Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Mitogen-activated protein kinase (MAPK) pathways represent ubiquitous cellular signal transduction pathways that regulate all aspects of life (such as development of eye) and are frequently altered in disease. Once activated through phosphorylation, these MAPKs in turn phosphorylate and activate transcription factors present either in the cytoplasm or in the nucleus, leading to the expression of target genes and, as a consequence, they elicit various biological responses.
- MAPK
- ERK
- p38
- JNK
- eye
- ocular
- MEK
- inhibitors
1. Introduction
The mitogen-activated protein kinase (MAPK or MAP kinase) family consists of protein kinases that phosphorylate their own dual serine (Ser) and threonine (Thr) residues (autophosphorylation), or those found on their substrate downstream kinases, to activate or de-activate their target [1]. MAPKs are ubiquitously expressed and evolutionarily conserved in eukaryotes. Each group of MAPKs contains a multi-tiered signaling cascade of kinases: at the top upstream level of the canonical MAPK pathways there are the MAPK kinase kinase kinases (MAPKKKKs, or MAP4Ks), which act upon MAPK kinase kinases (MAPKKK, or MAP3Ks), which then act in turn on MAPK kinases (MAPKKs, or MAP2Ks), with a final effector MAPK as their target (Figure 1). MAP3Ks are Ser/Thr protein kinases that are activated through phosphorylation., which, in turn, leads to the phosphorylation and activation of MAP2Ks in their Ser/Thr activation site (Ser-X-X-X-Ser/Thr motif). Activated MAP2Ks then stimulate MAPK activity through dual phosphorylation on Thr and Tyr residues within a conserved Thr-X-Tyr motif located in the activation loop of the MAPK domain [2] (Figure 1). A comprehensive list of MAPKs, MAP2Ks and MAPKs is presented in Table 1. MAPKs mainly include four subfamilies based on the conserved Thr-X-Tyr motif: ERK1/2, the JNK1/2/3, the p38 (α, β, γ, and δ), and the ERK5 branches, which are all ultimately activated by signaling cascades initiated by multiple factors such as growth factors and stress. More details on the signaling pathway members are given elsewhere [3]. Once activated through phosphorylation, these MAPKs in turn phosphorylate and activate an array of transcription factors present in the cytoplasm and nucleus, leading to the expression of target genes and resulting in a biological response. MAPKs are involved in multiple cellular processes, such as cell differentiation, proliferation, apoptosis, inflammation, stress responses, and immune defense [4]. In general, the activation of ERK by growth factors, hormones and proinflammatory stimuli promotes cell proliferation, whereas the activation of p38 and JNK by cellular and environmental stresses promotes multiple cellular processes such as proliferation, apoptosis, immunological effects, insulin signaling and neuronal activity [5]. The ERK pathway was the first MAPK cascade to be elucidated and is the best characterized. The canonical intracellular part of the activation pathway starts when a Ras GTPase exchanges a guanosine diphosphate (GDP) for a guanosine triphosphate (GTP) molecule [2]. This is facilitated upon the binding of extracellular mitogens to a cell surface receptor such as EGFR and the subsequent docking and activation of intracellular complexes, for instance GRB2-SOS [2]. This switching of Ras allows it to activate a MAP3K (e.g., Raf) and initiate the cascade of a MEK1/2 (MAP2Ks) and ERK1/2 (MAPK) activation (Figure 1). More generally, the ERK1/2 pathway is stimulated in mammalian cells by tyrosine kinase receptors and G-protein-coupled receptors through both Ras-dependent and Ras-independent pathways [6]. ERK1/2 is also activated by growth factors, mitogens, cytokines, osmotic stress, and in response to insulin [2]. Given its central role in cell proliferation, differentiation and survival, the MAPK pathway network and its inhibition has attracted great pharmacological interest in the context of cancer research, and a plethora of compounds have been developed/identified that can directly act on this pathway to influence cell fate.
