The essential oils and their components, such as terpenes, and/or phenols, possess anti-inflammatory and antioxidant activities. It is demonstrated that some essential oils may increase the number of immunocompetent cells, including PMNs, macrophages, dendritic cells, natural killer cells, and B and T lymphocytes. Allium sativum essential oil and some of its organosulfur components are shown to have a positive effect on macrophage phagocytosis and can activate macrophage chemotaxis, human neutrophil responses with reactive oxygen species (ROS) production, and lymphocyte proliferation. Eucalyptus essential oil also shows the same properties of stimulating phagocytosis by macrophages. On the other hand, “Roman coriander” (Nigella sativa L., Ranunculaceae) essential oil has not been found to have any beneficial or detrimental effects on neutrophil activities.
- essential oils
- antifungal activity
- immune system
1. Introduction
2. Antimicrobial Potential of Essential Oils
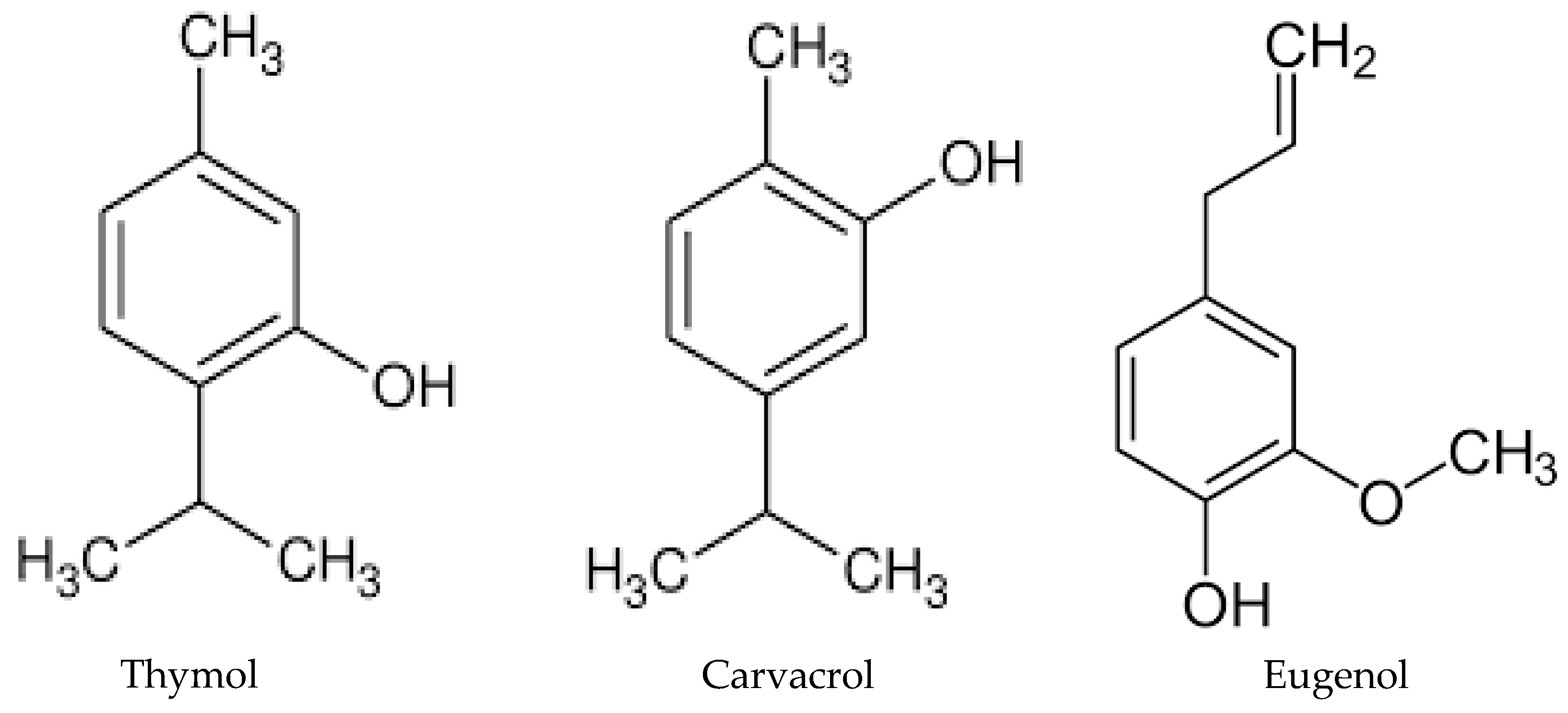
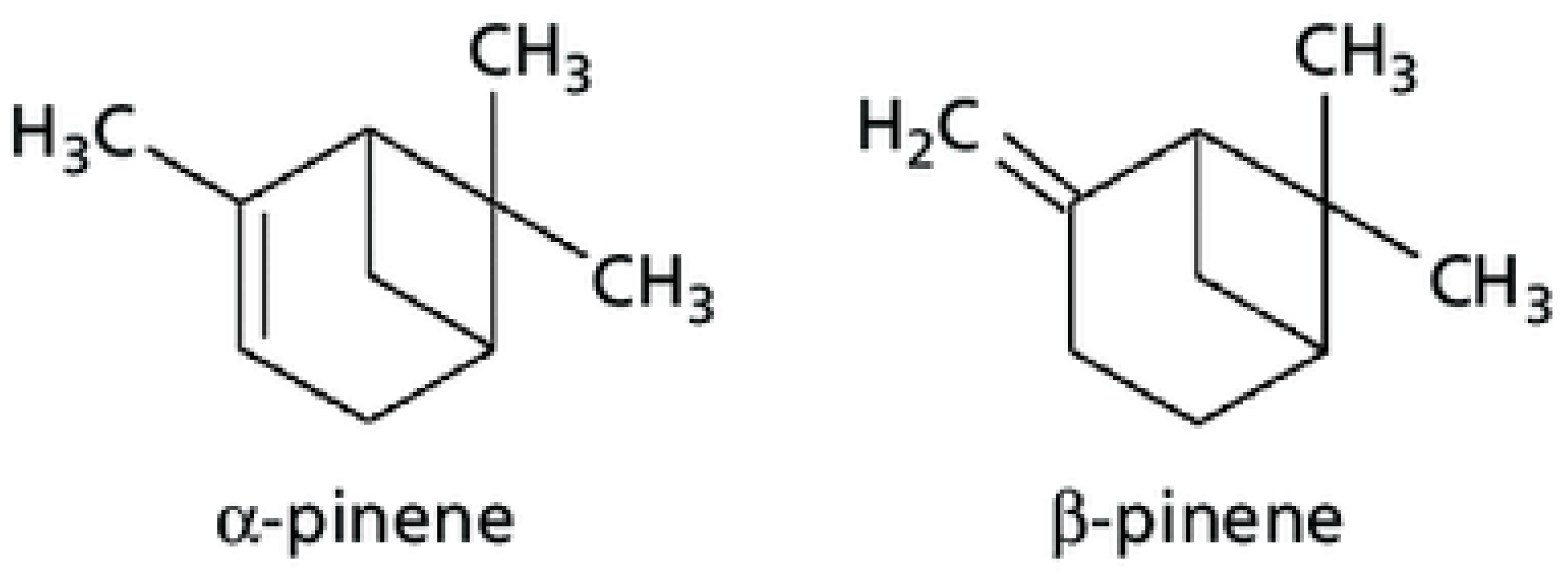
This entry is adapted from the peer-reviewed paper 10.3390/molecules28010435
References
- Wang, C.H.; Hsieh, Y.H.; Powers, Z.M.; Kao, C.Y. Defeating antibiotic-resistant bacteria: Exploring alternative therapies for a post-antibiotic era. Int. J. Mol. Sci. 2020, 21, 1061.
- Palmeira-de-Oliveira, A.; Salgueiro, L.; Palmeira-de-Oliveira, R.; Martinez-de-Oliveira, J.; Pina-Vaz, C.; Queiroz, J.A.; Rodrigues, A.G. Anti-Candida activity of essential oils. Mini. Rev. Med. Chem. 2009, 9, 1292–1305.
- Pina-Vaz, C.; Gonçalves Rodrigues, A.; Pinto, E.; Costa-de-Oliveira, S.; Tavares, C.; Salgueiro, L.; Cavaleiro, C.; Gonçalves, M.J.; Martinez-de-Oliveira, J. Antifungal activity of Thymus oils and their major compounds. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 73–78.
- Ali, B.; Ali, N.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop Biomed. 2015, 5, 601–611.
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Alternat. Med. 2016, 2016, 3012462.
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents-myth or real alternative? Molecules 2019, 24, 2130.
