moreover, as above mentioned, cortisol electrochemical immunosensors have been employed to analyze human/artificial sweat samples [8][9][10][11][24][41][42][43][44]. The underlying immunoassay principle and the electrochemical detection/signal principle of each sensor have been included in Table 1, while cortisol concentrations corresponding to the working range and/or limit of detection (LOD) have been listed as well.
| Immunoassay Principle | Signal Transduction Principle |
Biological Sample | Range/LoD | Reference |
|---|---|---|---|---|
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab 1, immobilized onto gold microelectrodes) |
Cyclic voltammetry | Buffer | 10 pM–500 nM/ 1 pM |
[15] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab, immobilized onto silver/silver oxide (Ag/AgO)—polyaniline nanocomposites) |
Cyclic voltammetry | Buffer | 1 pM–1 μM 0.64 pM |
[16] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab co-immobilized with HRP on the sensor electrode) |
Amperometry | Buffer | 10−7–10−5 M | [1] |
| Competitive (Competition between free cortisol and a BSA-cortisol conjugate immobilized onto magnetic beads for binding to an anti-cortisol Ab labeled with silver nanoclusters (AgNCs) |
Photoelectrochemistry | Saliva | 0.0001–100 ng/mL/ 0.06 pg/mL |
[18] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab immobilized on polyaniline-modified graphene electrodes) |
Electrochemical impedance spectroscopy | Saliva (canine) | 0.0005–50 μg/mL/ 3.57 fg/mL |
[17] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab labeled with ferrocene-tags and immobilized on a modified tin-doped indium oxide electrode) |
Cyclic voltammetry; square wave voltammetry | artificial saliva and zebrafish whole-body | 0.001–50 ng/mL/ 1.03 pg/mL |
[19] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab immobilized on a AuNP/MoS2/AuNP–modified screen-printed electrode) |
Differential pulse voltammetry | Saliva | 0.5–200 nM/ 0.11 nM |
[20] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab covalently immobilized on NiO thin film/ITO 2 electrodes) |
Cyclic voltammetry, Differential pulse voltammetry |
Saliva | 1 pg/mL–10 μg/mL/ 0.32 pg/mL |
[21] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab covalently immobilized onto micro-Au electrodes) |
Electrochemical impedance spectroscopy | Saliva | 1 pg/mL–10 ng/mL/ 0.87 ± 0.12 pg/mL |
[22] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab co-immobilized with BSA on glassy carbon electrodes that had been coated with tin disulfide nanoflakes) |
Cyclic voltammetry, differential pulse voltammetry | Saliva | 100 pM–100 μM/ 100 pM |
[23] |
| Competitive (Competition between an ALP-labeled cortisol conjugate and free cortisol for binding to an anti-cortisol Ab indirectly immobilized on disposable graphite screen-printed electrodes) |
Square wave voltammetry | Saliva | 0.5–55.1 ng/mL/ 1.7 ng/mL |
[25] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab immobilized on electroreduced graphene oxide deposited on screen-printed electrodes) |
Electrochemical chronoamperometry | Saliva, sweat | 0.1 ng/mL | [24] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab covalently immobilized on reduced graphene oxide channels between two planar electrodes) |
Resistance | Human saliva and buffer solution of rat adrenal gland acute slices | 10 pg/mL | [26] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab immobilized on Au-substrates modified with ZnO nanostructures (1D nanorods, 2D nanoflakes)) |
Cyclic voltammetry | Saliva | 1 pM | [27] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab covalently immobilized on microfabricated interdigitated microelectrodes) |
Cyclic voltammetry | Saliva | 10 pg/mL–100 ng/mL/ 10 pg/mL |
[28] |
| Competitive (Competition between a GOD 3–cortisol conjugate and free cortisol for binding to an anti-cortisol Ab immobilized on platinum electrodes; lateral and vertical fluid control mechanisms were integrated in the sensor) |
Amperometry | Saliva | 0.1–10 ng/mL | [29] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab immobilized on gold microelectrode arrays) |
Electrochemical impedance spectroscopy | Saliva and interstitial fluid | 1 pM–100 nM | [31] |
| Competitive (Competition between free cortisol and a cortisol analog covalently immobilized on single-walled carbon nanotubes and free cortisol for binding to an anti-cortisol Ab) |
Resistance/conductance | Saliva | 1 pg/mL–10 ng/mL/ 1 pg/mL |
[30] |
| Competitive (Competition between cortisol immobilized on naflon pretreated glassy carbon electrodes and free cortisol for binding to a biotinylated anti-cortisol Ab; detection was performed via reaction with HRP–streptavidin) |
Electrochemical impedance spectroscopy, cyclic voltammetry | Plasma | 0.1–1000 ng/mL/ 0.05 ng/mL |
[33] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab co-immobilized with GOD on gold electrodes) |