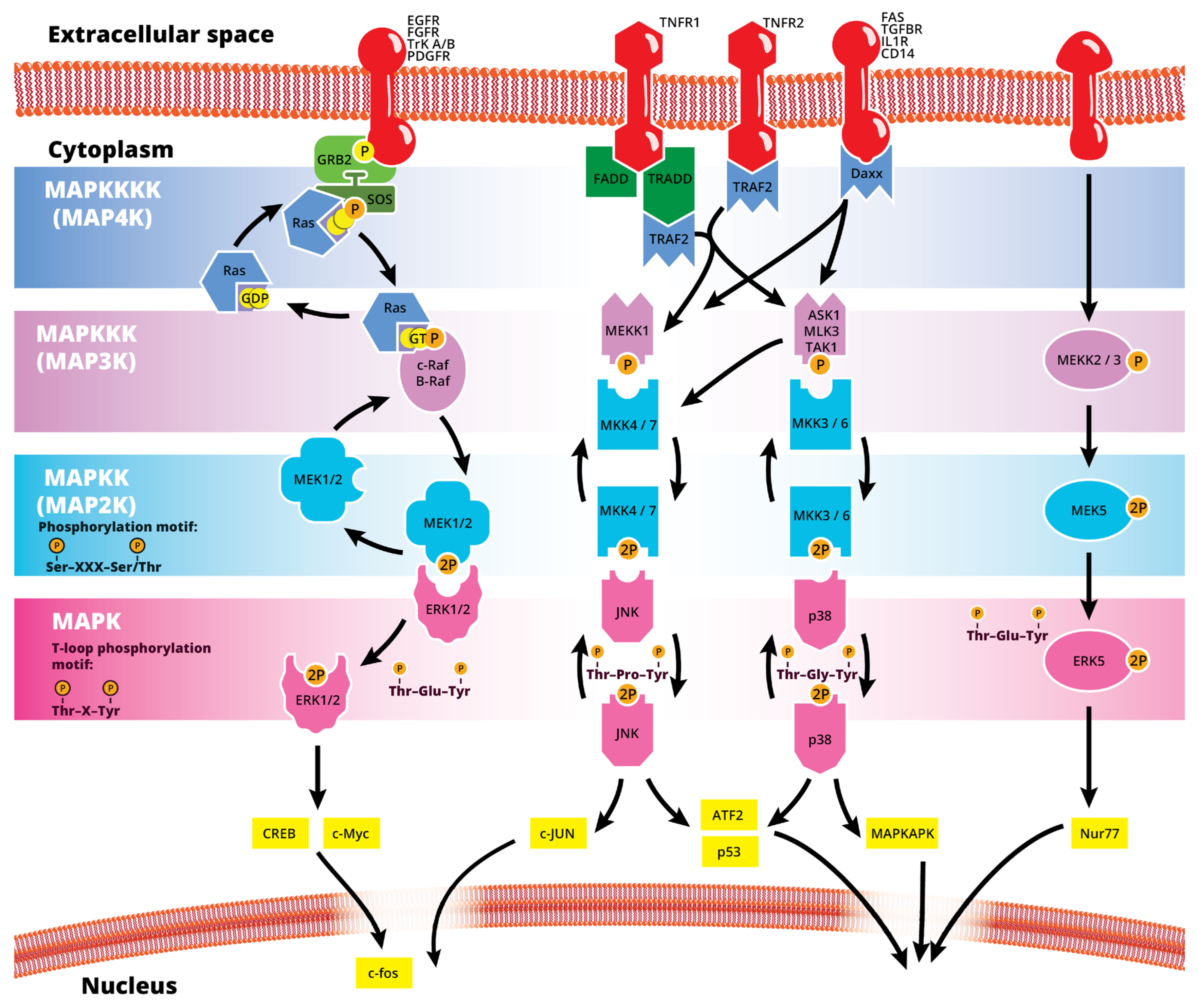
Figure 1. Simplified schematic summary of the main MAPK signaling pathways.
