Nanoparticles can be synthesized through physical, chemical, and biological routes, where biologically synthesized nanoparticles are also referred to as biogenic-synthesized nanoparticles or bionanoparticles. Bionanoparticles exploit the inherent reducing property of biological entities to develop cost-effective, non-toxic, time-efficient, sustainable, and stable nanosized particles. There is a wide array biomedical focus on metallic nanoparticles, especially silver nanoparticles, due to their distinctive physiochemical properties making them a suitable therapeutic molecule carrier. This article aims to provide a broad insight into the various classes of living organisms that can be exploited for the development of silver nanoparticles, and elaboratively review the interdisciplinary biomedical applications of biogenically synthesized silver nanoparticles in health and life sciences domains.
- nanotechnology
- bionanoparticles
- silver nanoparticles
- biological entities
- biomedical applications
1. Introduction
2. Biosynthesis of AgNPs
2.1. Methods of Biosynthesis: Physical, Chemical, and Biological
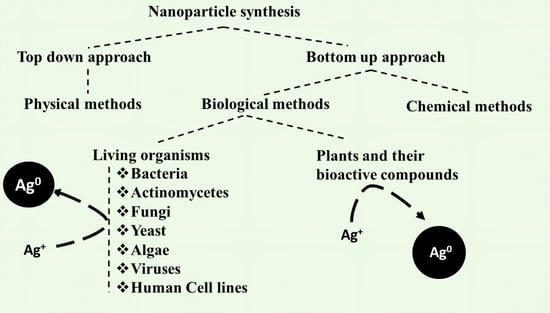
2.2. Biological Species for Nanoparticles Synthesis
2.2.1. Bacteria
2.2.2. Actinomycetes
2.2.3. Fungi
2.2.4. Yeast
2.2.5. Algae
2.2.6. Virus
2.2.7. Plants
2.2.8. Human Cell Line
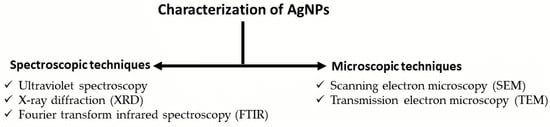
2.3. Characterization Techniques
3. Biomedical Applications
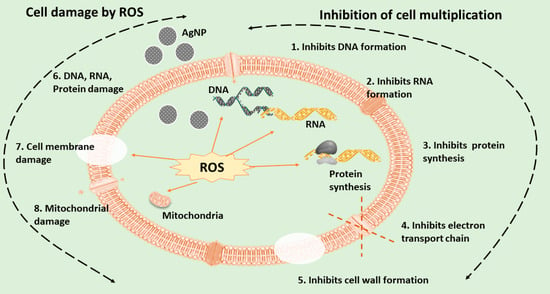
3.1. Antimicrobial Activity and Associated Applications
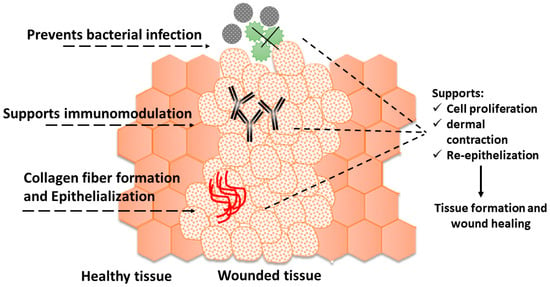
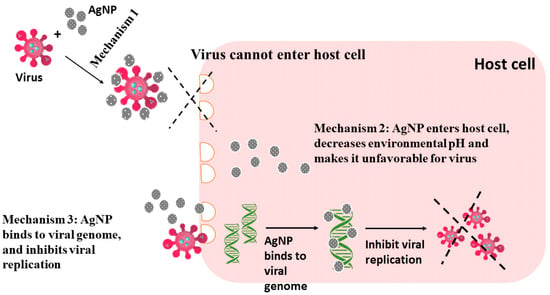
3.2. Antiviral Agents
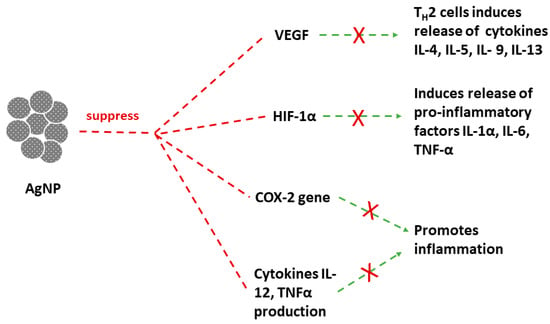
3.3. Other Biological Applications of AgNPs
4. Toxicity Associated with AgNPs
5. Outlook
This entry is adapted from the peer-reviewed paper 10.3390/suschem4010007
References
- Calderón-Jiménez, B.; Johnson, M.E.; Montoro Bustos, A.R.; Murphy, K.E.; Winchester, M.R.; Vega Baudrit, J.R. Silver Nanoparticles: Technological Advances, Societal Impacts, and Metrological Challenges. Front. Chem. 2017, 5, 6.
- Alemán, J.V.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829.
- Hofmann-Amtenbrink, M.; Grainger, D.W.; Hofmann, H. Nanoparticles in medicine: Current challenges facing inorganic nanoparticle toxicity assessments and standardizations. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1689–1694.
- Xu, R.; Wang, D.; Zhang, J.; Li, Y. Shape-dependent catalytic activity of silver nanoparticles for the oxidation of styrene. Chem. Asian J. 2006, 1, 888–893.
- Alshehri, A.H.; Jakubowska, M.; Młożniak, A.; Horaczek, M.; Rudka, D.; Free, C.; Carey, J.D. Enhanced Electrical Conductivity of Silver Nanoparticles for High Frequency Electronic Applications. ACS Appl. Mater. Interfaces 2012, 4, 7007–7010.
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677.
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106.
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780.
- Ramezanpour, M.; Leung, S.S.W.; Delgado-Magnero, K.H.; Bashe, B.Y.M.; Thewalt, J.; Tieleman, D.P. Computational and experimental approaches for investigating nanoparticle-based drug delivery systems. Biochim. Biophys. Acta 2016, 1858, 1688–1709.