Amperometry | Plasma (fish) |
1.25–200 ng/mL | [34] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab, immobilized on interdigitated gold microelectrodes) |
Cyclic voltammetry | Plasma | 10 pg/mL–500 ng/mL/ 10 pg/mL |
[35] |
| Competitive (Competition between an ALP-labeled cortisol conjugate and free cortisol for binding to an anti-cortisol Ab covalently immobilized on gold electrodes. |
Amperometry | Serum | 0–250 ng/mL/ 13.4 ng/mL |
[32] |
| Competitive (Competition between an ALP-labeled cortisol conjugate and free cortisol for binding to an anti-cortisol Ab immobilized through protein A on magnetic particles; the immunocomplexes formed were trapped on the surface of screen-printed electrodes with a small magnet and ALP activity was monitored) |
Differential pulse voltammetry | Serum | 5 × 10−3–150 ng/mL/ 3.5 pg/mL |
[37] |
| Competitive (Competition between an HRP-labeled cortisol conjugate and free cortisol for binding to an anti-cortisol Ab immobilized on gold electrodes functionalized with a AuNP–protein G–DTBP 4 scaffold) |
Square wave voltammetry | Buffer, Serum | 50–2,500 pg/mL/ 16 pg/mL |
[36] |
| Noncompetitive (Direct binding of cortisol and cortisone (transformed into cortisol via the enzyme 3α-hydroxysteroid dehydrogenase) to an anti-cortisol Ab immobilized on gold nanowires/working electrodes) |
Square wave voltammetry | Buffer, Serum | 10–80 μM | [38] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab immobilized on L-cys 5–AuNPs–MXene-modified electrodes) |
Amperometry | Artificial Sweat | 5–180 ng/mL/ 0.54 ng/mL |
[8] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab immobilized on the surface of flexible screen-printed electrodes coated with AuNPs) |
Differential pulse voltammetry | Sweat | 7.47 nM | [9] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab, immobilized on a flexible electrode prepared on polydimethylsiloxane modified with multiwalled carbon nanotubes and AuNPs) |
Cyclic voltammetry, differential pulse voltammetry | Sweat | 1 fg/mL–1 μg/mL/ 0.3 fg/mL |
[10] |
| Competitive (Competition between an HRP–cortisol conjugate and free cortisol for binding to an anti-cortisol antibody immobilized on graphene-based electrode) |
Amperometry | Sweat | 0.43–50.2 ng/mL | [11] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol antibody immobilized on a conductive carbon yarn functionalized with ellipsoidal Fe2O3 particles) |
Cyclic voltammetry | Sweat | 1 fg/mL–1 μg/mL/ 0.005 fg/mL |
[41] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab immobilized on MoS2 sheets integrated into a nanoporous flexible electrode system) |
Electrochemical impedance spectroscopy | Sweat | 1–500 ng/mL/ 1 ng/mL |
[42] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab immobilized on ZnO thin film deposited on a flexible nanoporous polyamide membrane; room temperature ionic liquids were employed to enhance sensor stability) |
Electrochemical impedance spectroscopy | Sweat | 10–200 ng/mL/ 10 ng/mL |
[43] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab immobilized on a ZnO thin film deposited on a flexible nanoporous polyamide membrane) |
Electrochemical impedance spectroscopy | Sweat | 10–200 ng/mL/ 1 ng/mL |
[44] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab, covalently immobilized on a gold microelectrode array) |
Electrochemical impedance spectroscopy | Interstitial fluid | 1 pM–100 nM | [39] |
| Competitive (Competition between free cortisol and an HRP–cortisol conjugate for binding to an anti-cortisol antibody immobilized on a platinum electrode) |
Amperometry | Dialysates of extracellular fluid of animal brain, amygdala region (sheep) |
0–100 ng/mL (in vitro measurement) |
[40] |
| Competitive (Competition between HRP–cortisol conjugate and free cortisol for binding to an anti-cortisol antibody immobilized on platinum electrodes) |
Potentiometry | Dialysates of animal circulating blood (sheep, cattle, rat) | 0.3 μg/100 mL | [2] |
2. Optical Cortisol Immunosensors
The first optical cortisol immunosensors were developed in the late 2000s. These sensors, which have been based on the surface plasmon resonance (SPR) detection principle [45][46][47] and applied to the detection of salivary cortisol, are mentioned in a recent review article concerning analysis of saliva as an ideal “health mirror” sample [48]. Since the late 2000s, several other optical cortisol immunosensors have been described in the literature.
In Table 2, we present most of the articles published in the last fifteen years regarding the development of optical immunosensors for detecting cortisol in a variety of matrices, from plain buffer solutions [49][50][51] to complex biological specimens. As shown (Table 2), complex biological samples analyzed for cortisol with optical immunosensors include mainly saliva [46][47][52][53][54][55][56][57][58] as well as blood plasma/serum [59][60] and urine [46].