Table 1. Summary of MAP kinases (up to MAP3Ks) with gene names, protein names and alternative names, the pathways they are known to interact with and their relative level at the signaling cascade.
| Gene Name | Protein Name | Alternative Protein Names | Pathway Involved | MAPK Level | Other Gene/Protein Names |
|---|---|---|---|---|---|
| MAPK1 | ERK2 | p42-MAPK | MEK/ERK | MAPK | MAPK2, p38, p40, p41, ERT1, NS13 |
| MAPK3 | ERK1 | p44-MAPK | MEK/ERK | MAPK | ERT2, PRKM3 |
| MAPK4 | ERK4 | p63-MAPK | atypical MAPK | MAPK | PRKM4 |
| MAPK6 | ERK3 | p97-MAPK | atypical MAPK | MAPK | PRKM6, HsT17250 |
| MAPK7 | ERK5 | ERK5 | MAPK | PRKM7, BMK1 | |
| MAPK8 | JNK1 | SAPK1 | JNK | MAPK | PRKM8 |
| MAPK9 | JNK2 | p54aSAPK | JNK | MAPK | PRKM9 |
| MAPK10 | JNK3 | p54bSAPK | JNK | MAPK | PRKM10, SAPK1b, p493F12 |
| MAPK11 | p38 beta | SAPK2, SAPK2B | p38 | MAPK | PRKM11 |
| MAPK12 | p38 gamma | ERK6, SAPK-3 | p38 | MAPK | PRKM12 |
| MAPK13 | p38 delta | SAPK4 | p38 | MAPK | PRKM13 |
| MAPK14 | p38 alpha | SAPK2A, Mxi2 | p38 | MAPK | PRKM14, PRKM15, CSBP, EXIP |
| MAPK15 | ERK7/8 | atypical MAPK | MAPK | ||
| MAP2K1 | MEK1 | MKK1, MAPKK1 | MEK/ERK | MAP2K | CFC3 |
| MAP2K2 | MEK2 | MKK2, MAPKK2 | MEK/ERK | MAP2K | CFC4 |
| MAP2K3 | MEK3 | MKK3, MAPKK3 | p38 | MAP2K | SAPKK2 |
| MAP2K4 | MEK4 | MKK4, MAPKK4 | JNK | MAP2K | SAPKK1, JNKK1, JNKK |
| MAP2K5 | MEK5 | MAPKK5 | ERK5 | MAP2K | |
| MAP2K6 | MEK6 | MKK6, MAPKK6 | p38 | MAP2K | SAPKK3 |
| MAP2K7 | MEK7 | MKK7, MAPKK7 | JNK | MAP2K | SAPKK4, JNKK2 |
| RAF1 | c-Raf | Raf-1 | MEK/ERK | MAP3K | |
| BRAF | B-Raf | BRAF-1, RAFB1 | MEK/ERK | MAP3K | NS7 |
| MAP3K1 | MEKK1 | JNK | MAP3K | ||
| MAP3K2 | MEKK2 | MEKK2B | ERK5 | MAP3K | |
| MAP3K3 | MEKK3 | MAPKKK3 | ERK5 | MAP3K | |
| MAP3K4 | MEKK4 | MAPKKK4 | MAP3K | MTK1, PRO0412 | |
| MAP3K5 | ASK1 | MEKK5, MAPKKK5 | JNK and p38 | MAP3K | |
| MAP3K6 | ASK2 | MEKK6, MAPKKK6 | MAP3K | ||
| MAP3K7 | TAK1 | MEKK7, TGF1a | JNK and p38 | MAP3K | CSCF, FMD2 |
| MAP3K8 | MEKK8 | Tpl-2, c-COT | MAP3K | COT, EST, ESTF, AURA2 | |
| MAP3K9 | MLK1 | MEKK9 | MAP3K | PRKE1 | |
| MAP3K10 | MLK2 | MEKK10 | MAP3K | MST | |
| MAP3K11 | MLK3 | MEKK11 | JNK and p38 | MAP3K | PTK1, SPRK |
| MAP3K12 | ZPK | MEKK12 | MAP3K | DLK, MUK, HP09298 | |
| MAP3K13 | LZK | MEKK13 | JNK | MAP3K | MLK |
| MAP3K14 | MAP3K | FTDCR1B, HS, HSNIK, NIK | |||
| MAP3K15 | ASK3 | MAP3K | bA723P2.3 | ||
| TAOK1 | PSK2 | MAP3K16, TAO1 | JNK | MAP3K | DDIB, KFC-B, MARKK, hKFC-B |
| TAOK2 | PSK | MAP3K17, TAO2 | MAP3K | Tao2beta, PSK1-BETA | |
| TAOK3 | MAP3K18 | p38 | MAP3K | DPK, JIK, hKFC-A | |
| MAP3K19 | MAP3K | RCK, YSK4 | |||
| MAP3K20 | MLK7 | mlklak, pk | MAP3K | AZK, MLT, MRK, ZAK, SFMMP | |
| MAP3K21 | MLK4 | dJ862P8.3 | MAP3K |
The eye is the central organ of the visual system of animals, allowing vision and other, vision-independent photo-response functions by collecting light from the environment and converting the light information to neuronal impulses, ultimately terminating in the brain’s visual cortex. In vertebrates, this is achieved via a complex system of structures which are organized in a spherical organ and serve distinct roles.
