Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Immunology
Physical exercise represents an effective preventive and therapeutic strategy beneficially modifying the course of multiple diseases. The protective mechanisms of exercise are manifold; primarily, they are elicited by alterations in metabolic and inflammatory pathways. Exercise intensity and duration strongly influence the provoked response. This narrative review aims to provide comprehensive up-to-date insights into the beneficial effects of physical exercise by illustrating the impact of moderate and vigorous exercise on innate and adaptive immunity.
- exercise
- inflammation
- metabolism
- physical activity
1. Moderate Exercise and Its Impact on the Immune System
1.1. Acute Effects
Exercise induces an increased oxygen demand in muscles, which can be redeemed by increased cardiac output and greater blood flow, stimulated by the sympathetic nervous system [10]. Hemodynamic shear stress, as well as the release of catecholamines and glucocorticoids, force leukocyte release from the vascular, pulmonary, hepatic, and splenic reservoirs and subsequently increase circulating leukocyte numbers [11,12]. Thus, acute moderate exercise induces a leukocytosis in the blood, primarily driven by lymphocytosis and granulocytosis. The amount of leukocytosis positively correlates with the intensity and duration of exercise and is independent of individual fitness levels [7,13]. In the post-exercise period, the return to baseline levels varies between different leukocyte subsets. While the blood count of natural killer (NK) cells, a subset of lymphoid cells, decreases after only one hour [14], neutrophil counts return back to baseline rather delayed after 24 h [7,13].
Phenotypical changes in leukocytes induced by moderate exercise have not been investigated extensively. Still, it has been reported that moderate exercise leads to a transient upregulation of the intracellular vitamin D receptor (VDR) on T lymphocytes [15]. Vitamin D, essential for T cell development, differentiation, and effector functions, plays a key role in immunomodulation. Initiated effects include T cell phenotype switching by suppressing pro-inflammatory TH1 and TH17 cells and promoting regulatory T cells (Treg) [16]. By transiently inducing the expression of VDR, moderate exercise represents a potential adjuvant in the treatment of vitamin D deficiency, a disease with a worldwide prevalence of one billion [17] that is associated with several autoimmune diseases, including diabetes and multiple sclerosis, and increased susceptibility to viral infections [18].
Moreover, moderate exercise promotes neutrophil activation and migration. During their departure from the circulation to the site of action, neutrophils undergo phenotypical changes required for pre-activation. Here, the integrin α-M (also known as CD11b) is upregulated, while the surface receptor CD62 is cleaved off by proteolytic shedding [19,20]. Physical exercise enhances the expression of CD11b in neutrophils acutely, as an immediate response to exercise [7], but also chronically, as individuals who exercise regularly report increased levels of CD11b [21].
1.2. Chronic Effects
In the long term, exercise particularly affects lymphocytes.
Immunosurveillance describes the ability of the immune system to recognize and fight against foreign pathogens, a process largely executed by patrolling CD8+ lymphocytes and NK cells [22,23]. After recognizing mutation-derived neoantigens, CD8+ cytotoxic cells induce apoptosis of malignant cells through the release of cytotoxic substances and activation of the death receptor [24]. The corresponding cells in innate immunity are NK cells [25]. To perform, CD8+ cells and NK cells must arrive at the site of action. Recently, it was reported that physical exercise improves the trafficking behavior of both CD8+ cells and NK cells. Moderate exercise increases the number of CD8+ cells expressing C-X-C motif chemokine receptor 3 (CXCR3) [26,27]. CXCR3 is an inflammatory chemokine receptor orchestrating the migration of activated T cells in the inflamed periphery [28]. Further, the increased release of catecholamines during exercise promotes the mobilization of NK cells while skeletal muscle-derived IL-6 encourages the redistribution of NK cells leading to an increased uptake in tumors [29,30]. The improved recruitment capacities of cytotoxic CD8+ cells and NK cells correlate with decreased tumor size [26,27] and improved survival in cancer patients [31]. Strikingly, the anti-neoplastic effects of exercise seem to be persistent: adoptively transferred CD8+ cells from exercising mice caused enhanced survival and reduced tumor growth in mice [26]. The recruitment profile of B lymphocytes remains unaffected by exercise [30].
