| Cancer Type |
Mutation Frequency (%) |
Reference |
| Malignant melanoma |
17.0–85.0 |
[61,63,71,72] |
| Genitourinary cancers |
|
|
| Bladder cancer |
59.0–85.0 |
[25,61,73,74,75,76] |
| Urothelial carcinomas |
29.5–64.5 |
[77,78] |
| Kidney cancers |
0 |
[61] |
| Prostate Cancer |
0 |
[79] |
| CNS tumors |
|
|
| Glioblastoma |
54.0–84.0 |
[61,70,73,78,80] |
| Other gliomas (ependymoma, astrocytoma, mixed glioma, oligodendroglioma) |
2.7–78.0 |
[25,64,70,78] |
| Medulloblastoma |
33.3–65.0 |
[70,78] |
| Hepatocellular carcinoma |
31.4–59.0 |
[25,78,81,82,83,84] |
| Thyroid cancer (papillary, follicular, poorly differentiated, and anaplastic carcinomas) |
3.4–46.3 |
[61,85,86,87] |
| Gastrointestinal stromal tumor |
0–3.8 |
[61,88] |
| Malignant pleural mesothelioma |
11.3 |
[89] |
| Atypical fibroxanthomas |
93.0 |
[90] |
| Sarcomas (chondrosarcoma, fibrosarcoma, myxofibrosarcoma, myxoid liposarcoma, osteosarcoma, pleomorphic dermal sarcomas) |
4.3–79.1 |
[25,90,91] |
| Basal cell carcinoma of the skin |
73.8 |
[92] |
| Squamous cell carcinoma of the skin |
20.0–74.0 |
[25,92,93] |
| Squamous cell carcinoma of esophageal |
1.6 |
[94] |
| Squamous cell carcinoma of penile |
48.6 |
[95] |
| Squamous cell carcinoma of the head and neck |
11.9–64.7 |
[3,4,13,15,25,26,27,28,29,32,93,96,97] |
| Squamous cell carcinoma of the cervix |
0–21.4 |
[25,26,93,96] |
| Breast cancer, colorectal cancer, ovarian cancer, esophageal adenocarcinoma, acute myeloid leukemia, chronic lymphoid leukemia, pancreatic cancer, and testicular carcinoma |
0–5.0 |
[61,78] |
5. TERT Promoter Mutations in Head and Neck Squamous Cell Carcinoma
5.1. The Frequency of TERT Promoter Mutations
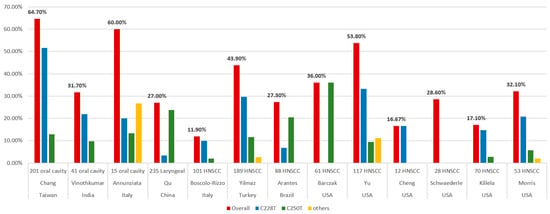
For HNCs, the frequency of TERT promoter mutations varied significantly among previous studies. These differences could be explained by the tumor subsite, sample size, methodological sensitivity, risk factors, and population ethnicity (Table 2).
Table 2. Summary of studies evaluating the association between head and neck cancers with TERT promoter mutations.
Killela et al. surveyed 70 oral cavity cancers and identified TERT promoter mutations in 12 of the tumors (17.1%) [
25]. Schwaederle et al. analyzed 423 cases of TERT promoter alterations using next-generation sequencing (NGS). Only 28 patients (6.6%) had HNCs. The incidence of TERT promoter alternations was 14.4% (61 of 423) in the overall population and 28.6% (8 of 28) in HNCs [
32]. Cheng et al. collected 84 cases of SCC from different sites, including 12 HNC and C228T mutations, which were detected in 16.67% (2 of 12) [
93]. Barczak et al. analyzed 61 HNC patients to determine the prevalence of the hTERT promoter C250T mutation. High-resolution melting mutation analysis was used to identify the C250T hTERT promoter mutation, followed by sequencing verification in 10% of the samples. The prevalence of the hTERT promoter C250T mutation was 36% [
15]. Yu et al. identified TERT promoter mutations in 117 patients with SCC of the oral cavity (N = 74), larynx (N = 24), hypopharynx (N = 5), and HPV-negative SCC of the oropharynx (N = 14) using NGS. Overall, 63 patients (53.8%) had TERT promoter alterations, and the most common mutations were C228T and C250T [
29]. Morris et al. collected 53 patients and 20 oral cavities, 18 oropharynges, 7 larynges, and 2 hypopharynges. Overall, the frequency of the TERT alteration was 32.1% (17 of 53), yet it was much higher in HPV-negative tumors (53% vs. 4.3%). Remarkably, 91% (10 of 11) of the HPV-negative tongue SCCs possessed TERT mutations [
97].