- Tullio, V.; Nostro, A.; Mandras, N.; Dugo, P.; Banche, G.; Cannatelli, M.A.; Cuffini, A.M.; Alonzo, V.; Carlone, N.A. Antifungal activity of essential oils against filamentous fungi determined by broth microdilution and vapour contact methods. J. Appl. Microbiol. 2007, 102, 1544–1550.
- Scalas, D.; Mandras, N.; Roana, J.; Tardugno, R.; Cuffini, A.M.; Ghisetti, V.; Benvenuti, S.; Tullio, V. Use of Pinus sylvestris L. (Pinaceae), Origanum vulgare L. (Lamiaceae), and Thymus vulgaris L. (Lamiaceae) essential oils and their main components to enhance itraconazole activity against azole susceptible/not-susceptible Cryptococcus neoformans strains. BMC Complement. Altern. Med. 2018, 18, 43.
- Ugazio, E.; Tullio, V.; Binello, A.; Tagliapietra, S.; Dosio, F. Ozonated oils as antimicrobial systems in topical applications. Their characterization, current applications, and advances in improved delivery techniques. Molecules 2020, 25, 334.
- Carlone, N.A.; Mandras, N.; Nicolosi, D.; Vitali, L. Farmaci antibatterici. In Microbiologia Farmaceutica, 3rd ed.; Carlone, N., Pompei, R., Tullio, V., Eds.; Edises Università S.r.l.: Naples, Italy, 2021; pp. 178–207.
- Carlone, N.A.; Mandras, N. Farmaci antifungini. In Microbiologia Farmaceutica, 3rd ed.; Carlone, N., Pompei, R., Tullio, V., Eds.; Edises Università S.r.l.: Naples, Italy, 2021; pp. 226–238.
- Tullio, V.; Roana, J.; Scalas, D.; Mandras, N. Evaluation of the antifungal activity of Mentha x piperita (Lamiaceae) of Pancalieri (Turin, Italy) essential oil and its synergistic interaction with azoles. Molecules 2019, 24, 3148.
- Garozzo, A.; Timpanaro, R.; Bisignano, B.; Furneri, P.M.; Bisignano, G.; Castro, A. In vitro antiviral activity of Melaleuca alternifolia essential oil. Lett. Appl. Microbiol. 2009, 49, 806–808.
- Li, Y.; Fu, X.; Ma, X.; Geng, S.; Jiang, X.; Huang, Q.; Hu, C.; Han, X. Intestinal microbiome-metabolome responses to essential oils in piglets. Front. Microbiol. 2018, 9, 1988.
- Carlone, N.A.; Cuffini, A.M.; Tullio, V.; Cavallo, G. Interactions of antibiotics with phagocytes in vitro. J. Chemother. 1991, 3 (Suppl. 1), 98–104.
- Tullio, V.; Cuffini, A.M.; Fazari, S.; Carlone, N.A. Cefonicid potentiation of human macrophage activity. Microbiologica 1992, 15, 219–226.
- Tullio, V.; Cuffini, A.M.; De Leo, C.; Perrone, F.; Carlone, N.A. Interaction of Candida albicans, macrophages and fluconazole: In vitro and ex vivo observations. J. Chemother. 1996, 8, 438–444.
- Labro, M.T. Interference of antibacterial agents with phagocyte functions: Immunomodulation or “immuno-fairy tales”? Clin. Microbiol. Rev. 2000, 13, 615–650.
- Tullio, V.; Cuffini, A.M.; Bonino, A.; Palarchio, A.I.; Roana, J.; Mandras, N.; Rossi, V.; Carlone, N.A. Influence of a new fluoroquinolone, AF3013 (the active metabolite of prulifloxacin), on macrophage functions against Klebsiella pneumoniae: An in vitro comparison with pefloxacin. J. Antimicrob. Chemother. 2000, 46, 241–247.
- Banche, G.; Allizond, V.; Mandras, N.; Tullio, V.; Cuffini, A.M. Host immune modulation by antimicrobial drugs: Current knowledge and implications for antimicrobial chemotherapy. Curr. Opin. Pharmacol. 2014, 18, 159–166.
- Scalas, D.; Banche, G.; Merlino, C.; Giacchino, F.; Allizond, V.; Garneri, G.; Patti, R.; Roana, J.; Mandras, N.; Tullio, V.; et al. Role of caspofungin in restoring the impaired phagocyte-dependent innate immunity towards Candida albicans in chronic haemodialysis patients. Int. J. Antimicrob. Agents 2012, 39, 73–76.