Additionally, regular physical exercise counters immunosenescence, the progressive age-related deterioration of the immune system. The age-related atrophy of the thymus, the organ primarily responsible for T cell homeostasis, leads to a reduced supply of naïve T lymphocytes, while antigen-specific memory T cells relatively increase [32]. Thus, T cells have an attenuated capability to interact with novel pathogens, to adequately respond to vaccinations, and to repress latent infections, such as varicella-zoster [33]. Moderate exercise, however, may ward off this process by preserving the thymic output. Increased frequencies of naïve T lymphocytes, less pronounced expansion of memory cells, and less accumulation of senescent cells have been reported in physically active older adults, demonstrating the exercise-induced “rejuvenation” of the immune system [13,34,35]. Thereby, exercise beneficially modifies the course of several inflammatory diseases because chronic inflammation and senescent cells are mutually dependent. By constantly activating immune cells, chronic inflammation promotes cellular senescence. Vice versa, senescent cells produce pro-inflammatory cytokines and foster inflammation [36,37]. Complementary, physical exercise helps to maintain a regular CD4/CD8 ratio [38] and elevates the number of immunomodulatory TREG cells [38,39]. On the other hand, exercise does not cause the preservation of naïve B lymphocytes [34]. Finally, no differences in surface receptors of neutrophils have been reported as a chronic effect of moderate exercise [21,40].
2. Vigorous Exercise and Its Impact on the Immune System
2.1. Acute Effects
As exercise-induced leukocytosis positively correlates with the intensity and duration of an activity, the amount of released cells during acute vigorous exercise is more pronounced [7,13]. Alterations in lymphocyte numbers are well studied and follow a biphasic course. First, exercise induces an instantaneous lymphocytosis lasting 45–60 min [41]. The release of catecholamines during exercise provokes a tremendous increase in NK cells (10×) and CD8+ T lymphocytes (2.5×) via ß2-adrenergic receptor (ß2-AR) signaling [4,42,43,44]. Mobilization of CD4+ cells occurs independently from ß2-AR and is less pronounced. Contrary to the effector status regulated release of T lymphocytes, the nearly twofold increase in B lymphocytes is mainly due to the enhanced release of immature subsets [45]. The pronounced increase in lymphocyte numbers is followed by a massive decrease within one to three hours post-exercise provoking a transient lymphopenia [13,39,46,47], which is primarily driven by a reduction in NK cells and CD8+ T lymphocytes [48]. This induced lymphopenia used to be commonly seen as part of a short-term suppression of the immune system leading to an increased susceptibility for infections, a phenomenon called the “open-window” theory [49]. However, the contemporary opinion is that transient lymphopenia is only a snapshot of the increased redeployment of lymphocytes promoting immunosurveillance and immunoregulation [4,12]. Another contributing factor to exercise-induced lymphopenia is enhanced apoptosis of highly differentiated T cells in the early post-exercise period, characterized by increased expression of the death receptor CD95 [50,51]. The induced gap is replenished by increased mobilization of naïve T cells indicating a regenerative effect of exercise [52].
Exercise does not only encourage the mobilization of lymphoid, but also of myeloid cells. The exercise-induced monocytosis is, similar to the lymphocytosis, highly dependent on the sensitivity to ß2-AR signaling and monocyte subsets; classical (CD14++ CD16-), non-classical (CD14+ CD16++), and intermediate (CD14+ CD16+) monocytes [53,54] respond differently. An acute bout of exercise preferably induces an increase in non-classical monocytes [44,55] while the rise of classical and intermediate monocytes is less pronounced [44]. The count of circulating monocytes rapidly returns back to baseline within one hour after exercise [55,56]. In contrast to the fast decline of lymphocytes and monocytes, neutrophil numbers continue to rise for one hour after exercise cessation and return to baseline delayed after 24 h [47,51,57].
By phenotypic changes, vigorous exercise creates an anti-inflammatory milieu. Vigorous exercise acutely elevates the expression of CD39 and CD73 on CD4+ T lymphocytes [58]. These surface-associated enzymes catalyze the conversion of adenosine triphosphate (ATP)/adenosine diphosphate (ADP) to adenosine monophosphate (AMP)/adenosine, respectively. While ATP promotes inflammation, fulfilling its role as a “danger” signal in inflammation, the nucleotide promotes the migration of phagocytes to damaged tissues, adenosine triggers a series of anti-inflammatory responses [39,59]. By promoting the degradation of ATP to adenosine via the CD39/CD73 pathway, vigorous exercise induces a shift toward an anti-inflammatory environment [58]. Further, vigorous exercise reduces the expression of Toll-like receptors (TLRs) 1, 2, and 4 on CD14+ monocytes in the early postexercise period [60]. Cells of innate immunity are equipped with TLR to rapidly fight against invading pathogens. However, excess activation of TLR leads to the sustained secretion of pro-inflammatory cytokines and chemokines which contributes to the progression of numerous inflammatory and auto-immune diseases [61,62]. By reducing the expression of TLR, exercise provokes anti-inflammatory effects which may beneficially alter the course of diseases, such as atherosclerosis, asthma, or systemic lupus erythematosus [63].