In Italy, Boscolo-Rizzo et al. analyzed cancer tissue and adjacent mucosa specimens from 101 patients with HNCs and evaluated the prevalence of the TERT promoter mutations by Sanger sequencing. The tumor subsites in the HNCs included the oral cavity (N = 27), oropharynx (N = 23), hypopharynx (N = 15), and larynx (N = 36). The TERT promoter harbored mutations in 12 tumors (11.9%), with C228T and C250T, which accounted for 83.3% and 16.7%, respectively. They also evaluated the TERT mRNA level and found no significant difference between the TERT mRNA level and the mutational status of the TERT promoter [
3]. Annunziata et al. analyzed tumor biopsies from 15 oral SCCs and nine oropharyngeal SCCs. The frequency of TERT promoter mutations was 60% (9 of 15) in oral SCCs and was absent in oropharyngeal SCCs. There were five hotspot mutations (three C228T and two C250T) and four other mutations. They also investigated the TERT mRNA levels and identified that the TERT mRNA levels were comparable to those detected in peri-tumor tissues. However, these data were from six oropharyngeal SCCs and illustrated that they all lacked mutations in the TERT promoter [
96].
In Turkey, Yilmaz et al. collected a total of 189 patients with HNCs, including 102 oral cavities, 22 oropharynges, 6 hypopharynges, and 59 larynges. The TERT gene expression was examined by polymerase chain reaction (PCR)-based direct sequencing. TERT promoter mutations were detected in 43.9% (83 of 189) of the cases. Three TERT promoter region mutations were detected: C228T (56 of 83; 67.5%), C250T (22 of 83; 26.5%), and C228A (5 of 83; 6%). The frequency of the C228T mutation was almost twice that of the C250T and C228A mutations [
4]. In Brazil, Arantes et al. collected 88 HNC patients and analyzed the TERT promoter mutations C228T and C250T using pyrosequencing. The overall prevalence of the TERT hotspot mutations is 27.3% (6.8% at locus C228T and 20.5% at C250T) [
13]. In India, Vinothkumar et al. analyzed 181 primary tumors of the uterine cervix and oral cavity using PCR amplification and sequencing. A high frequency of TERT hotspot mutations was observed in both cervical (30 of 140, 21.4%) and oral (13 of 41, 31.7%) SCCs. Among the oral cancer samples, the TERT promoter hotspot mutations were frequent, while the C228T mutation (69.2%) was twice as frequent as the C250T (30.8%) [
26].
In Taiwan, Chang et al. included 201 oral cavity SCC tumors and adjacent normal tissues to detect two TERT promoter mutations (C228T and C250T) using Sanger sequencing. Overall, the TERT hotspot promoter mutations occurred at a high frequency (64.7%) in patients with oral cavity SCCs. There were 52.5% (104 of 201) and 12.9% (26 of 201) oral cavity SCC tumor tissues containing that contained the C228T and C250T mutations, respectively [
28].
In China, Qu et al. obtained 235 laryngeal cancer tissues using a pyrosequencing assay to detect the TERT promoter mutations C228T and C250T. The TERT promoter hotspot mutations were present in 27% (64 of 235) of the samples. The TERT C250T mutations were more common (56 of 235) than the C228T mutations (8 of 235) [
27]. In
Figure 1, we summarized the reported frequencies of the TERT promoter mutations in HNCs from the various studies mentioned above.
Figure 1. Frequencies of TERT promoter mutations in head and neck cancers from different studies.
5.2. TERT Promoter Mutations in Different Anatomic Distribution
HNC is a heterogeneous group of tumors involving distinct anatomical sites and subsites with varying etiological factors. Yu et al. showed that TERT promoter mutations were more abundant in oral cavity SCCs than in laryngopharyngeal cancers (81.1% vs. 7.0%) [
29]. Boscolo-Rizzo et al. demonstrated that the prevalence of TERT hotspot promoter mutations is significantly higher in oral cavity SCCs (37%) [
3]. Annunziata et al. also showed that TERT promoter mutations were predominant in oral SCCs (60%), yet absent in oropharyngeal SCCs [
96]. Arantes et al. reported that 92% of the mutation cases were located in the oral cavity [
13]. Finally, Yilmaz et al. showed that the frequency of the TERT promoter mutations in oral SCCs (75.5%) was significantly higher than in the other locations [
4]. The anatomic distribution of cases is strongly associated with TERT promoter mutations, and the highest frequency is in oral cavity cancers.
As for the subsites in oral cavity SCCs, Arantes et al. noticed that 92% of the mutated cases were mainly in the tongue [
13]. Killela et al. also revealed that 11 out of the 12 cancers with TERT promoter mutations were in the oral tongue, although only 23 of the 70 oral cavity cancers originated in the oral tongue [
25]. However, Yilmaz et al. demonstrated that the highest rate was related to the buccal location and the lowest to the floor of the mouth (82.35% and 61.53%, respectively), although the difference was not statistically significant [
4].
5.3. TERT Promoter Mutation and Human Papillomavirus Status
An association between HPV infection and oropharyngeal SCC has been proven. It was also clear that the molecular landscape and clinical pattern were different between HPV-positive and HPV-negative oropharyngeal cancers [
10]. Only two studies have investigated the association between HPV status and TERT promoter mutations.