| Immunoassay Principle | Signal Transduction Principle |
Biological Sample | Range/LoD | Reference |
|---|---|---|---|---|
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab immobilized on a D-shaped, gold-coated silica optical fiber) |
Surface plasmon resonance (SPR) | Buffer | 0.01–100 ng/mL/ 1.46 ng/mL |
[49] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab immobilized on a plastic optical fiber coated with gold–palladium allοy) |
SPR | Buffer | 1 pg/mL | [50] |
| Competitive (Competition between a fluorescently labeled BSA–cortisol conjugate and free cortisol for binding to an anti-cortisol Ab immobilized on glass substrate coated with gold) |
Metal-enhanced fluorescence (MEF) | Buffer | 0.02 μg/mL | [51] |
| Noncompetitive (Direct binding between of cortisol to an anti-cortisol Ab or aptamer, immobilized on quantum dots) |
Fluorescence quenching | Saliva | 1 nM (aptamer-based) 100 pM (Ab-based) |
[52] |
| Lateral flow–type Competitive (Competition between a BSA–cortisol conjugate immobilized on the strip and free cortisol for binding to a Cy3-labeled anti-cortisol Ab) |
Fluorescence (detected with a smartphone-linked reader) |
Saliva | 0.1 ng/mL | [53] |
| Competitive (Competition between an HRP-labeled cortisol conjugate and free cortisol for binding to an anti-cortisol Ab indirectly immobilized on PDMS microfluidic channel) |
Colorimetry | Saliva | 0.01–20 ng/mL/ 18 pg/mL |
[54] |
| Lateral flow–type Competitive (Competition between an HRP-labeled cortisol conjugate and free cortisol for binding to an anti-cortisol Ab immobilized on the strip) |
Chemiluminescence (detected through a smartphone camera) |
Saliva | 0.3–60 ng/mL/ 0.3 ng/mL |
[55] |
| Lateral flow–type Competitive (Based on europium fluorescent particle conjugates) |
Fluorescence (Detected with a cassette reader transferring results through a Bluetooth device, manufactured by Oasis Diagnostics) |
Saliva | 0.91 ng/mL | [56] |
| Competitive (Competition between a cortisol analogue, hydrocortisone 3-(O-carboxymethyl)oxime, covalently immobilized on gold surface and free cortisol for binding to an anti-cortisol Ab) |
SPR | Saliva | 10 ppt–100 ppb/ 38 ppt |
[57] |
| Competitive (Competition between a BSA–cortisol conjugate immobilized on a disposable disk chip and free cortisol for binding to an ALP-labeled anti-cortisol Ab) |
Chemiluminescence | Saliva | 0.4–11.3 ng/mL | [58] |
| Competitive (Competition between an in-house prepared cortisol conjugate immobilized on a gold sensor surface and free cortisol for binding to the anti-cortisol Ab; a secondary Ab was used for signal increase) |
SPR | Saliva | 91–934 pg/mL/ 49 pg/mL |
[47] |
| Noncompetitive (Direct binding of cortisol to an anti-cortisol Ab covalently immobilized on the polycarboxylate hydrogel–coated sensing surface) |
SPR | Saliva, urine | 3 μg/L | [46] |
| Competitive (Competition between a BSA–cortisol conjugate immobilized on the SPR-sensor surface and free cortisol for binding to a monoclonal anti-cortisol Ab) |
SPR | Saliva, buffer | 1.0 ng/mL | [45] |
| Competitive (Competition between cortisol analogues, i.e., suitably prepared cortisol–ssDNA conjugates, and free cortisol for binding to a biotinylated anti-cortisol Ab immobilized on streptavidin-coated particles) |
Particle mobility (detected through dark field microscopy) | Blood plasma (filtered or microdialysis-sampled) | High nM–low μM | [59] |
| Competitive (Competition between a BSA–cortisol conjugate immobilized on paper and free cortisol for binding to gold nanoparticles loaded with the anti-cortisol Ab) |
Color | Blood serum | 21.5 μg/dL | [60] |
3. Conclusions – Future Perspectives
Cortisol homeostasis is essential for human health, while abnormal cortisol levels have been correlated to and may serve as a valuable biomarker for several disease-states. Thus, it is important to monitor cortisol concentrations in various biological samples by means of proper analytical methods. Cortisol immunoassays are currently considered the analytical method of choice for determining cortisol in biological specimens, such as blood serum/plasma, urine, saliva, or, more recently, hair. Transformation of the conventional immunoassays to technologically advanced antibody-based assays, which can easily be accomplished in a short time by non-skilled persons and are capable of being “integrated” into portable devices for point-of-care measurements, has led to the development of several cortisol immunosensors during the last three decades. Cortisol immunosensors can be divided according to the signal transduction principle they rely upon. Most of cortisol immunosensors are electrochemical and rely on signal measurement through cyclic voltammetry, impedance spectrometry, and amperometry. On the other hand, several optical immunosensors, including flow lateral-type strip sensors, have been developed, especially during the last decade; besides the usually measured optical signals, e.g., SPR signals, some of the most recently reported cortisol immunosensors [59] rely on the measurement of other parameters, e.g., on particle mobility monitoring with the aid of a microscope [61].
Some immunosensors could simultaneously detect cortisol along another biomarker, e.g., insulin [32], lactate [24] or IL-6 [43]; this “multi-analyte” approach, although technologically difficult to achieve and therefore rarely reported, is highly desirable from a clinical point of view. Thus, a dual electrochemical immunosensor proposed for the simultaneous detection of cortisol and insulin at the point-of-care [32] may eventually offer improved management of diabetes.