- Muhammad, Z.; Raza, A.; Ghafoor, S.; Naeem, A.; Naz, S.S.; Riaz, S.; Ahmed, W.; Rana, N.F. PEG capped methotrexate silver nanoparticles for efficient anticancer activity and biocompatibility. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2016, 91, 251–255.
- Petrov, P.D.; Yoncheva, K.; Gancheva, V.; Konstantinov, S.; Trzebicka, B. Multifunctional block copolymer nanocarriers for co-delivery of silver nanoparticles and curcumin: Synthesis and enhanced efficacy against tumor cells. Eur. Polym. J. 2016, 81, 24–33.
- Al-Obaidi, H.; Kalgudi, R.; Zariwala, M.G. Fabrication of inhaled hybrid silver/ciprofloxacin nanoparticles with synergetic effect against Pseudomonas aeruginosa. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2018, 128, 27–35.
- Kaur, A.; Goyal, D.; Kumar, R. Surfactant mediated interaction of vancomycin with silver nanoparticles. Appl. Surf. Sci. 2018, 449, 23.
- Arumai Selvan, D.; Mahendiran, D.; Senthil Kumar, R.; Kalilur Rahiman, A. Garlic, green tea and turmeric extracts-mediated green synthesis of silver nanoparticles: Phytochemical, antioxidant and in vitro cytotoxicity studies. J. Photochem. Photobiol. B 2018, 180, 243–252.
- Jiang, Q.; Yu, S.; Li, X.; Ma, C.; Li, A. Evaluation of local anesthetic effects of Lidocaine-Ibuprofen ionic liquid stabilized silver nanoparticles in Male Swiss mice. J. Photochem. Photobiol. B 2018, 178, 367–370.
- Karthik, C.S.; Manukumar, H.M.; Ananda, A.P.; Nagashree, S.; Rakesh, K.P.; Mallesha, L.; Qin, H.-L.; Umesha, S.; Mallu, P.; Krishnamurthy, N.B. Synthesis of novel benzodioxane midst piperazine moiety decorated chitosan silver nanoparticle against biohazard pathogens and as potential anti-inflammatory candidate: A molecular docking studies. Int. J. Biol. Macromol. 2018, 108, 489–502.
- Rai, M.; Ingle, A.P.; Gupta, I.; Brandelli, A. Bioactivity of noble metal nanoparticles decorated with biopolymers and their application in drug delivery. Int. J. Pharm. 2015, 496, 159–172.
- Stebounova, L.V.; Guio, E.; Grassian, V.H. Silver nanoparticles in simulated biological media: A study of aggregation, sedimentation, and dissolution. J. Nanoparticle Res. 2011, 13, 233–244.
- Argentiere, S.; Cella, C.; Cesaria, M.; Milani, P.; Lenardi, C. Silver nanoparticles in complex biological media: Assessment of colloidal stability and protein corona formation. J. Nanoparticle Res. 2016, 8, 1–13.
- Gliga, A.R.; Skoglund, S.; Odnevall Wallinder, I.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11.
- Gorham, J.M.; Rohlfing, A.B.; Lippa, K.A.; MacCuspie, R.I.; Hemmati, A.; David Holbrook, R. Storage Wars: How citrate-capped silver nanoparticle suspensions are affected by not-so-trivial decisions. J. Nanoparticle Res. 2014, 16, 2339.
- Kanti Das, T.; Ganguly, S.; Remanan, S.; Das, N.C. Temperature-Dependent Study of Catalytic Ag Nanoparticles Entrapped Resin Nanocomposite towards Reduction of 4-Nitrophenol. ChemistrySelect 2019, 4, 3665–3671.
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291.
- Prasher, P.; Singh, M.; Mudila, H. Silver nanoparticles as antimicrobial therapeutics: Current perspectives and future challenges. 3 Biotech 2018, 8, 411.
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A Review on Plants and Microorganisms Mediated Synthesis of Silver Nanoparticles, Role of Plants Metabolites and Applications. Int. J. Environ. Res. Public. Health 2022, 19, 674.
- Ali, S.; Chen, X.; Ajmal Shah, M.; Ali, M.; Zareef, M.; Arslan, M.; Ahmad, S.; Jiao, T.; Li, H.; Chen, Q. The avenue of fruit wastes to worth for synthesis of silver and gold nanoparticles and their antimicrobial application against foodborne pathogens: A review. Food Chem. 2021, 359, 129912.
- Kaabipour, S.; Hemmati, S. A review on the green and sustainable synthesis of silver nanoparticles and one-dimensional silver nanostructures. Beilstein J. Nanotechnol. 2021, 12, 102–136.
- Gudikandula, K.; Charya Maringanti, S. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016, 11, 714–721.
- Lee, J.; Park, E.Y.; Lee, J. Non-toxic nanoparticles from phytochemicals: Preparation and biomedical application. Bioprocess Biosyst. Eng. 2014, 37, 983–989.
- Saifuddin, N.; Wong, C.W.; Yasumira, A.A.N. Rapid Biosynthesis of Silver Nanoparticles Using Culture Supernatant of Bacteria with Microwave Irradiation. J. Chem. 2009, 6, 61–70.
- Mousavi-Khattat, M.; Keyhanfar, M.; Razmjou, A. A comparative study of stability, antioxidant, DNA cleavage and antibacterial activities of green and chemically synthesized silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1022–S1031.
- Kummara, S.; Patil, M.B.; Uriah, T. Synthesis, characterization, biocompatible and anticancer activity of green and chemically synthesized silver nanoparticles—A comparative study. Biomed. Pharmacother. Biomed. Pharmacother. 2016, 84, 10–21.
- Spagnoletti, F.N.; Kronberg, F.; Spedalieri, C.; Munarriz, E.; Giacometti, R. Protein corona on biogenic silver nanoparticles provides higher stability and protects cells from toxicity in comparison to chemical nanoparticles. J. Environ. Manag. 2021, 297, 113434.
- Sreelekha, E.; George, B.; Shyam, A.; Sajina, N.; Mathew, B. A Comparative Study on the Synthesis, Characterization, and Antioxidant Activity of Green and Chemically Synthesized Silver Nanoparticles. BioNanoScience 2021, 11, 489–496.
