Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Resectable gastric or gastroesophageal (G/GEJ) cancer is a heterogeneous disease with no defined molecularly based treatment strategy. Unfortunately, nearly half of patients experience disease recurrence despite standard treatments (neoadjuvant and/or adjuvant chemotherapy/chemoradiotherapy and surgery).
- perioperative GC
- HER2
- MSI-H
1. Introduction
Gastric cancer (GC) is the fifth most common cancer and the third cause of cancer-related mortality worldwide [1,2]. When diagnosis occurs in metastatic disease, the prognosis is poor and radical surgery is not routinely recommended [3].
In the setting of resectable disease, multimodal treatments, including perioperative chemotherapy, showed a significant survival improvement compared with surgery alone. At first, the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) and FNCLCC/FFCD trials demonstrated the benefit of perioperative chemotherapy in survival and in recurrence-rate reduction. Patients with resectable gastric or gastro-esophageal junction (G/GEJ) adenocarcinoma were assigned to either surgery alone or perioperative chemotherapy (i.e., epirubicin, cisplatin and fluorouracil [ECF] in the MAGIC trial and cisplatin and fluorouracil in the FNCLCC/FFCD trial). Five-year survival rates were higher in the perioperative-chemotherapy group compared to the surgery group (36% vs. 23% in MAGIC, 38% vs. 24% in FNCLCC/FFCD trial) [4,5].
Although docetaxel showed a remarkable efficacy in first- and further-line treatments for metastatic G/GEJ adenocarcinoma, both alone and in combination with cisplatin and fluorouracil [6,7], toxicities associated with combination regimens were so relevant to suggest replacement of cisplatin with oxaliplatin. This new combination, which consisted of perioperative fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT), was evaluated in the phase III FLOT4-AIO trial that demonstrated the efficacy of FLOT as a perioperative therapy over ECF in G/GEJ adenocarcinoma. The median overall survival (OS) was 50 months in the FLOT arm vs. 35 months in the ECF arm, and the pathological complete regression (pCR) rate was 16% vs. 6%, respectively [8,9]. A recent Italian case series (RealFLOT study) confirmed the feasibility and safety of the FLOT regimen in a less prognostically favorable selected patient population.
In advanced G/GEJ cancer, the introduction of human epidermal growth factor receptor-2 (HER2)-directed therapies and immune checkpoint inhibitors (ICIs) in combination with chemotherapy has improved survival. Moreover, novel targets could significantly change the continuum of care [11,12,13,14,15].
2. Tailored Treatments
2.1. HER2-Directed Therapies
Over the past few years, increasing attention has developed around targeted therapies, and substantial changes have been made in therapeutic choices and strategies in most malignant tumors [16]. One of the first molecular targets was HER2. The development of the specific monoclonal antibody (moAb) trastuzumab, the first targeted agent for solid malignancies, led to a drastic change in the clinical course of HER2-positive breast cancer patients [17]. In GC patients, previous studies have reported that the HER2 protein expression level ranged from 7.3% to 22.1% [18]. The association between HER2 status and prognosis has been described in several studies, with a general agreement on the negative prognostic role of HER2 overexpression [19,20,21]. The randomized controlled ToGA trial showed that the addition of trastuzumab to chemotherapy offered a meaningful survival gain in advanced GC patients overexpressing HER2 (mOS 13.8 months vs. 11.1 months, HR 0.74; 95% CI 0.60–0.91; p = 0.0046) [13]. Kurokawa and colleagues later demonstrated that HER2 overexpression was an independent prognostic factor in patients with resectable GC (HR 1.59; 95% CI 1.24–2.02; p = 0.001), and they also showed that HER2 intra-tumoral heterogeneity was frequent and did not affect prognosis. In this study, 180 HER2-positive cases were identified among 1148 GC patients, assessed by immunohistochemistry (IHC) and fluorescence in situ hybridization. The trial showed that HER2-positive GC was more commonly related to intestinal-type adenocarcinoma and upper stomach location. Other factors, including the pT and pN stages, did not show any correlation with HER2 overexpression [22]. As previously described, the results from the AIO-FLOT4 trial have set a new standard of care for resectable GC patients fit for intensive treatment. However, more than half of the patients in the FLOT group experienced disease recurrence within 3 years and the estimated OS at 5 years was 45% [9] (Figure 1).