A great number of cortisol immunosensors have been applied to the analysis of saliva samples and less to urine or blood plasma/serum, while too little information concerning immunosensors for hair cortisol is currently available, at least to our knowledge. This tendency might be attributed, at least partly, to special requirements for the collection and/or treatment of the relevant samples: e.g., the requirement of 24 h-urine collection renders urine samples weak candidates for real-time detection of cortisol through a point-of-care immunosensor device [7]. Other factors supporting this trend may include matrix complexity, need for careful pH adjustment before sample analysis, etc. On the other hand, a special group of electrochemical immunosensors (e.g., miniaturized, and flexible/wearable sensors that are based on new materials, such as two-dimensional nanosheets of MoS2 [42]) have allowed real time and even continuous monitoring of cortisol in sweat. As reported, free (protein-unbound) cortisol seems to be present in sweat glands, through a mechanism resembling transportation of free cortisol by the bloodstream to the salivary glands. From sweat glands, cortisol is thought to reach sweat by passive transportation through the cell lipid-bilayer membrane [62]. Sweat cortisol concentrations have been reported to range from 8 to 142 ng/mL [3][6], the highest levels being found in the morning and correlating with salivary levels [62]. So far, sweat cortisol has not been detected with conventional methods -at least, not routinely, possibly due to difficulties in collecting and properly storing the corresponding samples for subsequent laboratory analysis.
At present, routine analysis of biological samples for cortisol monitoring is only performed in lab-settings [63]. Further research efforts are needed before cortisol immunosensors have become fully commercialized and available to clinical and self-monitoring applications. A first challenge of such efforts would be to perform large validation studies, in order to ensure that the analytical characteristics of the immunosensors developed are of high quality. Although there is always space for improvement, sensitivity/LOD is usually not a problem, while specificity and/or simplicity of production and cost-related issues associated with the anti-cortisol antibodies, which are inherent in all immunochemical analytical techniques, might be addressed by antibodies’ replacement with aptamers [52][64] or molecularly imprinted polymers (MIPs) [65]. However, special attention should be paid on the validation of repeatability/reproducibility [66] as well as operational stability, especially when complex biological samples, such as blood plasma/serum are to be analyzed (which may, e.g., affect integrity and deteriorate functioning of electrodes in electrochemical immunosensors); conditions of reusability as well as storage stability/durability/life span are also issues to be studied. Wearable immunosensors for detection of cortisol mainly in sweat samples seem to provide exciting prospects for further progress in the field, but particular aspects have to be addressed in the years to come [67]. These aspects include accurate sample collection, potential toxicity and biocompatibility of sensor materials, and appropriate power supply of the flexible electronics these sensors require, while data processing and communication constitute a separate research field, which may be further elaborated and improved. Moreover, thorough knowledge of the biological and chemical characteristics of the samples to be analyzed along with a deep insight in cortisol physiology and partitioning/kinetics/dynamics of the hormone in different compartments / fluids of the organism in normal and disease states would nicely supplement research efforts in the field (Figure 1) and relevant studies may be parallel performed. To achieve this, close collaboration among clinicians, physicists, chemists, bio scientists and engineers is a prerequisite.
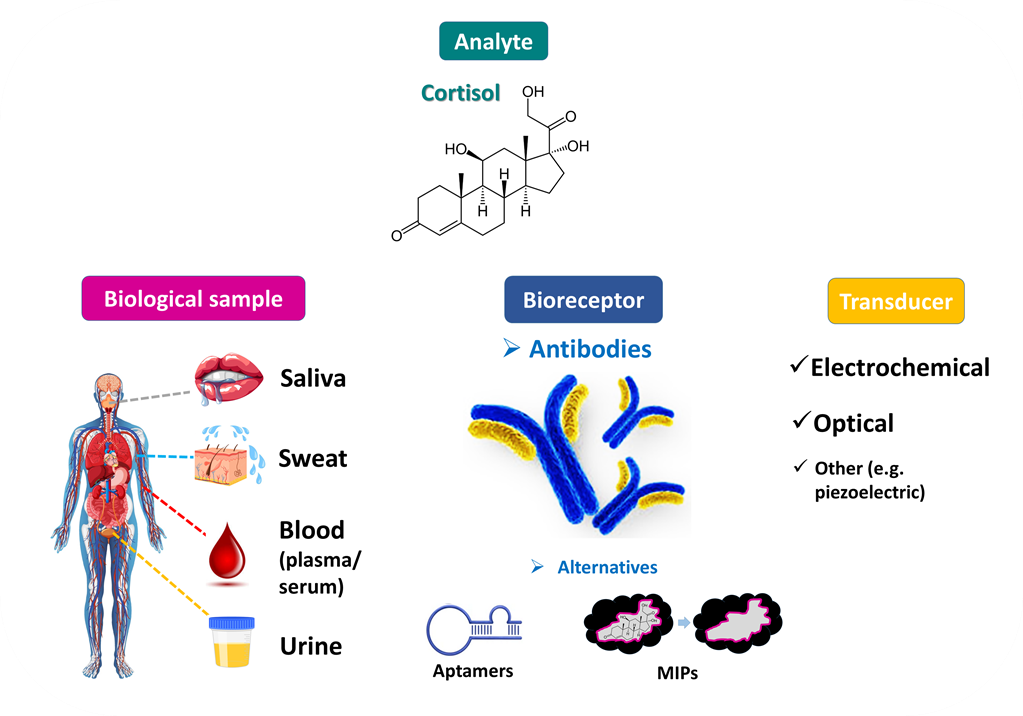
Figure 1. Cortisol has been detected in various biological fluids and cortisol levels may serve as a valuable biomarker (e.g., of stress). Cortisol determination has been achieved mainly with analytical methods based on specific cortisol-binders, and especially anti-cortisol antibodies. Cortisol immunosensors based on different signal transduction principles are expected to be eventually commercialized and serve as an easy-to-handle, reliable tool for the point-of-care clinical analysis of cortisol.