- Veeragoni, D.; Deshpande, S.; Rachamalla, H.K.; Ande, A.; Misra, S.; Mutheneni, S.R. In Vitro and In Vivo Anticancer and Genotoxicity Profiles of Green Synthesized and Chemically Synthesized Silver Nanoparticles. ACS Appl. Bio Mater. 2022, 5, 2324–2339.
- Durán, N.; Marcato, P.D.; Durán, M.; Yadav, A.; Gade, A.; Rai, M. Mechanistic aspects in the biogenic synthesis of extracellular metal nanoparticles by peptides, bacteria, fungi, and plants. Appl. Microbiol. Biotechnol. 2011, 90, 1609–1624.
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96.
- Long, D.; Wu, G.; Chen, S. Preparation of oligochitosan stabilized silver nanoparticles by gamma irradiation. Radiat. Phys. Chem. 2007, 76, 1126–1131.
- Chen, J.; Wang, J.; Zhang, X.; Jin, Y. Microwave-assisted green synthesis of silver nanoparticles by carboxymethyl cellulose sodium and silver nitrate. Mater. Chem. Phys. 2008, 108, 421–424.
- Francis, S.; Joseph, S.; Koshy, E.P.; Mathew, B. Microwave assisted green synthesis of silver nanoparticles using leaf extract of elephantopus scaber and its environmental and biological applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 795–804.
- Srikar, S.K.; Giri, D.D.; Pal, D.B.; Mishra, P.K.; Upadhyay, S.N. Green Synthesis of Silver Nanoparticles: A Review. Green Sustain. Chem. 2016, 06, 34–56.
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406.
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638.
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356.
- Naghdi, M.; Taheran, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y.; Valero, J.R. Green and energy-efficient methods for the production of metallic nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 2354–2376.
- Manivasagan, P.; Nam, S.Y.; Oh, J. Marine microorganisms as potential biofactories for synthesis of metallic nanoparticles. Crit. Rev. Microbiol. 2016, 42, 1007–1019.
- Golinska, P.; Wypij, M.; Ingle, A.P.; Gupta, I.; Dahm, H.; Rai, M. Biogenic synthesis of metal nanoparticles from actinomycetes: Biomedical applications and cytotoxicity. Appl. Microbiol. Biotechnol. 2014, 98, 8083–8097.
- Hulkoti, N.I.; Taranath, T.C. Biosynthesis of nanoparticles using microbes- a review. Colloids Surf. B Biointerfaces 2014, 121, 474–483.
- Patil, M.P.; Kim, G.-D. Marine microorganisms for synthesis of metallic nanoparticles and their biomedical applications. Colloids Surf. B Biointerfaces 2018, 172, 487–495.
- Ammar, H.A.; El Aty, A.A.A.; El Awdan, S.A. Extracellular myco-synthesis of nano-silver using the fermentable yeasts Pichia kudriavzeviiHA-NY2 and Saccharomyces uvarumHA-NY3, and their effective biomedical applications. Bioprocess Biosyst. Eng. 2021, 44, 841–854.
- Fredrickson, J.K.; Zachara, J.M.; Balkwill, D.L.; Kennedy, D.; Li, S.W.; Kostandarithes, H.M.; Daly, M.J.; Romine, M.F.; Brockman, F.J. Geomicrobiology of high-level nuclear waste-contaminated vadose sediments at the hanford site, washington state. Appl. Environ. Microbiol. 2004, 70, 4230–4241.
- Kalimuthu, K.; Suresh Babu, R.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. B Biointerfaces 2008, 65, 150–153.
- Shivaji, S.; Madhu, S.; Singh, S. Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process Biochem. 2011, 46, 1800–1807.
- Liu, J.; Qiao, S.Z.; Hu, Q.H.; Lu, G.Q.M. Magnetic nanocomposites with mesoporous structures: Synthesis and applications. Small Weinh. Bergstr. Ger. 2011, 7, 425–443.
- Vaseghi, Z.; Nematollahzadeh, A.; Tavakoli, O. Green methods for the synthesis of metal nanoparticles using biogenic reducing agents: A review. Rev. Chem. Eng. 2018, 34, 529–559.
- Chumpol, J.; Siri, S. Simple green production of silver nanoparticles facilitated by bacterial genomic DNA and their antibacterial activity. Artif. Cells Nanomed. Biotechnol. 2018, 46, 619–625.
- Saravanan, M.; Barik, S.K.; MubarakAli, D.; Prakash, P.; Pugazhendhi, A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb. Pathog. 2018, 116, 221–226.
- Yurtluk, T.; Akçay, F.A.; Avcı, A. Biosynthesis of silver nanoparticles using novel Bacillus sp. SBT8. Prep. Biochem. Biotechnol. 2018, 48, 151–159.
- Arzoo, S.; Naqvi, Z.; Hussain, M.; Shamim, S.; Zeb, T.F.; Ali, S. Production and antimicrobial activity of silver nanoparticles synthesized from indigenously isolated Pseudomonas aeruginosa from Rhizosphere. Pak. J. Pharm. Sci. 2020, 33, 2815–2822.
- Sable, S.V.; Kawade, S.; Ranade, S.; Joshi, S. Bioreduction mechanism of silver nanoparticles. Mater. Sci. Eng. C 2020, 107, 110299.
- Saleem, S.; Iqbal, A.; Hasnain, S. Bacterial mediated silver nanoparticles and their efficacy against MRSA. Trop. Biomed. 2020, 37, 482–488.
- Singh, P.; Pandit, S.; Mokkapati, V.; Garnæs, J.; Mijakovic, I. A Sustainable Approach for the Green Synthesis of Silver Nanoparticles from Solibacillus isronensis sp. and Their Application in Biofilm Inhibition. Molecules 2020, 25, 2783.
- Li, J.; Tian, B.; Li, T.; Dai, S.; Weng, Y.; Lu, J.; Xu, X.; Jin, Y.; Pang, R.; Hua, Y. Biosynthesis of Au, Ag and Au-Ag bimetallic nanoparticles using protein extracts of Deinococcus radiodurans and evaluation of their cytotoxicity. Int. J. Nanomed. 2018, 13, 1411–1424.