2. Physiological Role of MAPKs in the Eye
MAPK/ERK signaling, as a master proliferation and cellular differentiation regulation pathway, is indispensable for the formation of the organism as a whole during development [7]. More precisely, ERK kinases play important roles in promoting embryonic survival and regulate the development of the eye in vertebrates. Of note, although the process depicted in Figure 2 is largely conserved within vertebrates, fish such as zebrafish do not form a lens pit and vesicle; rather, the cells from the lens placode proliferate and migrate inwards, directly forming a solid spherical mass that detaches from the surface ectoderm. The formation of the neural retina, RPE and the cornea follow the same process and lineage. In adult goldfish, ERKs are highly expressed in multiple ocular tissues including the lens epithelial cells, lens fiber cells and the retina, whereas its inhibition promotes early apoptosis, preventing the formation of the eye [8]. Underscoring the importance of the ERK pathway in development, all RASopathies, which are pathologies due to mutations in the RAS-MAPK pathway, are confined to only gain-of-function mutational defects that lead to inefficient inhibition of the pathway, while there is no documented RASopathy caused by mutational pathway knockout [9]; since such mutations should be more common than gain-of-function mutations, their absence signifies that when they occur, are most likely non-viable. Regarding ocular development, morphology and function, RASopathies present only minor clinical manifestations such as the appearance of Lisch nodules, which are aggregates of dendritic melanocytes forming papules in the iris [10]. Given its importance for cellular functions, MAPK/ERK signaling has been implicated in multiple organisms in the processes of wound healing and regeneration. For instance, ERK2 is essential for retinal pigment epithelium (RPE) cell proliferation in vitro [11][12]. Although in mammals, the RPE is post-mitotic in the adult, the mechanisms underlying RPE proliferation are important for stem cell applications and for developmental understanding. MEK–ERK signaling is strengthened by auto-regulation of the expression of constituent molecules in the pathway [13], but blockade of initial MEK–ERK signaling inhibits the cell-cycle re-entry of newt RPE cells [14], and after wounding in the adult newt [15]. The MEK pathway is also essential to switch adult newt RPE cells to neural cells. [16]. Regeneration of a complete neural retina can be achieved in larval Xenopus Leavis through the activation of the MAPK signaling pathway by administering exogenous FGF-2 [17]. In zebrafish, retina regeneration after injury depends on Müller glia (MG) dedifferentiation into a cycling population of multipotent progenitors via an EGFR/MAPK signal transduction cascade that regulates the expression of regeneration-associated genes such as PAX6 [18][19]. It should be noted, however, that mammals, unlike teleost fish, do not possess the innate ability for retinal regeneration; rather, mammals develop gliosis after retinal damage. Thus, this knowledge is relevant to humans in the context of stem cell research, the potential for interventions to induce regeneration, or in developmental research. During rat embryogenesis, the ERK1/2 pathway is required for the proper development of retino-geniculate connections [20]. FGF2 stimulates PAX6 expression during the induction of transdifferentiation of the RPE through a FGFR/MEK/ERK signaling cascade into a neural-like epithelium [21]. Similar transdifferentiation is obtained in chicks through the ectopic expression of a constitutively-activated allele of MEK-1 [22]. In the injured chick retina, the MG showed an accumulation of p-ERK1/2 [23]. Regarding the JNK activation pathway, the upstream kinases MKK4 and MKK7 have redundant and unique roles in molecular signaling that are important for retinal development, RGC maturation and the response to axonal injury signaling [24]. JNK and p38 phosphorylation is increased after retinal ischemia, mainly in amacrine, ganglion and bipolar cells while ERK is activated in MG cells [25]. Specific blockage of ERK and p38 phosphorylation, but not of JNK, prevents ischemia-induced apoptosis and improves retinal function in a rat model [25]. Other studies have demonstrated, for instance, that in vivo inhibition of p38 MAPK activity may be detrimental to injured photoreceptor cells [26]. Thus, the use of p38 MAPK inhibitors for therapeutic purposes must take into account the possible side effects. p38 is activated in retinal ganglion cells (RGCs) after optic nerve axotomy, and this activation is in the signaling pathway for RGC apoptosis [27]. MAPK also plays a significant role in MG cell proliferation and differentiation within the retina, in a stage-dependent manner. Prior work strongly supports a model whereby activation of the MAPK signaling pathway promotes the entry of progenitors into a MG cell differentiation pathway during embryonic retinal development, but not after birth [28]. For example, Shp2 protein phosphatase deletion abolished ERK phosphorylation in the neural retina, leading to extensive retinal cell death and degeneration. Additionally, Shp2 mediated a basal level of Ras-MAPK signaling in MG cells during postnatal development and in an adult retina under normal physiological conditions [29]. Also, the ERK1/2 and p38 MAPK pathways are key regulators of growth cone guidance in vitro [30].