References
- Xu, Y.; Suleiman, A.A. Reusable Amperometric Immunosensor for the Determination of Cortisol. Analytical Letters 1997, 30, 2675-2689, doi:10.1080/00032719708001813.
- Cook, C.J. Real-time measurements of corticosteroids in conscious animals using an antibody-based electrode. Nat Biotechnol 1997, 15, 467-471, doi:10.1038/nbt0597-467.
- Zea, M.; Bellagambi, F.G.; Ben Halima, H.; Zine, N.; Jaffrezic-Renault, N.; Villa, R.; Gabriel, G.; Errachid, A. Electrochemical sensors for cortisol detections: Almost there. TrAC Trends in Analytical Chemistry 2020, 132, 116058, doi:https://doi.org/10.1016/j.trac.2020.116058.
- Falk, M.; Psotta, C.; Cirovic, S.; Shleev, S. Non-Invasive Electrochemical Biosensors Operating in Human Physiological Fluids. Sensors (Basel) 2020, 20, doi:10.3390/s20216352.
- Chung, M.; Fortunato, G.; Radacsi, N. Wearable flexible sweat sensors for healthcare monitoring: A review. Journal of the Royal Society, Interface 2019, 16, 20190217, doi:10.1098/rsif.2019.0217.
- Madhu, S.; Ramasamy, S.; Sekhar, P.; Bhansali, S.; Nagamony, P.; Manickam, P.; Viswanathan, C. Review—Towards Wearable Sensor Platforms for the Electrochemical Detection of Cortisol. Journal of The Electrochemical Society 2020, 167, 067508, doi:10.1149/1945-7111/ab7e24.
- Kaushik, A.; Vasudev, A.; Arya, S.K.; Pasha, S.K.; Bhansali, S. Recent advances in cortisol sensing technologies for point-of-care application. Biosens Bioelectron 2014, 53, 499-512, doi:10.1016/j.bios.2013.09.060.
- Laochai, T.; Yukird, J.; Promphet, N.; Qin, J.; Chailapakul, O.; Rodthongkum, N. Non-invasive electrochemical immunosensor for sweat cortisol based on L-cys/AuNPs/ MXene modified thread electrode. Biosensors and Bioelectronics 2022, 203, 114039, doi:https://doi.org/10.1016/j.bios.2022.114039.
- Cheng, C.; Li, X.; Xu, G.; Lu, Y.; Low, S.S.; Liu, G.; Zhu, L.; Li, C.; Liu, Q. Battery-free, wireless, and flexible electrochemical patch for in situ analysis of sweat cortisol via near field communication. Biosens Bioelectron 2021, 172, 112782, doi:10.1016/j.bios.2020.112782.
- Liu, Q.; Shi, W.; Tian, L.; Su, M.; Jiang, M.; Li, J.; Gu, H.; Yu, C. Preparation of nanostructured PDMS film as flexible immunosensor for cortisol analysis in human sweat. Anal Chim Acta 2021, 1184, 339010, doi:10.1016/j.aca.2021.339010.
- Torrente-Rodríguez, R.M.; Tu, J.; Yang, Y.; Min, J.; Wang, M.; Song, Y.; Yu, Y.; Xu, C.; Ye, C.; IsHak, W.W.; et al. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mHealth system. Matter 2020, 2, 921-937, doi:10.1016/j.matt.2020.01.021.
- Bian, L.; Shao, W.; Liu, Z.; Zeng, Z.; Star, A. Detection of Stress Hormone with Semiconducting Single-Walled Carbon Nanotube-Based Field-Effect Transistors. Journal of The Electrochemical Society 2022, 169, 057519, doi:10.1149/1945-7111/ac6e8d.
- Madhu, S.; Anthuuvan, A.J.; Ramasamy, S.; Manickam, P.; Bhansali, S.; Nagamony, P.; Chinnuswamy, V. ZnO Nanorod Integrated Flexible Carbon Fibers for Sweat Cortisol Detection. ACS Applied Electronic Materials 2020, 2, 499-509, doi:10.1021/acsaelm.9b00730.
- Cardinell, B.A.; Spano, M.L.; La Belle, J.T. Toward a Label-Free Electrochemical Impedance Immunosensor Design for Quantifying Cortisol in Tears. Crit Rev Biomed Eng 2019, 47, 207-215, doi:10.1615/CritRevBiomedEng.2019026109.
- Cruz, A.F.; Norena, N.; Kaushik, A.; Bhansali, S. A low-cost miniaturized potentiostat for point-of-care diagnosis. Biosens Bioelectron 2014, 62, 249-254, doi:10.1016/j.bios.2014.06.053.
- Kaushik, A.; Vasudev, A.; Arya, S.K.; Bhansali, S. Mediator and label free estimation of stress biomarker using electrophoretically deposited Ag@AgO-polyaniline hybrid nanocomposite. Biosens Bioelectron 2013, 50, 35-41, doi:10.1016/j.bios.2013.06.012.
- Perkins, H.; Higgins, M.; Marcato, M.; Galvin, P.; Teixeira, S.R. Immunosensor for Assessing the Welfare of Trainee Guide Dogs. Biosensors (Basel) 2021, 11, doi:10.3390/bios11090327.