- Gupta, A.; Singh, D.; Singh, S.K.; Singh, V.K.; Singh, A.V.; Kumar, A. Role of actinomycetes in bioactive and nanoparticle synthesis. In Role of Plant Growth Promoting Microorganisms in Sustainable Agriculture and Nanotechnology; Woodhead Publishing: Amsterdam, The Netherlands; Elsevier: Amsterdam, The Netherlands, 2019; pp. 163–182. ISBN 978-0-12-817004-5.
- Das, S.; Lyla, P.S.; Khan, S.A. Distribution and generic composition of culturable marine actinomycetes from the sediments of Indian continental slope of Bay of Bengal. Chin. J. Oceanol. Limnol. 2008, 26, 166–177.
- Abdeen, S.; Geo, S.; Praseetha, P.K.; Dhanya, R.P. Biosynthesis of silver nanoparticles from Actinomycetes for therapeutic applications. Int. J. Nano Dimens. 2014, 5, 155–162.
- Bhatti, A.A.; Haq, S.; Bhat, R.A. Actinomycetes benefaction role in soil and plant health. Microb. Pathog. 2017, 111, 458–467.
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Ramani, R.; Srinivas, V.; Sastry, M. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology 2003, 14, 824–828.
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular Biosynthesis of Monodisperse Gold Nanoparticles by a Novel Extremophilic Actinomycete, Thermomonospora sp. Langmuir 2003, 19, 3550–3553.
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.-K. Actinobacteria mediated synthesis of nanoparticles and their biological properties: A review. Crit. Rev. Microbiol. 2016, 42, 209–221.
- Wypij, M.; Czarnecka, J.; Świecimska, M.; Dahm, H.; Rai, M.; Golinska, P. Synthesis, characterization and evaluation of antimicrobial and cytotoxic activities of biogenic silver nanoparticles synthesized from Streptomyces xinghaiensis OF1 strain. World J. Microbiol. Biotechnol. 2018, 34, 23.
- Pallavi, S.S.; Rudayni, H.A.; Bepari, A.; Niazi, S.K.; Nayaka, S. Green synthesis of Silver nanoparticles using Streptomyces hirsutus strain SNPGA-8 and their characterization, antimicrobial activity, and anticancer activity against human lung carcinoma cell line A549. Saudi J. Biol. Sci. 2022, 29, 228–238.
- Mabrouk, M.; Elkhooly, T.A.; Amer, S.K. Actinomycete strain type determines the monodispersity and antibacterial properties of biogenically synthesized silver nanoparticles. J. Genet. Eng. Biotechnol. 2021, 19, 57.
- Dhillon, G.S.; Brar, S.K.; Kaur, S.; Verma, M. Green approach for nanoparticle biosynthesis by fungi: Current trends and applications. Crit. Rev. Biotechnol. 2012, 32, 49–73.
- Guilger-Casagrande, M.; Lima, R. de Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7.
- Korbekandi, H.; Asghari, G.; Chitsazi, M.R.; Bahri Najafi, R.; Badii, A.; Iravani, S. Green biosynthesis of silver nanoparticles using Althaea officinalis radix hydroalcoholic extract. Artif. Cells Nanomed. Biotechnol. 2016, 44, 209–215.
- Fernández, J.G.; Fernández-Baldo, M.A.; Berni, E.; Camí, G.; Durán, N.; Raba, J.; Sanz, M.I. Production of silver nanoparticles using yeasts and evaluation of their antifungal activity against phytopathogenic fungi. Process Biochem. 2016, 51, 1306–1313.
- Yadav, A.; Kon, K.; Kratosova, G.; Duran, N.; Ingle, A.P.; Rai, M. Fungi as an efficient mycosystem for the synthesis of metal nanoparticles: Progress and key aspects of research. Biotechnol. Lett. 2015, 37, 2099–2120.
- Soleimani, P.; Mehrvar, A.; Michaud, J.P.; Vaez, N. Optimization of silver nanoparticle biosynthesis by entomopathogenic fungi and assays of their antimicrobial and antifungal properties. J. Invertebr. Pathol. 2022, 190, 107749.
- Koli, S.H.; Mohite, B.V.; Suryawanshi, R.K.; Borase, H.P.; Patil, S.V. Extracellular red Monascus pigment-mediated rapid one-step synthesis of silver nanoparticles and its application in biomedical and environment. Bioprocess Biosyst. Eng. 2018, 41, 715–727.
- Ansari, A.; Pervez, S.; Javed, U.; Abro, M.I.; Nawaz, M.A.; Qader, S.A.U.; Aman, A. Characterization and interplay of bacteriocin and exopolysaccharide-mediated silver nanoparticles as an antibacterial agent. Int. J. Biol. Macromol. 2018, 115, 643–650.
- Li, P.-J.; Pan, J.-J.; Tao, L.-J.; Li, X.; Su, D.-L.; Shan, Y.; Li, H.-Y. Green Synthesis of Silver Nanoparticles by Extracellular Extracts from Aspergillus japonicus PJ01. Molecules 2021, 26, 4479.
- Wang, D.; Xue, B.; Wang, L.; Zhang, Y.; Liu, L.; Zhou, Y. Fungus-mediated green synthesis of nano-silver using Aspergillus sydowii and its antifungal/antiproliferative activities. Sci. Rep. 2021, 11, 10356.
- Yeast—Industrial Applications; Morata, A.; Loira, I. (Eds.) InTech: Rijeka, Croatia, 2017; ISBN 978-953-51-3599-9.
- Jha, A.K.; Prasad, K.; Kulkarni, A.A.R. Yeast Mediated Synthesis of Silver Nanoparticles. Int. J. Nanosci. Nanotechnol. 2008, 4, 17–22.
- Boroumand Moghaddam, A.; Namvar, F.; Moniri, M.; Tahir, P.; Azizi, S.; Mohamad, R. Nanoparticles Biosynthesized by Fungi and Yeast: A Review of Their Preparation, Properties, and Medical Applications. Molecules 2015, 20, 16540–16565.
- Breierová, E.; Vajcziková, I.; Sasinková, V.; Stratilová, E.; Fišera, M.; Gregor, T.; Šajbidor, J. Biosorption of Cadmium Ions by Different Yeast Species. Z. Für Nat. C 2002, 57, 634–639.
- Skalickova, S.; Baron, M.; Sochor, J. Nanoparticles Biosynthesized by Yeast: A Review of their application. Kvas. Prum. 2017, 63, 290–292.