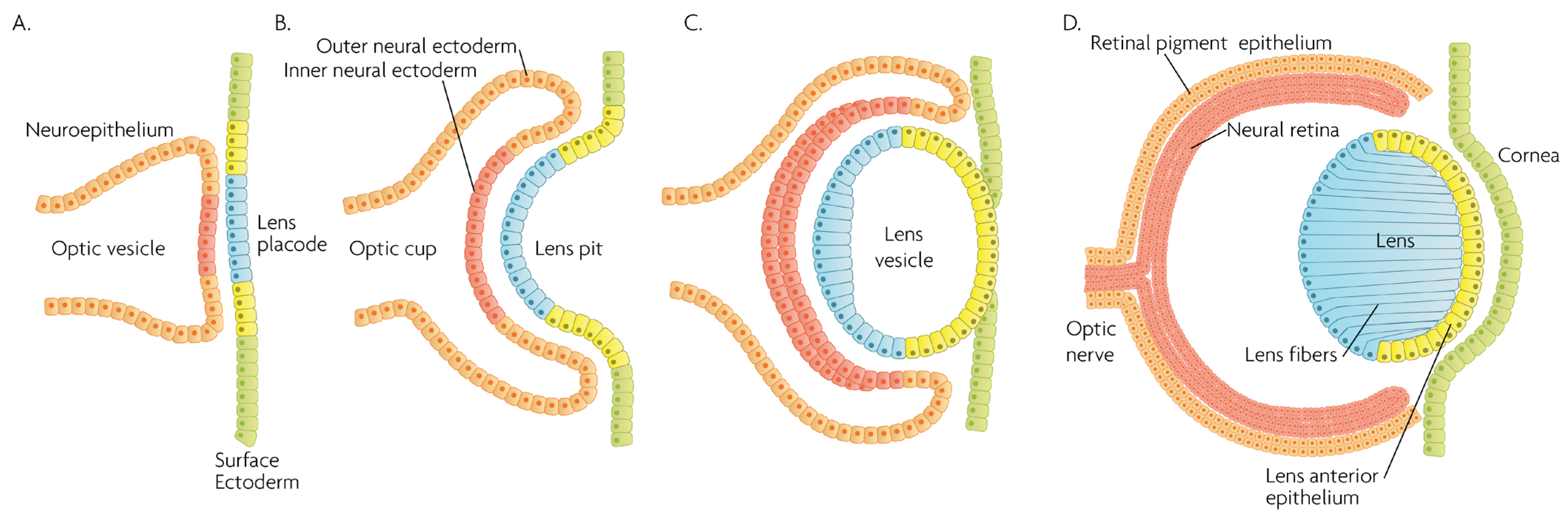
Figure 2. Schematic overview of developmental events during mammalian eye development, and germ layer origin of structures in the eye. (A) The optic vesicle, derived from the neuroepithelium of ectodermal lineage, approaches the surface ectoderm where the lens placode (blue cells) forms at the area of proximity between the layers. (B) The optic vesicle forms the optic cup, by the concurrent invagination of both the lens placode, forming the lens pit, and the proximal layer of the optic vesicle to the surface ectoderm, forming the presumptive neural retina (red cells). (C) The lens pit closes up onto itself forming the lens vesicle, with the cells from the central part of the lens pit (blue cells) directed posteriorly, and the cells from the lens pit periphery (yellow cells) directed anteriorly. The optic cup continues to invaginate. (D) The invaginated (inner) layer of the optic cup differentiates into the neural retina (red cells), while the outer layer forms the retinal pigment epithelium, RPE (orange cells). Cells in the posterior surface of the lens vesicle elongate towards the opposite pole, forming the lens fibers and filling the central volume of the lens, while the cells on the anterior side form the lens anterior epithelium. The surface ectoderm closes after the lens vesicle detaches, and the now continuous surface ectoderm forms the cornea.