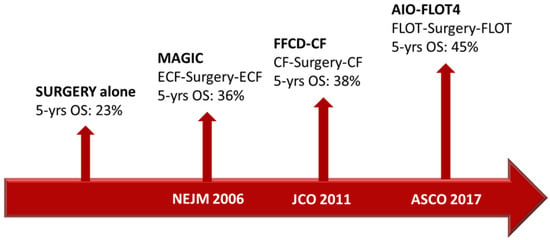
Figure 1. Timeline of the main trials that change the clinical practice in perioperative GC treatment.
Given the results of the AIO-FLOT4 trial in the perioperative setting, the next step was to evaluate the combination of trastuzumab and FLOT as perioperative treatment in patients with HER2-positive, locally advanced esophagogastric adenocarcinomas.
HER-FLOT was a multicenter, phase II study aiming at evaluating the toxicity and activity of trastuzumab in combination with chemotherapy in the perioperative setting. This trial reached the primary endpoint (pCR > 20%) achieving a pCR rate of 21.4%. In fact, 12 out of 56 patients had no viable neoplastic cells at the time of surgery, as assessed by a central pathologist, and 14 patients (25.0%) had subtotal response (<10% residual tumor). The most frequently observed grade (G) 3–4 adverse events (AEs) were quite similar to the FLOT4 trial, and only one case of severe heart failure related to trastuzumab was reported. pCR was also a surrogate of survival outcome. Overall, median disease-free survival (DFS) was 42.5 months and the 3-year OS rate was 82.1% [23].
The PETRARCA trial explored the role of dual HER2 blockade (trastuzumab and pertuzumab) in combination with chemotherapy (FLOT) compared to chemotherapy alone in HER2-positive GC patients who were amenable to a perioperative strategy. Trastuzumab and pertuzumab were administered every 3 weeks for 3 preoperative and 3 postoperative cycles, followed by 9 maintenance cycles. The primary endpoint was the pCR rate that was achieved in 35% of patients (n = 14) in the experimental arm vs. 12% of patients (n = 5) in the control group (p = 0.019). Among secondary endpoints, node-negativity and radical-surgery (i.e., R0) rates were met. The 2-year OS rate was 84% in the experimental arm and 77% in the control arm. Median DFS was not reached in the experimental arm vs. 26 months in the FLOT arm (HR 0.58, 95% CI 0.28–1.19, p = 0.130), with 2-year DFS of 70% and 54%, respectively. The safety profile of the experimental group was characterized by a predominance of diarrhea, neutropenia and leukopenia as high G AEs [24].
The negative results from the JACOB trial, which evaluated the addition of dual anti-HER2 blockade to a taxane-free polychemotherapy regimen, led to a premature stop of the PETRARCA trial and failure to transition to a phase III study [25]. However, PETRARCA’s investigators pointed out the high pCR and pN0 rates in the experimental arm at the cost of slightly higher gastrointestinal and hematopoietic toxicity.
The ongoing, international, randomized phase II EORTC INNOVATION trial has the purpose to evaluate the addition of trastuzumab or trastuzumab plus pertuzumab to chemotherapy in the perioperative treatment of patients with HER2-positive G/GEJ adenocarcinoma. Preliminary results are expected in 2023 [26].
In support of the anti-HER2 blockade strategy, results from some retrospective and prospective experiences conducted in Asian patients have recently been published.
Data from 45 Asian patients with HER2-positive stage II-III GC were retrospectively analyzed. Twenty-nine patients received trastuzumab plus FLOT and sixteen received chemotherapy alone. The primary endpoint was the objective response rate (ORR). In the trastuzumab + FLOT arm, the ORR was 72.4% and the disease control rate (DCR) was 89.7%, while in the FLOT group the ORR and DCR were 43.8% and 87.5%, respectively. The 2-year OS rates were 78.1% and 73.9%, respectively (p = 0.932). Although the study did not reach statistical significance in primary and ancillary endpoints, the addition of trastuzumab provided a numerically high tumor response rate and a promising pathological regression compared to the control group: the tumor regression rate to grade Ia/Ib was obtained in 44.8% of patients [27].