- Cunha, F.A.; da, C.S.O. Cunha, M.; da Frota, S.M.; Mallmann, E.J.J.; Freire, T.M.; Costa, L.S.; Paula, A.J.; Menezes, E.A.; Fechine, P.B.A. Biogenic synthesis of multifunctional silver nanoparticles from Rhodotorula glutinis and Rhodotorula mucilaginosa: Antifungal, catalytic and cytotoxicity activities. World J. Microbiol. Biotechnol. 2018, 34, 127.
- Sharma, P.; Sharma, N. Industrial and Biotechnological Applications of Algae: A Review. J. Adv. Plant Biol. 2017, 1, 1–25.
- Sharma, A.; Sharma, S.; Sharma, K.; Chetri, S.P.K.; Vashishtha, A.; Singh, P.; Kumar, R.; Rathi, B.; Agrawal, V. Algae as crucial organisms in advancing nanotechnology: A systematic review. J. Appl. Phycol. 2016, 28, 1759–1774.
- Schröfel, A.; Kratošová, G.; Bohunická, M.; Dobrocka, E.; Vávra, I. Biosynthesis of gold nanoparticles using diatoms—Silica-gold and EPS-gold bionanocomposite formation. J. Nanoparticle Res. 2011, 13, 3207–3216.
- Dahoumane, S.A.; Mechouet, M.; Wijesekera, K.; Filipe, C.D.M.; Sicard, C.; Bazylinski, D.A.; Jeffryes, C. Algae-mediated biosynthesis of inorganic nanomaterials as a promising route in nanobiotechnology—A review. Green Chem. 2017, 19, 552–587.
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600.
- Sathishkumar, R.S.; Sundaramanickam, A.; Srinath, R.; Ramesh, T.; Saranya, K.; Meena, M.; Surya, P. Green synthesis of silver nanoparticles by bloom forming marine microalgae Trichodesmium erythraeum and its applications in antioxidant, drug-resistant bacteria, and cytotoxicity activity. J. Saudi Chem. Soc. 2019, 23, 1180–1191.
- Dahoumane, S.A.; Wujcik, E.K.; Jeffryes, C. Noble metal, oxide and chalcogenide-based nanomaterials from scalable phototrophic culture systems. Enzym. Microb. Technol. 2016, 95, 13–27.
- Rao, S.S.; Saptami, K.; Venkatesan, J.; Rekha, P.D. Microwave-assisted rapid synthesis of silver nanoparticles using fucoidan: Characterization with assessment of biocompatibility and antimicrobial activity. Int. J. Biol. Macromol. 2020, 163, 745–755.
- Bao, Z.; Cao, J.; Kang, G.; Lan, C.Q. Effects of reaction conditions on light-dependent silver nanoparticle biosynthesis mediated by cell extract of green alga Neochloris oleoabundans. Environ. Sci. Pollut. Res. Int. 2019, 26, 2873–2881.
- Husain, S.; Verma, S.K.; Yasin, D.; Hemlata, N.A.N.; Rizvi, M.M.; Fatma, T. Facile green bio-fabricated silver nanoparticles from Microchaete infer dose-dependent antioxidant and anti-proliferative activity to mediate cellular apoptosis. Bioorganic Chem. 2021, 107, 104535.
- Narayanan, K.B.; Han, S.S. Helical plant viral nanoparticles-bioinspired synthesis of nanomaterials and nanostructures. Bioinspir. Biomim. 2017, 12, 031001.
- Kobayashi, M.; Tomita, S.; Sawada, K.; Shiba, K.; Yanagi, H.; Yamashita, I.; Uraoka, Y. Chiral meta-molecules consisting of gold nanoparticles and genetically engineered tobacco mosaic virus. Opt. Express 2012, 20, 24856–24863.
- Young, M.; Debbie, W.; Uchida, M.; Douglas, T. Plant Viruses as Biotemplates for Materials and Their Use in Nanotechnology. Annu. Rev. Phytopathol. 2008, 46, 361–384.
- Gahlawat, G.; Choudhury, A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019, 9, 12944–12967.
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161.
- Marchiol, L. Synthesis of metal nanoparticles in living plants. Ital. J. Agron. 2012, 7, 37.
- Park, Y.; Hong, Y.N.; Weyers, A.; Kim, Y.S.; Linhardt, R.J. Polysaccharides and phytochemicals: A natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol. 2011, 5, 69.
- Rajeshkumar, S.; Bharath, L.V. Mechanism of plant-mediated synthesis of silver nanoparticles—A review on biomolecules involved, characterisation and antibacterial activity. Chem. Biol. Interact. 2017, 273, 219–227.
- Rice-evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The Relative Antioxidant Activities of Plant-Derived Polyphenolic Flavonoids. Free Radic. Res. 1995, 22, 375–383.
- Tamuly, C.; Hazarika, M.; Bordoloi, M.; Bhattacharyya, P.K.; Kar, R. Biosynthesis of Ag nanoparticles using pedicellamide and its photocatalytic activity: An eco-friendly approach. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2014, 132, 687–691.
- Yang, X.; Feng, Y.; He, Z.; Stoffella, P.J. Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2005, 18, 339–353.
- Milner, M.J.; Kochian, L.V. Investigating heavy-metal hyperaccumulation using Thlaspi caerulescens as a model system. Ann. Bot. 2008, 102, 3–13.
- Kumar, V.; Yadav, S.K. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 2009, 84, 151–157.
- Mukunthan, K.S.; Balaji, S. Cashew Apple Juice ( Anacardium occidentale L.) Speeds Up the Synthesis of Silver Nanoparticles. Int. J. Green Nanotechnol. 2012, 4, 71–79.
- Ahmed, K.B.A.; Subramaniam, S.; Veerappan, G.; Hari, N.; Sivasubramanian, A.; Veerappan, A. β-Sitosterol-D-glucopyranoside isolated from Desmostachya bipinnata mediates photoinduced rapid green synthesis of silver nanoparticles. RSC Adv. 2014, 4, 59130–59136.