- Luo, D.; Fu, Q.; Gao, R.; Su, L.; Su, Y.; Liu, B. Signal-on photoelectrochemical immunoassay for salivary cortisol based on silver nanoclusters-triggered ion-exchange reaction with CdS quantum dots. Anal Bioanal Chem 2022, 414, 3033-3042, doi:10.1007/s00216-022-03893-z.
- Abdulsattar, J.O.; Greenway, G.M.; Wadhawan, J.D. Electrochemical immunoassay for the detection of stress biomarkers. Heliyon 2020, 6, e03558, doi:10.1016/j.heliyon.2020.e03558.
- Liu, J.; Xu, N.; Men, H.; Li, S.; Lu, Y.; Low, S.S.; Li, X.; Zhu, L.; Cheng, C.; Xu, G.; et al. Salivary Cortisol Determination on Smartphone-Based Differential Pulse Voltammetry System. Sensors (Basel) 2020, 20, doi:10.3390/s20051422.
- Dhull, N.; Kaur, G.; Gupta, V.; Tomar, M. Highly sensitive and non-invasive electrochemical immunosensor for salivary cortisol detection. Sensors and Actuators B: Chemical 2019, 293, 281-288, doi:https://doi.org/10.1016/j.snb.2019.05.020.
- Khan, M.S.; Dighe, K.; Wang, Z.; Srivastava, I.; Schwartz-Duval, A.S.; Misra, S.K.; Pan, D. Electrochemical-digital immunosensor with enhanced sensitivity for detecting human salivary glucocorticoid hormone. Analyst 2019, 144, 1448-1457, doi:10.1039/C8AN02085J.
- Liu, X.; Hsu, S.P.C.; Liu, W.C.; Wang, Y.M.; Liu, X.; Lo, C.S.; Lin, Y.C.; Nabilla, S.C.; Li, Z.; Hong, Y.; et al. Salivary Electrochemical Cortisol Biosensor Based on Tin Disulfide Nanoflakes. Nanoscale Res Lett 2019, 14, 189, doi:10.1186/s11671-019-3012-0.
- Tuteja, S.K.; Ormsby, C.; Neethirajan, S. Noninvasive Label-Free Detection of Cortisol and Lactate Using Graphene Embedded Screen-Printed Electrode. Nanomicro Lett 2018, 10, 41, doi:10.1007/s40820-018-0193-5.
- Kämäräinen, S.; Mäki, M.; Tolonen, T.; Palleschi, G.; Virtanen, V.; Micheli, L.; Sesay, A.M. Disposable electrochemical immunosensor for cortisol determination in human saliva. Talanta 2018, 188, 50-57, doi:10.1016/j.talanta.2018.05.039.
- Kim, Y.H.; Lee, K.; Jung, H.; Kang, H.K.; Jo, J.; Park, I.K.; Lee, H.H. Direct immune-detection of cortisol by chemiresistor graphene oxide sensor. Biosens Bioelectron 2017, 98, 473-477, doi:10.1016/j.bios.2017.07.017.
- Vabbina, P.K.; Kaushik, A.; Pokhrel, N.; Bhansali, S.; Pala, N. Electrochemical cortisol immunosensors based on sonochemically synthesized zinc oxide 1D nanorods and 2D nanoflakes. Biosens Bioelectron 2015, 63, 124-130, doi:10.1016/j.bios.2014.07.026.
- Pasha, S.K.; Kaushik, A.; Vasudev, A.; Snipes, S.A.; Bhansali, S. Electrochemical Immunosensing of Saliva Cortisol. Journal of The Electrochemical Society 2014, 161, B3077, doi:10.1149/2.017402jes.
- Yamaguchi, M.; Matsuda, Y.; Sasaki, S.; Sasaki, M.; Kadoma, Y.; Imai, Y.; Niwa, D.; Shetty, V. Immunosensor with fluid control mechanism for salivary cortisol analysis. Biosens Bioelectron 2013, 41, 186-191, doi:10.1016/j.bios.2012.08.016.
- Tlili, C.; Myung, N.V.; Shetty, V.; Mulchandani, A. Label-free, chemiresistor immunosensor for stress biomarker cortisol in saliva. Biosens Bioelectron 2011, 26, 4382-4386, doi:10.1016/j.bios.2011.04.045.
- Venugopal, M.; Arya, S.K.; Chornokur, G.; Bhansali, S. A Realtime and Continuous Assessment of Cortisol in ISF Using Electrochemical Impedance Spectroscopy. Sens Actuators A Phys 2011, 172, 154-160, 32. doi:10.1016/j.sna.2011.04.028.
- Vargas, E.; Povedano, E.; Krishnan, S.; Teymourian, H.; Tehrani, F.; Campuzano, S.; Dassau, E.; Wang, J. Simultaneous cortisol/insulin microchip detection using dual enzyme tagging. Biosens Bioelectron 2020, 167, 112512, doi:10.1016/j.bios.2020.112512.
- Sun, B.; Gou, Y.; Ma, Y.; Zheng, X.; Bai, R.; Ahmed Abdelmoaty, A.A.; Hu, F. Investigate electrochemical immunosensor of cortisol based on gold nanoparticles/magnetic functionalized reduced graphene oxide. Biosens Bioelectron 2017, 88, 55-62, doi:10.1016/j.bios.2016.07.047.