This entry is adapted from the peer-reviewed paper 10.3390/cells12040617
References
- Peti, W.; Page, R. Molecular basis of MAP kinase regulation. Protein Sci. 2013, 22, 1698–1710.
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83.
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618.
- Jeffrey, K.L.; Camps, M.; Rommel, C.; Mackay, C.R. Targeting dual-specificity phosphatases: Manipulating MAP kinase signalling and immune responses. Nat. Rev. Drug Discov. 2007, 6, 391–403.
- Gkouveris, I.; Nikitakis, N.G. Role of JNK signaling in oral cancer: A mini review. Tumor Biol. 2017, 39, 1–9.
- Büscher, D.; Hipskind, R.A.; Krautwald, S.; Reimann, T.; Baccarini, M. Ras-dependent and -independent pathways target the mitogen-activated protein kinase network in macrophages. Mol. Cell. Biol. 1995, 15, 466–475.
- Brown, J.L.; Sones, J.L.; Angulo, C.N.; Abbott, K.; Miller, A.D.; Boehm, U.; Roberson, M.S. Conditional loss of ERK1 and ERK2 results in abnormal placentation and delayed parturition in the mouse. Sci. Rep. 2019, 9, 9641.
- Li, L.; Wang, L.; Li, T.-T.; Li, X.; Huang, X.-Q.; Chen, X.-W.; Li, Z.-L.; Lv, X.-M.; Liu, F.-Y.; Luo, Z.-W.; et al. ERK Signaling Pathway Regulates Embryonic Survival and Eye Development in Goldfish, Carassius auratus. Curr. Mol. Med. 2013, 13, 959–967.
- Jafry, M.; Sidbury, R. RASopathies. Clin. Dermatol. 2020, 38, 455–461.
- Cao, H.; Alrejaye, N.; Klein, O.D.; Goodwin, A.F.; Oberoi, S. A review of craniofacial and dental findings of the RASopathies. Orthod. Craniofac. Res. 2017, 20 (Suppl. S1), 32–38.
- Hecquet, C.; Lefevre, G.; Valtink, M.; Engelmann, K.; Mascarelli, F. Activation and role of MAP kinase-dependent pathways in retinal pigment epithelial cells: ERK and RPE cell proliferation. Investig. Opthalmol. Vis. Sci. 2002, 43, 3091–3098.
- Yasumuro, H.; Sakurai, K.; Toyama, F.; Maruo, F.; Chiba, C. Implications of a Multi-Step Trigger of Retinal Regeneration in the Adult Newt. Biomedicines 2017, 5, 25.
- Kochańczyk, M.; Kocieniewski, P.; Kozłowska, E.; Jaruszewicz-Błońska, J.; Sparta, B.; Pargett, M.; Albeck, J.G.; Hlavacek, W.S.; Lipniacki, T. Relaxation oscillations and hierarchy of feedbacks in MAPK signaling. Sci. Rep. 2017, 7, 38244.
- Mizuno, A.; Yasumuro, H.; Yoshikawa, T.; Inami, W.; Chiba, C. MEK–ERK signaling in adult newt retinal pigment epithelium cells is strengthened immediately after surgical induction of retinal regeneration. Neurosci. Lett. 2012, 523, 39–44.