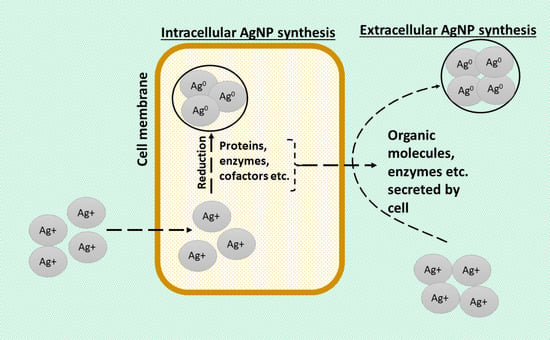
- Subramanian, P.; Shanmugam, K. Extracellular and intracellular synthesis of silver nanoparticles. Asian J. Pharm. Clin. Res. 2016, 9, 133–139.
- Rabia Kanwar, R.F.; Khalid, A. 2. Biological, physical and chemical synthesis of silver nanoparticles and their non-toxic bio-chemical application: A brief review. Pure Appl. Biol. PAB 2021, 11, 421–438.
- Sharma, N.K.; Vishwakarma, J.; Rai, S.; Alomar, T.S.; AlMasoud, N.; Bhattarai, A. Green Route Synthesis and Characterization Techniques of Silver Nanoparticles and Their Biological Adeptness. ACS Omega 2022, 7, 27004–27020.
- Tomaszewska, E.; Ranoszek-Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection Limits of DLS and UV-Vis Spectroscopy in Characterization of Polydisperse Nanoparticles Colloids. J. Nanomater. 2013, 2013, 313081.
- Link, S.; El-Sayed, M.A. Optical Properties and Ultrafast Dynamics of Metallic Nanocrystals. Annu. Rev. Phys. Chem. 2003, 54, 331–366.
- Noginov, M.A.; Zhu, G.; Bahoura, M.; Adegoke, J.; Small, C.; Ritzo, B.A.; Drachev, V.P.; Shalaev, V.M. The effect of gain and absorption on surface plasmons in metal nanoparticles. Appl. Phys. B 2007, 86, 455–460.
- He, R.; Qian, X.; Yin, J.; Zhu, Z. Preparation of polychrome silver nanoparticles in different solvents. J. Mater. Chem. 2002, 12, 3783–3786.
- Rani, P.; Kumar, V.; Singh, P.P.; Matharu, A.S.; Zhang, W.; Kim, K.-H.; Singh, J.; Rawat, M. Highly stable AgNPs prepared via a novel green approach for catalytic and photocatalytic removal of biological and non-biological pollutants. Environ. Int. 2020, 143, 105924.
- Majeed Khan, M.A.; Kumar, S.; Ahamed, M.; Alrokayan, S.A.; AlSalhi, M.S. Structural and thermal studies of silver nanoparticles and electrical transport study of their thin films. Nanoscale Res. Lett. 2011, 6, 434.
- Parit, S.B.; Karade, V.C.; Patil, R.B.; Pawar, N.V.; Dhavale, R.P.; Tawre, M.; Pardesi, K.; Jadhav, U.U.; Dawkar, V.V.; Tanpure, R.S.; et al. Bioinspired synthesis of multifunctional silver nanoparticles for enhanced antimicrobial and catalytic applications with tailored SPR properties. Mater. Today Chem. 2020, 17, 100285.
- Mehta, B.K.; Chhajlani, M.; Shrivastava, B.D. Green synthesis of silver nanoparticles and their characterization by XRD. J. Phys. Conf. Ser. 2017, 836, 012050.
- Fissan, H.; Ristig, S.; Kaminski, H.; Asbach, C.; Epple, M. Comparison of different characterization methods for nanoparticle dispersions before and after aerosolization. Anal. Methods 2014, 6, 7324–7334.
- Hall, J.B.; Dobrovolskaia, M.A.; Patri, A.K.; McNeil, S.E. Characterization of nanoparticles for therapeutics. Nanomed. 2007, 2, 789–803.
- Reymond-Laruinaz, S.; Saviot, L.; Potin, V.; Marco de Lucas, M. del C. Protein–nanoparticle interaction in bioconjugated silver nanoparticles: A transmission electron microscopy and surface enhanced Raman spectroscopy study. Appl. Surf. Sci. 2016, 389, 17–24.
- Qayyum, S.; Khan, A.U. Biofabrication of broad range antibacterial and antibiofilm silver nanoparticles. IET Nanobiotechnol. 2016, 10, 349–357.
- Lateef, A. The microbiology of a pharmaceutical effluent and its public health implications. World J. Microbiol. Biotechnol. 2004, 20, 167–171.
- Lateef, A.; Oloke, J.K.; Gueguim-Kana, E.B. Antimicrobial resistance of bacterial strains isolated from orange juice products. Afr. J. Biotechnol. 2004, 3, 334–338.
- Adewoye, S.O.; Lateef, A. Assessment of the Microbiological Quality of Clarias gariepinus Exposed to an Industrial Effluent in Nigeria. Environmentalist 2004, 24, 249–254.
- Eltarahony, M.; Zaki, S.; ElKady, M.; Abd-El-Haleem, D. Biosynthesis, Characterization of Some Combined Nanoparticles, and Its Biocide Potency against a Broad Spectrum of Pathogens. J. Nanomater. 2018, 2018, 5263814.
- Chandrasekaran, R.; Seetharaman, P.; Krishnan, M.; Gnanasekar, S.; Sivaperumal, S. Carica papaya (Papaya) latex: A new paradigm to combat against dengue and filariasis vectors Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). 3 Biotech 2018, 8, 83.
- Arunasri, K.; Mohan, S.V. Biofilms. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 295–313. ISBN 978-0-444-64052-9.
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512.
- Malheiro, J.; Simões, M. Antimicrobial resistance of biofilms in medical devices. In Biofilms and Implantable Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; pp. 97–113. ISBN 978-0-08-100382-4.
- Almaguer-Flores, A. Biofilms in the oral environment. In Bio-Tribocorrosion in Biomaterials and Medical Implants; Woodhead Publishing Limited: Sawston, UK; Elsevier: Sawston, UK, 2013; pp. 169–186. ISBN 978-0-85709-540-4.
- Loza-Correa, M.; Ramírez-Arcos, S. Detection of bacterial adherence and biofilm formation on medical surfaces. In Biofilms and Implantable Medical Devices; Woodhead Publishing: Duxford, UK; Woodhead Publishing: Cambridge, MA, USA; Woodhead Publishing: Kidlington, UK; Elsevier: Duxford, UK; Elsevier: Cambridge, MA, USA; Elsevier: Kidlington, UK, 2017; pp. 181–193. ISBN 978-0-08-100382-4.