The Japan Clinical Oncology Group (JCOG) conducted the multi-institutional two-arm open label randomized phase II Trigger trial to evaluate the efficacy and safety of S-1-cisplatin in combination with trastuzumab vs. S-1-cisplatin alone for patients with HER2-positive locally advanced GC. The primary endpoint was OS. The study enrolled 46 patients of the preplanned 130 due to slow accrual and was prematurely ended in 2021. The ORR was higher in the experimental group than in the control group (66.7% vs. 36.4%, p = 0.08), even though the difference was not statistically significant. pCR rates were 50.0% and 22.7% (p = 0.07), respectively, and the percentages of patients who experienced pathological downstage were 22.7% and 50.0% (p = 0.07), respectively [28]. Although of interest, this study did not add conclusive data on the role of anti-HER2 blockade in patients with locally advanced disease.
After several disappointing or inconclusive results about the use of anti-HER2 agents other than trastuzumab, the recent results from the phase II DESTINY-Gastric01 trial with the antibody-drug conjugate trastuzumab deruxtecan (T-DXd) renewed interest in advanced GC. The excellent performance of T-DXt in terms of ORR (51.3% vs. 14.3%) and OS (median 12.5 vs. 8.4 months; IC 95% 0.39–0.88, HR 0.59; p = 0.01) compared with physicians’ choice of chemotherapy has led to planning the phase II EPOC2003 study (NCT05034887) with the aim to evaluate T-DXd as a neoadjuvant treatment for patients with HER2-positive G/GEJ adenocarcinoma in six Japanese centers [29,30].
Finally, the randomized phase III RTOG 1010 study included a total of 202 patients diagnosed with locally advanced HER2-positive esophageal adenocarcinoma. Patients were randomized to receive either chemoradiotherapy (CROSS scheme) plus trastuzumab or chemoradiotherapy alone. The primary endpoint was DFS, which was not met. Median DFS was 19.6 months (95% CI 13.5–26.2) with chemoradiotherapy plus trastuzumab compared to 14.2 months (10.5–23.0) for chemoradiotherapy alone (HR 0.99, 95% CI 0.71–1.39, p = 0.97). In conclusion, although well tolerated, the addition of trastuzumab to the neoadjuvant trimodality treatment did not result in any benefit, neither in terms of pCR nor of DFS [31].
2.2. Immune Checkpoint Blockade
As in many solid tumors, the standard of care in G/GEJ cancer is about to change with the introduction of ICIs in combination with chemotherapy as the first line of treatment [15].
Programmed cell death ligand 1 (PD-L1) is a protein expressed in different immune system cells (e.g., T lymphocytes, epithelial cells, endothelial cells, macrophages, dendritic cells). Cancer cells also use PD-L1 to evade the anti-tumor immune response inhibiting cytotoxic T-cell activity. Moreover, neoplastic cells have the ability to upregulate anti-cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), leading to the formation of a co-inhibitory pathway to avoid host immune responses. Antibodies against the checkpoint proteins programmed death-1 (PD-1) (nivolumab, pembrolizumab), PD-L1 (atezolizumab, avelumab, durvalumab) and CTLA-4 (ipilimumab, tremelimumab) were shown to be effective in reactivating protective T-cell activity [32].
One of the most important predictors of benefit from immunotherapy is the deficient mismatch repair/microsatellite instability-high (dMMR/MSI-H) status, leading to the agnostic approval of pembrolizumab for patients with dMMR advanced tumor [33]. Moreover, several pieces of evidence showed no benefit or harm from peri-/post-operative chemotherapies in resectable MSI-H G/GEJ cancers.