In a cohort of 53 patients with advanced HNCs, performed by Morris et al., a very high TERT alternation rate (53%, 16 of 30) was present in 30 HPV-negative tumors, however, there was only one TERT alternation (4.3%), which was a TERT amplification rather than a hotspot mutation, in 23 HPV-positive tumors. HPV-negative tongue SCCs showed the highest TERT mutation rate (91%). This demonstrated that TERT mutations and HPV infection may represent parallel mechanisms of telomerase activation in HNCs [
97]. In another cohort study conducted by Annunziata et al., among the 9 patients with TERT promoter mutations in 15 oral SCC patients, 7 were HPV-negative and 2 were HPV-positive (
p = 0.486). The frequency of TERT mutations was also independent of HPV tumor status in oral cancer [
96].
5.4. TERT Promoter Mutation and Tobacco, Alcohol, and Betel Quid
Aside from HPV infection, tobacco smoking, alcohol consumption, and betel quid chewing are the other three main etiological factors of HNC [
4,
5]. Until now, the relationship between the TERT promoter mutations and these three factors remains inconclusive.
In a Brazilian cohort of 88 patients with HNC conducted by Arantes et al., the frequency of the C250T mutation appeared to be higher in alcohol consumers. Of the patients harboring the TERT promoter mutation C250T, 94.4% were alcohol consumers, and 66.7% of the patients harboring the TERT promoter mutation C228T did not consume alcohol [
13]. In a Chinese cohort of 235 laryngeal cancer cases reported by Qu et al., hotspot mutations were not significantly correlated with any clinicopathological variables. However, TERT promoter mutations, particularly the C250T mutation, were more frequent in smoking patients (47 of 130) than in non-smoking patients (9 of 49), although no statistical significance was noted [
27]. In a cohort of 201 patients with oral cavity SCC performed by Chang et al. in Taiwan, the C228T mutation was significantly associated with betel nut chewing [
28]. In contrast, in a Turkish cohort of 189 HNC patients performed by Yilmaz et al., TERT promoter region mutations in HNC were inversely related to smoking and alcohol consumption [
4].
5.5. TERT Promoter Mutation and Other Factors
Schwaederle et al. demonstrated that TERT promoter alterations are more frequent in men. They were also associated with brain cancers, skin/melanoma, head, and neck tumors, and increased median numbers of alterations in the univariate analysis. However, this association in head and neck tumors was not found in further multivariate analyses [
32]. Yilmaz et al. reported that TERT promoter region mutations in HNCs are associated with younger age and female genders in a cohort from Turkey [
4]. Barczak et al. demonstrated a significant association between the frequency of the homozygous C250T mutation and tumor grade (T1 = 27%, T2 = 36%, T3 = 35%, T4 = 46%,
p ≤ 0.0001) [
15]. However, in a cohort of 41 patients with oral SCCs, performed by Vinothkumar et al. in India, no significant correlation was observed between any of the genotypes and the clinicopathological characteristics [
26].
5.6. TERT Promoter Mutation and Survival
TERT promoter mutations in various reports of different cancers have been associated with aggressive characteristics, poor outcomes, and shorter survival [
98,
99,
100,
101]. In HNC, Qu et al. showed that TERT promoter mutations significantly affected the overall survival of laryngeal cancer patients, particularly those with the C250T mutation. TERT promoter mutations were significant predictors of poor prognosis in patients with laryngeal cancer, as an independent variable, with respect to age, tumor localization, TNM stage, tumor invasion, lymph node metastasis, and smoking history [
27]. Schwaederle et al. also demonstrated a significantly shorter overall survival in patients harboring the TERT promoter alterations in the overall population in a univariate analysis. Subanalyses of the three tumor types with the highest prevalence of TERT alterations consistently showed a trend toward shorter survival for patients with altered TERT promoters in brain tumors, head, and neck cancers, and melanoma/skin tumors [
32]. Arantes et al. demonstrated no statistically significant association between the presence of hotspot mutations (C228T and C250T) and survival. However, the presence of the C228T mutation impacted patient outcomes, with a significant decrease in 5-year disease-free survival (20.0 vs. 63.0%) and 5-year overall survival (16.7 vs. 45.1%) [
13].
Similar results were reported by Yu et al. [
29]. They reported that the TERT promoter mutations were associated with locoregional failure (LRF) in the overall cohort and in oral cavity SCCs. This increased risk for LRF is independent of the oral cavity primary site, TP53 mutation status, extracapsular extension, and positive surgical margins suggesting that the TERT promoter mutations are an independent biomarker of LRF rather than a surrogate for OSCCs, or other known prognostic markers. The cumulative incidence of LRF was similar between the two types of TERT promoter mutations (C250T and C228A/T groups), and both were associated with a higher cumulative incidence of LRF compared to wildtype tumors. Overall, they demonstrated that TERT promoter mutations were associated with an increased risk of LRF, although not with distant failure or overall survival [
29].
In contrast, Yilmaz et al. did not find a significant association between the presence of TERT mutations and OS, despite patients with HNCs harboring TERT mutations exhibiting a slightly shorter median OS [
4]. Boscolo-Rizzo et al. showed no significant association between the TERT promoter status and overall survival, although the TERT mRNA level had an impact on clinical outcomes [
3]. Chang et al. also reported that there was no significant difference in overall survival, disease-specific survival, and disease-free survival between TERT promoter mutations and the wildtype [
28].