- Alt, V.; Bechert, T.; Steinrücke, P.; Wagener, M.; Seidel, P.; Dingeldein, E.; Domann, E.; Schnettler, R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials 2004, 25, 4383–4391.
- van de Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Infection of orthopedic implants and the use of antibiotic-loaded bone cements. A review. Acta Orthop. Scand. 2001, 72, 557–571.
- Liu, Y.; Zheng, Z.; Zara, J.N.; Hsu, C.; Soofer, D.E.; Lee, K.S.; Siu, R.K.; Miller, L.S.; Zhang, X.; Carpenter, D.; et al. The antimicrobial and osteoinductive properties of silver nanoparticle/poly (DL-lactic-co-glycolic acid)-coated stainless steel. Biomaterials 2012, 33, 8745–8756.
- Zheng, Z.; Yin, W.; Zara, J.N.; Li, W.; Kwak, J.; Mamidi, R.; Lee, M.; Siu, R.K.; Ngo, R.; Wang, J.; et al. The use of BMP-2 coupled—Nanosilver-PLGA composite grafts to induce bone repair in grossly infected segmental defects. Biomaterials 2010, 31, 9293–9300.
- Magalhães, A.P.R.; Santos, L.B.; Lopes, L.G.; Estrela, C.R.D.A.; Estrela, C.; Torres, M.; Bakuzis, A.F.; Cardoso, P.C.; Carrião, M.S. Nanosilver Application in Dental Cements. Int. Sch. Res. Not. 2012, 2012, e365438.
- Akhavan, A.; Sodagar, A.; Mojtahedzadeh, F.; Sodagar, K. Investigating the effect of incorporating nanosilver/nanohydroxyapatite particles on the shear bond strength of orthodontic adhesives. Acta Odontol. Scand. 2013, 71, 1038–1042.
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent. Sci. 2017, 3, 163–175.
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460.
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874.
- Singh, R. Prospects of Nanobiomaterials for Biosensing. Int. J. Electrochem. 2011, 2011, 125487.
- Ehlerding, E.B.; Grodzinski, P.; Cai, W.; Liu, C.H. Big Potential from Small Agents: Nanoparticles for Imaging-Based Companion Diagnostics. ACS Nano 2018, 12, 2106–2121.
- Liu, Y.; Zeng, S.; Liu, Y.; Wu, W.; Shen, Y.; Zhang, L.; Li, C.; Chen, H.; Liu, A.; Shen, L.; et al. Synthesis and antidiabetic activity of selenium nanoparticles in the presence of polysaccharides from Catathelasma ventricosum. Int. J. Biol. Macromol. 2018, 114, 632–639.
- Franková, J.; Pivodová, V.; Vágnerová, H.; Juránová, J.; Ulrichová, J. Effects of silver nanoparticles on primary cell cultures of fibroblasts and keratinocytes in a wound-healing model. J. Appl. Biomater. Funct. Mater. 2016, 14, 137–142.
- Lee, C.G.; Link, H.; Baluk, P.; Homer, R.J.; Chapoval, S.; Bhandari, V.; Kang, M.J.; Cohn, L.; Kim, Y.K.; McDonald, D.M.; et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat. Med. 2004, 10, 1095–1103.
- Barnes, P.J. Th2 cytokines and asthma: An introduction. Respir. Res. 2001, 2, 64.
- Imtiyaz, H.Z.; Simon, M.C. Hypoxia-Inducible Factors as Essential Regulators of Inflammation. In Diverse Effects of Hypoxia on Tumor Progression; Simon, M.C., Ed.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 345, pp. 105–120. ISBN 978-3-642-13328-2.
- Lin, N.; Simon, M.C. Hypoxia-inducible factors: Key regulators of myeloid cells during inflammation. J. Clin. Investig. 2016, 126, 3661–3671.
- Kanipandian, N.; Kannan, S.; Ramesh, R.; Subramanian, P.; Thirumurugan, R. Characterization, antioxidant and cytotoxicity evaluation of green synthesized silver nanoparticles using Cleistanthus collinus extract as surface modifier. Mater. Res. Bull. 2014, 49, 494–502.
- Reddy, N.J.; Nagoor Vali, D.; Rani, M.; Rani, S.S. Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 115–122.
- Shanmugasundaram, T.; Radhakrishnan, M.; Gopikrishnan, V.; Pazhanimurugan, R.; Balagurunathan, R. A study of the bactericidal, anti-biofouling, cytotoxic and antioxidant properties of actinobacterially synthesised silver nanoparticles. Colloids Surf. B Biointerfaces 2013, 111, 680–687.
- Karami Mehrian, S.; Heidari, R.; Rahmani, F. Effect of silver nanoparticles on free amino acids content and antioxidant defense system of tomato plants. Indian J. Plant Physiol. 2015, 20, 257–263.
- Alavi, M.; Karimi, N.; Valadbeigi, T. Antibacterial, Antibiofilm, Antiquorum Sensing, Antimotility, and Antioxidant Activities of Green Fabricated Ag, Cu, TiO2, ZnO, and Fe3O4 NPs via Protoparmeliopsis muralis Lichen Aqueous Extract against Multi-Drug-Resistant Bacteria. ACS Biomater. Sci. Eng. 2019, 5, 4228–4243.
- Jones, A.-A.D.; Mi, G.; Webster, T.J. A Status Report on FDA Approval of Medical Devices Containing Nanostructured Materials. Trends Biotechnol. 2019, 37, 117–120.
- El Moussaoui, A.; Jawhari, F.Z.; Almehdi, A.M.; Elmsellem, H.; Fikri Benbrahim, K.; Bousta, D.; Bari, A. Antibacterial, antifungal and antioxidant activity of total polyphenols of Withania frutescens L. Bioorganic Chem. 2019, 93, 103337.
- Sharma, H.; Mishra, P.K.; Talegaonkar, S.; Vaidya, B. Metal nanoparticles: A theranostic nanotool against cancer. Drug Discov. Today 2015, 20, 1143–1151.