In this regard, an exploratory subgroup analysis from the MAGIC trial showed that patients with an MSI-H/dMMR tumor had a better prognosis (mOS not reached 95% CI, 11.5-NR months) compared to those with an MSS tumor in the surgery-alone arm (mOS 20.5 months; 95% CI, 16.7–27.8 months; HR, 0.42; p = 0.09) [34]. Similarly, a post hoc analysis from the ITACA-S trial suggested that MSI-H status represents an independent prognostic factor, being associated with better DFS (p = 0.02) and OS (p = 0.01) [35]. It is noteworthy that a post hoc analysis from the CLASSIC trial showed that MSI-H (p = 0.008) and PD-L1 (p = 0.044) were independent prognostic factors and no DFS benefit was achieved by adjuvant chemotherapy for patients with an MSI-H tumor (5-year DFS 83.9% vs. 85.7%; p = 0.931) [36]. Finally, a meta-analysis focusing on patients with an MSI-H tumor including MAGIC, CLASSIC, ARTIST and ITACA-S trials was recently conducted. The authors described an advantage in DFS (5-year DFS 71.8% vs. 52.3%; p < 0.001) and OS (5-year OS 77.5% vs. 59.3%; p < 0.001) for patients with an MSI-H tumor compared to those with a microsatellite stable (MSS)/MSI-low tumor [37].
Immunotherapy in Perioperative Treatment of G/GEJ Cancer
The turning point of adjuvant therapy in esophageal or GEJ cancer was represented by the phase III, global, randomized, double-blind, placebo-controlled arm CheckMate 577 trial. The study included patients with stage II/III esophageal or GEJ cancer. After chemoradiotherapy and radical surgery, patients with at least ypT1 and/or ypN1 histologically confirmed cancer were randomized to receive nivolumab 240 mg every 2 weeks for 16 weeks, then 480 mg every 4 weeks (up to 1 year of therapy) or placebo. The primary endpoint was DFS, which was found to be superior in the experimental arm for all the intention-to-treat (ITT) population (22.4 vs. 11.0 months; HR, 0.69; 95% CI, 0.56–0.86; p < 0.001). The benefit was observed in all subgroups, regardless of histological type and PD-L1 level. However, in the subgroup of GEJ patients, the benefit was less marked, with an mDFS of 22.4 months in the nivolumab arm vs. 20.6 months in the placebo arm (HR 0.87, IC 95% 0.63–1.21) as compared to that obtained in esophageal cancer (mDFS 24.0, vs. 8.3, respectively, HR 0.61) [40].
In the phase II PERFECT trial, 40 patients with resectable esophageal adenocarcinoma received atezolizumab in combination with chemoradiotherapy followed by surgery in 83% of cases. Immune-related AEs were observed in 15% (n = 6) of patients in the experimental arm. Ten patients achieved a partial response (PR). Despite being a single-arm study, this represented the first trial to propose the combination of the CROSS scheme and an anti-PD-L1 agent, and it proved the feasibility of adding atezolizumab to CROSS-based neoadjuvant chemoradiotherapy [41].
The efficacy of atezolizumab in the perioperative setting was also evaluated in the randomized multicenter phase IIb DANTE trial from the German and Swiss groups in which patients with resectable G/GEJ adenocarcinoma were assigned to receive atezolizumab in combination with FLOT or FLOT alone. The primary endpoint was progression-free survival (PFS)/DFS after 5 years of observation. Secondary endpoints were recently summarized in an interim analysis presented at the 2022 ASCO meeting, reporting atezolizumab plus FLOT as feasible and safe in the perioperative setting. Surgical mobility and mortality, and R0 rates were comparable between the two arms, while a higher rate of downsizing and pathological regression for the experimental arm (pT0, 23% vs. 15%; pN0, 68% vs. 54%) was reached, especially in patients whose tumor had a high PD-L1 expression or MSI-H status. Indeed, in the MSI-H and PD-L1 combined positive score (CPS) ≥ 10, tumor regression grade (TRG) 1a/b, assessed by central pathologists, was observed in 70–71% of cases in the experimental arm vs. 47–52% in the control arm. These data surely provide the rationale for implementation towards a phase III study [42,43].