- Wu, H.; Ohnuki, H.; Ota, S.; Murata, M.; Yoshiura, Y.; Endo, H. New approach for monitoring fish stress: A novel enzyme-functionalized label-free immunosensor system for detecting cortisol levels in fish. Biosens Bioelectron 2017, 93, 57-64, doi:10.1016/j.bios.2016.10.001.
- Kaushik, A.; Yndart, A.; Jayant, R.D.; Sagar, V.; Atluri, V.; Bhansali, S.; Nair, M. Electrochemical sensing method for point-of-care cortisol detection in human immunodeficiency virus-infected patients. Int J Nanomedicine 2015, 10, 677-685, doi:10.2147/ijn.s75514.
- Liu, X.; Zhao, R.; Mao, W.; Feng, H.; Liu, X.; Wong, D.K.Y. Detection of cortisol at a gold nanoparticle|Protein G–DTBP-scaffold modified electrochemical immunosensor. Analyst 2011, 136, 5204-5210, doi:10.1039/C1AN15411G.
- Moreno-Guzmán, M.; Eguílaz, M.; Campuzano, S.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Disposable immunosensor for cortisol using functionalized magnetic particles. Analyst 2010, 135, 1926-1933, doi:10.1039/c0an00206b.
- Kumar, A.; Aravamudhan, S.; Gordic, M.; Bhansali, S.; Mohapatra, S.S. Ultrasensitive detection of cortisol with enzyme fragment complementation technology using functionalized nanowire. Biosens Bioelectron 2007, 22, 2138-2144, doi:10.1016/j.bios.2006.09.035.
- Arya, S.K.; Chornokur, G.; Venugopal, M.; Bhansali, S. Dithiobis(succinimidyl propionate) modified gold microarray electrode based electrochemical immunosensor for ultrasensitive detection of cortisol. Biosens Bioelectron 2010, 25, 2296-2301, doi:10.1016/j.bios.2010.03.016.
- Cook, C.J. Measuring of extracellular cortisol and corticotropin-releasing hormone in the amygdala using immunosensor coupled microdialysis. J Neurosci Methods 2001, 110, 95-101, doi:10.1016/s0165-0270(01)00421-6.
- Sekar, M.; Pandiaraj, M.; Bhansali, S.; Ponpandian, N.; Viswanathan, C. Carbon fiber based electrochemical sensor for sweat cortisol measurement. Sci Rep 2019, 9, 403, doi:10.1038/s41598-018-37243-w.
- Kinnamon, D.; Ghanta, R.; Lin, K.C.; Muthukumar, S.; Prasad, S. Portable biosensor for monitoring cortisol in low-volume perspired human sweat. Sci Rep 2017, 7, 13312, doi:10.1038/s41598-017-13684-7.
- Munje, R.D.; Muthukumar, S.; Jagannath, B.; Prasad, S. A new paradigm in sweat based wearable diagnostics biosensors using Room Temperature Ionic Liquids (RTILs). Sci Rep 2017, 7, 1950, doi:10.1038/s41598-017-02133-0.
- Munje, R.D.; Muthukumar, S.; Panneer Selvam, A.; Prasad, S. Flexible nanoporous tunable electrical double layer biosensors for sweat diagnostics. Scientific Reports 2015, 5, 14586, doi:10.1038/srep14586.
- Stevens, R.C.; Soelberg, S.D.; Near, S.; Furlong, C.E. Detection of Cortisol in Saliva with a Flow-Filtered, Portable Surface Plasmon Resonance Biosensor System. Analytical Chemistry 2008, 80, 6747-6751, doi:10.1021/ac800892h.
- Frasconi, M.; Mazzarino, M.; Botrè, F.; Mazzei, F. Surface plasmon resonance immunosensor for cortisol and cortisone determination. Anal Bioanal Chem 2009, 394, 2151-2159, doi:10.1007/s00216-009-2914-6.
- Mitchell, J.S.; Lowe, T.E.; Ingram, J.R. Rapid ultrasensitive measurement of salivary cortisol using nano-linker chemistry coupled with surface plasmon resonance detection. Analyst 2009, 134, 380-386, doi:10.1039/b817083p.
- Ilea, A.; Andrei, V.; Feurdean, C.N.; Băbțan, A.M.; Petrescu, N.B.; Câmpian, R.S.; Boșca, A.B.; Ciui, B.; Tertiș, M.; Săndulescu, R.; et al. Saliva, a Magic Biofluid Available for Multilevel Assessment and a Mirror of General Health-A Systematic Review. Biosensors (Basel) 2019, 9, doi:10.3390/bios9010027.
- Soares, M.S.; Silva, L.C.B.; Vidal, M.; Loyez, M.; Facão, M.; Caucheteur, C.; Segatto, M.E.V.; Costa, F.M.; Leitão, C.; Pereira, S.O.; et al. Label-free plasmonic immunosensor for cortisol detection in a D-shaped optical fiber. Biomed Opt Express 2022, 13, 3259-3274, doi:10.1364/boe.456253.
- Leitão, C.; Leal-Junior, A.; Almeida, A.R.; Pereira, S.O.; Costa, F.M.; Pinto, J.L.; Marques, C. Cortisol AuPd plasmonic unclad POF biosensor. Biotechnol Rep (Amst) 2021, 29, e00587, doi:10.1016/j.btre.2021.e00587.