- Patra, S.; Mukherjee, S.; Barui, A.K.; Ganguly, A.; Sreedhar, B.; Patra, C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 53, 298–309.
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S.; B, V.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 2014, 4, 316–335.
- Davalos, D.; Akassoglou, K. Fibrinogen as a key regulator of inflammation in disease. Semin. Immunopathol. 2012, 34, 43–62.
- Levi, M.; Schultz, M.; van der Poll, T. Disseminated intravascular coagulation in infectious disease. Semin. Thromb. Hemost. 2010, 36, 367–377.
- Wang, L.; Zhang, T.; Li, P.; Huang, W.; Tang, J.; Wang, P.; Liu, J.; Yuan, Q.; Bai, R.; Li, B.; et al. Use of Synchrotron Radiation-Analytical Techniques To Reveal Chemical Origin of Silver-Nanoparticle Cytotoxicity. ACS Nano 2015, 9, 6532–6547.
- Tolaymat, T.M.; El Badawy, A.M.; Genaidy, A.; Scheckel, K.G.; Luxton, T.P.; Suidan, M. An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: A systematic review and critical appraisal of peer-reviewed scientific papers. Sci. Total Environ. 2010, 408, 999–1006.
- Park, J.; Lim, D.-H.; Lim, H.-J.; Kwon, T.; Choi, J.; Jeong, S.; Choi, I.-H.; Cheon, J. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem. Commun. Camb. Engl. 2011, 47, 4382–4384.
- Sung, J.H.; Ji, J.H.; Song, K.S.; Lee, J.H.; Choi, K.H.; Lee, S.H.; Yu, I.J. Acute inhalation toxicity of silver nanoparticles. Toxicol. Ind. Health 2011, 27, 149–154.
- Cho, Y.-M.; Mizuta, Y.; Akagi, J.; Toyoda, T.; Sone, M.; Ogawa, K. Size-dependent acute toxicity of silver nanoparticles in mice. J. Toxicol. Pathol. 2018, 31, 73–80.
- Moradi-Sardareh, H.; Basir, H.R.G.; Hassan, Z.M.; Davoudi, M.; Amidi, F.; Paknejad, M. Toxicity of silver nanoparticles on different tissues of Balb/C mice. Life Sci. 2018, 211, 81–90.
- Roshan, R.; Ranjbar, S. Toxicological effects of silver nanoparticles in rats. Afr. J. Microbiol. Res. 2012, 6, 5587–5593.
- Wen, H.; Dan, M.; Yang, Y.; Lyu, J.; Shao, A.; Cheng, X.; Chen, L.; Xu, L. Acute toxicity and genotoxicity of silver nanoparticle in rats. PLoS ONE 2017, 12, e0185554.
- Mazen, N.F.; Saleh, E.Z.; Mahmoud, A.A.; Shaalan, A.A. Histological and immunohistochemical study on the potential toxicity of sliver nanoparticles on the structure of the spleen in adult male albino rats. Egypt. J. Histol. 2017, 40, 374–387.
- Trickler, W.J.; Lantz, S.M.; Murdock, R.C.; Schrand, A.M.; Robinson, B.L.; Newport, G.D.; Schlager, J.J.; Oldenburg, S.J.; Paule, M.G.; Slikker, W., Jr.; et al. Silver Nanoparticle Induced Blood-Brain Barrier Inflammation and Increased Permeability in Primary Rat Brain Microvessel Endothelial Cells. Toxicol. Sci. 2010, 118, 160–170.
- Khan, A.M.; Korzeniowska, B.; Gorshkov, V.; Tahir, M.; Schrøder, H.; Skytte, L.; Rasmussen, K.L.; Khandige, S.; Møller-Jensen, J.; Kjeldsen, F. Silver nanoparticle-induced expression of proteins related to oxidative stress and neurodegeneration in an in vitro human blood-brain barrier model. Nanotoxicology 2019, 13, 221–239.
- Xu, F.; Piett, C.; Farkas, S.; Qazzaz, M.; Syed, N.I. Silver nanoparticles (AgNPs) cause degeneration of cytoskeleton and disrupt synaptic machinery of cultured cortical neurons. Mol. Brain 2013, 6, 29.
- Repar, N.; Li, H.; Aguilar, J.S.; Li, Q.Q.; Damjana, D.; Hong, Y. Silver nanoparticles induce neurotoxicity in a human embryonic stem cell-derived neuron and astrocyte network. Nanotoxicology 2018, 12, 104–116.
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Köller, M. The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv. 2012, 2, 6981–6987.
- Zanette, C.; Pelin, M.; Crosera, M.; Adami, G.; Bovenzi, M.; Larese, F.F.; Florio, C. Silver nanoparticles exert a long-lasting antiproliferative effect on human keratinocyte HaCaT cell line. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2011, 25, 1053–1060.
- Huang, H.; Lai, W.; Cui, M.; Liang, L.; Lin, Y.; Fang, Q.; Liu, Y.; Xie, L. An Evaluation of Blood Compatibility of Silver Nanoparticles. Sci. Rep. 2016, 6, 25518.
- Chen, L.Q.; Fang, L.; Ling, J.; Ding, C.Z.; Kang, B.; Huang, C.Z. Nanotoxicity of silver nanoparticles to red blood cells: Size dependent adsorption, uptake, and hemolytic activity. Chem. Res. Toxicol. 2015, 28, 501–509.
- Shathviha, P.C.; Ezhilarasan, D.; Rajeshkumar, S.; Selvaraj, J. β-sitosterol Mediated Silver Nanoparticles Induce Cytotoxicity in Human Colon Cancer HT-29 Cells. Avicenna J. Med. Biotechnol. 2021, 13, 42–46.
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290.
- Foldbjerg, R.; Dang, D.A.; Autrup, H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch. Toxicol. 2011, 85, 743–750.
- Singh, S.; Bhargava, D.; Dubey, V.; Mishra, A.; Singh, Y. Silver nanoparticles: Biomedical applications, toxicity, and safety issues. Int. J. Res. Pharm. Pharm. Sci. 2017, 2, 1–10.
- Zhang, T.; Wang, L.; Chen, Q.; Chen, C. Cytotoxic Potential of Silver Nanoparticles. Yonsei Med. J. 2014, 55, 283.