- Mandras, N.; Nostro, A.; Roana, J.; Scalas, D.; Banche, G.; Ghisetti, V.; Del Re, S.; Fucale, G.; Cuffini, A.M.; Tullio, V. Liquid and vapour-phase antifungal activities of essential oils against Candida albicans and non-albicans Candida. BMC Complement. Altern. Med. 2016, 16, 330.
- Mandras, N.; Roana, J.; Scalas, D.; Del Re, S.; Cavallo, L.; Ghisetti, V.; Tullio, V. The inhibition of non-albicans Candida species and uncommon yeast pathogens by selected essential oils and their major compounds. Molecules 2021, 26, 4937.
- Tadtong, S.; Puengseangdee, C.; Prasertthanawut, S.; Hongratanaworakit, T. Antimicrobial constituents and effects of blended Eucalyptus, Rosemary, Patchouli, Pine, and Cajuput essential oils. Nat. Prod. Commun. 2016, 11, 267–270.
- Benameur, Q.; Gervasi, T.; Pellizzeri, V.; Pľuchtová, M.; Tali-Maama, H.; Assaous, F.; Guettou, B.; Rahal, K.; Gruľová, D.; Dugo, G.; et al. Antibacterial activity of Thymus vulgaris essential oil alone and in combination with cefotaxime against blaESBL producing multidrug resistant Enterobacteriaceae isolates. Nat. Prod. Res. 2019, 33, 2647–2654.
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857.
- Tullio, V.; Mandras, N.; Allizond, V.; Nostro, A.; Roana, J.; Merlino, C.; Banche, G.; Scalas, D.; Cuffini, A.M. Positive interaction of thyme (red) essential oil with human polymorphonuclear granulocytes in eradicating intracellular Candida albicans. Planta. Med. 2012, 78, 1633–1635.
- Bertino, A. Oli Essenziali: Valutazione Dell’attività Antifungina e Della Loro Eventuale Capacità di Modulare le Funzioni dei PMN nei Confronti dei Lieviti Patogeni/Essential Oils: Evaluation of Antifungal Activity and their Potential Ability to Modulate the Functions of PMNs against Pathogenic Yeasts. Level 1. Bachelor’s Thesis in Drug Chemistry and Technology, 5-Years Postgraduate Degree, Second Cycle EHEA Equivalent, University of Torino, Torino, Italy, 2014.
- Mandras, N.; Roana, J.; Scalas, D.; Nostro, A.; Allizond, V.; Banche, G.; Cuffini, A.M.; Tullio, V. Azione sinergica tra olio essenziale di timo rosso e granulociti polimorfonucleati nell’eradicare Candida albicans e C. krusei intracellulari/Synergistic action between red thyme essential oil and polymorphonuclear granulocytes in eradicating Candida albicans and C. krusei intracellularly. In Proceedings of the X Congresso Naz Soc It Microbiol Farmaceut (SIMiF), Chieti, Italy, 6–7 June 2014.
- Tullio, V.; Mandras, N.; Scalas, D.; Allizond, V.; Banche, G.; Roana, J.; Greco, D.; Castagno, F.; Cuffini, A.M.; Carlone, N.A. Synergy of caspofungin with human polymorphonuclear granulocytes for killing Candida albicans. Antimicrob. Agents Chemother. 2010, 54, 3964–3966.
- Demir, K.K.; Butler-Laporte, G.; Del Corpo, O.; Ekmekjian, T.; Sheppard, D.C.; Lee, T.C.; Cheng, M.P. Comparative effectiveness of amphotericin B, azoles and echinocandins in the treatment of candidemia and invasive candidiasis: A systematic review and network meta-analysis. Mycoses 2021, 64, 1098–1110.
- Tullio, V.; Cuffini, A.M.; Giacchino, F.; Mandras, N.; Roana, J.; Comune, L.; Merlino, C.; Carlone, N.A. Combined action of fluconazole and PMNs from uremic patients in clearing intracellular Candida albicans. J. Chemother. 2003, 15, 301–303.
- Tullio, V.; Roana, J.; Scalas, D.; Mandras, N. Enhanced killing of Candida krusei by polymorphonuclear leucocytes in the presence of subinhibitory concentrations of Melaleuca alternifolia and “Mentha of Pancalieri” essential oils. Molecules 2019, 24, 3824.