- Safarian, S.M.; Kusov, P.A.; Kosolobov, S.S.; Borzenkova, O.V.; Khakimov, A.V.; Kotelevtsev, Y.V.; Drachev, V.P. Surface-specific washing-free immunosensor for time-resolved cortisol monitoring. Talanta 2021, 225, 122070, doi:10.1016/j.talanta.2020.122070.
- Liu, Y.; Wu, B.; Tanyi, E.K.; Yeasmin, S.; Cheng, L.-J. Label-Free Sensitive Detection of Steroid Hormone Cortisol Based on Target-Induced Fluorescence Quenching of Quantum Dots. Langmuir 2020, 36, 7781-7788, doi:10.1021/acs.langmuir.0c00513.
- Shin, J.; Kim, S.; Yoon, T.; Joo, C.; Jung, H.-I. Smart Fatigue Phone: Real-time estimation of driver fatigue using smartphone-based cortisol detection. Biosensors and Bioelectronics 2019, 136, 106-111, doi:https://doi.org/10.1016/j.bios.2019.04.046.
- Pinto, V.; Sousa, P.; Catarino, S.O.; Correia-Neves, M.; Minas, G. Microfluidic immunosensor for rapid and highly-sensitive salivary cortisol quantification. Biosens Bioelectron 2017, 90, 308-313, doi:10.1016/j.bios.2016.11.067.
- Zangheri, M.; Cevenini, L.; Anfossi, L.; Baggiani, C.; Simoni, P.; Di Nardo, F.; Roda, A. A simple and compact smartphone accessory for quantitative chemiluminescence-based lateral flow immunoassay for salivary cortisol detection. Biosens Bioelectron 2015, 64, 63-68, doi:10.1016/j.bios.2014.08.048.
- Shirtcliff, E.A.; Buck, R.L.; Laughlin, M.J.; Hart, T.; Cole, C.R.; Slowey, P.D. Salivary cortisol results obtainable within minutes of sample collection correspond with traditional immunoassays. Clin Ther 2015, 37, 505-514, doi:10.1016/j.clinthera.2015.02.014.
- Tahara, Y.; Huang, Z.; Kiritoshi, T.; Onodera, T.; Toko, K. Development of Indirect Competitive Immuno-Assay Method Using SPR Detection for Rapid and Highly Sensitive Measurement of Salivary Cortisol Levels. Front Bioeng Biotechnol 2014, 2, 15, doi:10.3389/fbioe.2014.00015.
- Yamaguchi, M.; Katagata, H.; Tezuka, Y.; Niwa, D.; Shetty, V. Automated-immunosensor with centrifugal fluid valves for salivary cortisol measurement. Sens Biosensing Res 2014, 1, 15-20, doi:10.1016/j.sbsr.2014.07.001.
- van Smeden, L.; Saris, A.; Sergelen, K.; de Jong, A.M.; Yan, J.; Prins, M.W.J. Reversible Immunosensor for the Continuous Monitoring of Cortisol in Blood Plasma Sampled with Microdialysis. ACS Sensors 2022, 7, 3041-3048, doi:10.1021/acssensors.2c01358.
- Apilux, A.; Rengpipat, S.; Suwanjang, W.; Chailapakul, O. Paper-based immunosensor with competitive assay for cortisol detection. Journal of Pharmaceutical and Biomedical Analysis 2020, 178, 112925, doi:https://doi.org/10.1016/j.jpba.2019.112925.
- Visser, E.W.A.; Yan, J.; van, I.L.J.; Prins, M.W.J. Continuous biomarker monitoring by particle mobility sensing with single molecule resolution. Nat Commun 2018, 9, 2541, doi:10.1038/s41467-018-04802-8.
- Sonner, Z.; Wilder, E.; Heikenfeld, J.; Kasting, G.; Beyette, F.; Swaile, D.; Sherman, F.; Joyce, J.; Hagen, J.; Kelley-Loughnane, N.; et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics 2015, 9, 031301, doi:10.1063/1.4921039.
- Hogenelst, K.; Soeter, M.; Kallen, V. Ambulatory measurement of cortisol: Where do we stand, and which way to follow? Sensing and Bio-Sensing Research 2019, 22, 100249, doi:https://doi.org/10.1016/j.sbsr.2018.100249.
- Martin, J.A.; Chávez, J.L.; Chushak, Y.; Chapleau, R.R.; Hagen, J.; Kelley-Loughnane, N. Tunable stringency aptamer selection and gold nanoparticle assay for detection of cortisol. Anal Bioanal Chem 2014, 406, 4637-4647, doi:10.1007/s00216-014-7883-8.
- Ertuğrul Uygun, H.D.; Uygun, Z.O.; Canbay, E.; Gi̇rgi̇n Sağın, F.; Sezer, E. Non-invasive cortisol detection in saliva by using molecularly cortisol imprinted fullerene-acrylamide modified screen printed electrodes. Talanta 2020, 206, 120225, doi:https://doi.org/10.1016/j.talanta.2019.120225.
- Justino, C.I.L.; Duarte, A.C.; Rocha-Santos, T.A.P. Critical overview on the application of sensors and biosensors for clinical analysis. Trends Analyt Chem 2016, 85, 36-60, doi:10.1016/j.trac.2016.04.004.
- Yang, Y.; Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chemical Society Reviews 2019, 48, 1465-1491, doi:10.1039/C7CS00730B.
This entry is adapted from the peer-reviewed paper 10.3390/bios13020285