2.2. Chronic Effects
In the long term, too, exercise promotes anti-inflammatory effects.
Vigorous exercise encourages an anti-inflammatory phenotype switching in lymphocytes and monocytes. The balance between TH1 and TH2 cells shifts in favor of TH2 [46,47]. Within the monocyte population, vigorous exercise decreases the frequency of intermediate and non-classical monocytes by diminishing the expression of CD16 [64,65]. Similar to moderate exercise, vigorous exercise leads to a downregulation of TLR [65]. Thus, the age-associated decline in monocyte function, characterized by increased expression of CD16 and impaired TLR expression [66,67], might be counteracted by exercise. Similar to moderate exercise, vigorous exercise preserves thymic output. VO2, the maximal oxygen uptake during physical exertion, positively correlates with the extent of naïve T lymphocytes [68] and the number of Interleukin-10 (IL-10)-producing TREG cells [39,47]. Finally, no difference in surface receptors of neutrophils has been reported [21,40].
In conclusion, exercise promotes mainly anti-inflammatory effects and hence may primarily exert advantageous effects in diseases with strong inflammatory components. Consequently, we now focus on how physical exercise potentially impacts the development and progression of atherosclerosis, a chronic inflammatory disease of the vessel wall. The multiple effects of exercise on immunity are summarized in Figure 1.
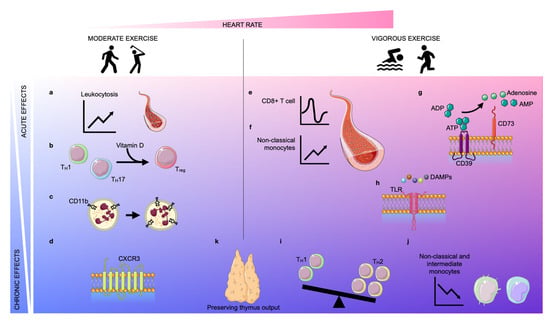
Figure 1. Acute and chronic effects of moderate and vigorous exercise. Depending on the increase in heart rate (pink area), exercise can be classified as moderate exercise or vigorous exercise. The effects of exercise can be classified as acute (light blue area) or chronic (blue area). Acute moderate exercise induces a leukocytosis in the blood (a) and a transient upregulation of the intracellular vitamin D receptor (VDR) in T lymphocytes, inducing T cell phenotype switching (b). Further, moderate exercise enhances the expression of CD11b on neutrophils acutely and chronically (c). In the long term, physical exercise improves the trafficking behavior of both CD8+ cells and natural killer (NK) cells by regulating the expression of CXCR3 (d). Vigorous exercise induces alterations in lymphocyte numbers in a biphasic way: an instantaneous lymphocytosis is followed by transient lymphopenia (e). Further, non-classical monocytes are increased during exercise (f). Vigorous exercise acutely elevates the expression of CD39 and CD73 on CD4+ T lymphocytes which assist in the conversion of adenosine triphosphate (ATP)/adenosine diphosphate (ADP) into adenosine monophosphate (AMP)/adenosine, respectively (g). Vigorous exercise reduces the expression of Toll-like receptors (TLRs) 1, 2, and 4 on CD14+ monocytes in the early post-exercise period, inducing anti-inflammatory effects (h). In the long term, exercise also promotes anti-inflammatory effects by encouraging anti-inflammatory phenotype switching in lymphocytes and monocytes. The balance between TH1 and TH2 cells shifts in favor of TH2 (i) and the frequency of intermediate and non-classical monocytes decreases due to diminished expression of CD16 (j). Finally, both moderate and vigorous exercise preserve thymic output (k).
This entry is adapted from the peer-reviewed paper 10.3390/ijms24043394
This entry is offline, you can click here to edit this entry!