The randomized, double-blind, phase III MATTERHORN trial aims at assessing the efficacy and safety of durvalumab in combination with perioperative FLOT, followed by adjuvant durvalumab monotherapy in patients with resectable G/GEJ cancer. The study is currently ongoing, with an estimated sample size of 900 patients at approximately 180 sites worldwide. The primary endpoint is event-free survival; secondary endpoints include OS and the pCR rate. Safety assessment will be evaluated [44].
A remarkable topic is the combination of immune checkpoint therapy, based on the possible synergistic effect of blocking both the PD-1/PD-L1 and the CTLA-4 pathways [45].
To this purpose, the phase II/III trial EA 2174, of the ECOG-ACRIN Cancer Research Group, is evaluating the perioperative treatment with nivolumab and ipilimumab in addition to standard chemotherapy and radiation therapy in patients with locoregional esophageal and GEJ adenocarcinoma. The primary neoadjuvant endpoint is the pCR rate, and the primary adjuvant endpoint is DFS. The safety run-in phase enrolled 31 patients divided into the two arms with no disproportional difference in safety or number of patients who proceeded to surgery [46].
The phase II VESTIGE trial has the primary objective to assess if the combination of nivolumab plus ipilimumab as an adjuvant treatment after neoadjuvant chemotherapy with FLOT and surgery improves DFS in patients with G/GEJ adenocarcinoma [47].
The immunotherapy combination strategy is also under evaluation in patients selected for biomarkers of response. The phase II NEONIPIGA trial enrolled 32 patients with resectable MSI-H/dMMR G/GEJ adenocarcinoma to receive neoadjuvant nivolumab in combination with ipilimumab for six cycles, followed by surgery and subsequent adjuvant treatment with single-agent nivolumab for 9 months. All patients received neoadjuvant treatment, and 29 of them proceeded to surgery. After a median follow-up of 12 months, 30 patients were free from progressive disease (PD), 1 patient died due to PD after five cycles of neoadjuvant therapy, and 1 patient died without relapse. Neoadjuvant therapy with dual checkpoint inhibition led to a pCR rate of 58.6%, meeting the primary endpoint. The combination of nivolumab and ipilimumab is promising as a neoadjuvant treatment in patients with MSI-H tumor and highlights the possibility of delaying or even avoiding surgery in highly selected subgroups of patients [48]. Furthermore, the Italian multicenter, non-randomized, single-arm, multi-cohort, open-label, phase II INFINITY study is evaluating the efficacy and tolerability of the combination of tremelimumab and durvalumab as a neoadjuvant (cohort 1) treatment or as a definitive treatment (cohort 2) in patients with MSI-H/dMMR resectable G/GEJ adenocarcinoma [49]. This study may provide new evidence on the therapeutic management of resectable G/GEJ cancer in patients selected for biomarkers highly predictive of response, particularly in regard to the possibility of non-surgical management.
Among the ongoing trials, the phase II, randomized IMAGINE trial (NCT04062656) is evaluating various immunotherapy or chemo-immunotherapy treatments (nivolumab, nivolumab plus ipilimumab, and nivolumab plus relatlimab, an anti-LAG3 antibody) in the perioperative set, while the KEYNOTE-585 trial (NCT03221426) is evaluating perioperative pembrolizumab plus chemotherapy for resectable G/GEJ adenocarcinoma. Notably, as there is no international standard adjuvant chemotherapy, pembrolizumab can be administered with cisplatin plus capecitabine or 5-fluorouracil, or, in a separate safety cohort, with the FLOT regimen.
The phase II, multicenter, 4-cohort, IMHOTEP trial (NCT04795661) is also evaluating the use of pembrolizumab in the neoadjuvant phase. Patients with resectable MSI-H/dMMR or EBV-positive GC will receive a single dose of pembrolizumab 400 mg 6 weeks before the surgery and a clinician’s choice adjuvant therapy. The primary endpoint will be pCR.
This entry is adapted from the peer-reviewed paper 10.3390/ijms24054877
This entry is offline, you can click here to edit this entry!