- Yoshikawa, T.; Mizuno, A.; Yasumuro, H.; Inami, W.; Vergara, M.N.; Del Rio-Tsonis, K.; Chiba, C. MEK-ERK and heparin-susceptible signaling pathways are involved in cell-cycle entry of the wound edge retinal pigment epithelium cells in the adult newt. Pigment Cell Melanoma Res. 2012, 25, 66–82.
- Susaki, K.; Chiba, C. MEK mediates in vitro neural transdifferentiation of the adult newt retinal pigment epithelium cells: Is FGF2 an induction factor? Pigment Cell Res. 2007, 20, 364–379.
- Vergara, M.N.; Del Rio-Tsonis, K. Retinal regeneration in the Xenopus laevis tadpole: A new model system. Mol. Vis. 2009, 15, 1000–1013.
- Wan, J.; Zhao, X.-F.; Vojtek, A.; Goldman, D. Retinal injury, growth factors, and cytokines converge on β-catenin and pStat3 signaling to stimulate retina regeneration. Cell Rep. 2014, 9, 285–297.
- Wan, J.; Ramachandran, R.; Goldman, D. HB-EGF is Necessary and Sufficient for Müller Glia Dedifferentiation and Retina Regeneration. Dev. Cell 2012, 22, 334–347.
- Naska, S.; Cenni, M.C.; Menna, E.; Maffei, L. ERK signaling is required for eye-specific retino-geniculate segregation. Development 2004, 131, 3559–3570.
- Spence, J.R.; Madhavan, M.; Aycinena, J.-C.; Del Rio-Tsonis, K. Retina regeneration in the chick embryo is not induced by spontaneous Mitf downregulation but requires FGF/FGFR/MEK/Erk dependent upregulation of Pax6. Mol. Vis. 2007, 13, 57–65.
- Galy, A.; Néron, B.; Planque, N.; Saule, S.; Eychène, A. Activated MAPK/ERK kinase (MEK-1) induces transdifferentiation of pigmented epithelium into neural retina. Dev. Biol. 2002, 248, 251–264.
- Fischer, A.J.; Scott, M.A.; Tuten, W. Mitogen-activated protein kinase-signaling stimulates Müller glia to proliferate in acutely damaged chicken retina. Glia 2009, 57, 166–181.
- Syc-Mazurek, S.B.; Rausch, R.L.; Fernandes, K.A.; Wilson, M.P.; Libby, R.T. Mkk4 and Mkk7 are important for retinal development and axonal injury-induced retinal ganglion cell death. Cell Death Dis. 2018, 9, 1095.
- Roth, S.; Shaikh, A.R.; Hennelly, M.M.; Li, Q.; Bindokas, V.; Graham, C.E. Mitogen-Activated Protein Kinases and Retinal is chemia. Investig. Opthalmol. Vis. Sci. 2003, 44, 5383–5395.
- Agca, C.; Gubler, A.; Traber, G.; Beck, C.; Imsand, C.; Ail, D.; Caprara, C.; Grimm, C. p38 MAPK signaling acts upstream of LIF-dependent neuroprotection during photoreceptor degeneration. Cell Death Dis. 2013, 4, e785.
- Kikuchi, M.; Tenneti, L.; Lipton, S.A. Role of p38 Mitogen-Activated Protein Kinase in Axotomy-Induced Apoptosis of Rat Retinal Ganglion Cells. J. Neurosci. 2000, 20, 5037–5044.
- Zhang, S.S.-M.; Li, H.; Huang, P.; Lou, L.X.; Fu, X.-Y.; Barnstable, C.J. MAPK signaling during Müller glial cell development in retina explant cultures. J. Ocul. Biol. Dis. Inform. 2010, 3, 129–133.
- Cai, Z.; Simons, D.L.; Fu, X.-Y.; Feng, G.-S.; Wu, S.M.; Zhang, X. Loss of Shp2-Mediated Mitogen-Activated Protein Kinase Signaling in Müller Glial Cells Results in Retinal Degeneration. Mol. Cell. Biol. 2011, 31, 2973–2983.
- Campbell, D.S.; Holt, C.E. Apoptotic Pathway and MAPKs Differentially Regulate Chemotropic Responses of Retinal Growth Cones. Neuron 2003, 37, 939–952.
This entry is offline, you can click here to edit this entry!
