Developing therapeutics for inflammatory diseases is challenging due to physiological mucosal barriers, systemic side effects, and the local microbiota. In the search for novel methods to overcome some of these problems, drug delivery systems that improve tissue-targeted drug delivery and modulate the microbiota are highly desirable. Microbial metabolites are known to regulate immune responses, an observation that has resulted in important conceptual advances in areas such as metabolite pharmacology and metabolite therapeutics. Indeed, the doctrine of “one molecule, one target, one disease” that has dominated the pharmaceutical industry in the 20th century is being replaced by developing therapeutics which simultaneously manipulate multiple targets through novel formulation approaches, including the multitarget-directed ligands. Thus, metabolites may not only represent biomarkers for disease development, but also, being causally linked to human diseases, an unexploited source of therapeutics.
1. Introduction
Changes in gastrointestinal microbiome composition and function can adversely impact host physiology and are associated with local and systemic inflammatory diseases, cancer, metabolic disorders, and opportunistic and recurrent pathogen infections. It is estimated that noncommunicable illnesses contribute to 70% of worldwide mortality. Due to the presence of biosynthetic or metabolic gene clusters encoding the biosynthetic machinery to produce primary and specialized metabolites [
1], microbes are highly metabolically active and produce trans-kingdom signaling molecules that interact with competing microorganisms and the host [
2]. The fermentation of undigested protein in the large intestine results in the formation of branched-chain amino acids, biogenic amines, short-chain fatty acids, ammonia, phenolic and indolic compounds, hydrogen sulfide, and nitric oxide [
3]. All these metabolites play important pathophysiological roles [
4] such that their identification along with the study of their effector pathways can lead to the discovery of incredibly innovative diagnostics and therapeutics.
2. Metabolite-Based Therapeutics: The Postbiotic Concept
The metabolome is contributed by diet, the host, and its microbiota. Under homeostatic conditions, the intestinal microbiota produce, modify, and degrade metabolites which serve as an effective means of communication in host–microbe interactions and profoundly affect human health. Metabolites are bioactive, and their functional activity includes the modulation of signaling and metabolic pathways that contribute to mucosal immune homeostasis [
10,
11]. Despite the fact that the microbiome is estimated to account for more than half of all fecal and urinary metabolites, only about a dozen metabolites currently have a well-characterized effect on the host; these include target cells, receptors, signaling pathways, and physiological outcomes [
12].
Quantitative or qualitative variation in metabolites is perceived by the host as an alteration of its microbial community, potentially an early indication of dysbiosis. Dysbiosis leads to subsequent alterations in metabolite composition, which has been shown to have direct consequences on host health in the context of multiple diseases. Thus, metabolite-based therapeutics, or “postbiotics”, may work as replacement therapy that is potentially effective against dysbiosis and its inflammatory consequences [
11].
Metabolites have a clear chemical structure and a long shelf life, occur naturally in a broad range of concentrations, are functionally pleiotropic, are easy to administer, are suitable for different routes of administration, show tissue bioavailability, are present at most body sites, are generally stable in the blood, and are therefore amenable for scalable modulation of their concentration. Because of these properties, they are attractive and uniquely suited for therapeutic strategies. However, although highly promising, biopharmaceutical approaches for their in vivo delivery are critically needed to turn beneficial pleiotropic activity into effective therapeutics for human illnesses. This demands for drug delivery systems (DSS) that pivotally contribute to the development of an ideal postbiotic capable of fostering the holistic balance for the homeostatic control of inflammation at the mucosal and tissue levels.
3. Tissue-Specific Drug Delivery
The field of drug delivery systems, including routes of delivery and delivery vehicles, has advanced dramatically in the past few decades, thanks to the increased understanding of the physiological barriers for efficient drug delivery and the development of several new modes of drug delivery that have entered clinical practice. These include lipid nanoparticles (essential for effective delivery of mRNA), the microneedle patch (for sustained delivery to low-income people or people with mental-health conditions), the robotic pill (for oral drug delivery of complex drugs), and cellular delivery via red blood cells or bacteria, to name a few [14]. However, despite improving the drug’s efficacy, side effects may still occur because the unwanted drugs interact with healthy organs or tissues. Thus, targeted drug delivery approaches are of great interest in the pharmaceutical sciences, as they enable the concentrated delivery of a drug compound to its desired target—increasing efficacy and reducing off-target effects as compared to conventional formulations [14]. DDS have enabled the development of many pharmaceutical products that improve patient health by enhancing the delivery of a therapeutic to its target site, minimizing off-target accumulation and facilitating patient compliance [15].
4. The Ubiquitous AhR Signaling Pathway
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that acts as a heterodimeric transcriptional regulator [
19]. Animal and human data indicate that the AhR is involved in various signaling pathways critical to normal cell homeostasis, which covers multiple aspects of physiology, such as cell proliferation and differentiation, gene regulation, cell motility and migration, inflammation, and host–microbiota interplay [
20,
21]. Signal molecules function as ligands for AhR, and activated AhR forms heterodimers at promoter recognition sequences of the target genes. The AhR/AhR nuclear translocator complex may then require coactivators (including members of other families of transcription factors) in order to initiate transcription and to unwind histone-bound DNA for exposing additional promoter recognition sites via their histone acetyltransferase function. Within this scenario, three major factors appear to contribute to the outcome of gene transcriptional regulation by AhR: the nature of the ligand, the local tissue microenvironment, and the presence of coactivators in the cell [
19,
20].
5. The Tryptophan-Indole Microbial Pathway
Trp is an essential amino acid supplied by dietary protein whose degradation occurs throughout the small intestine [
27,
28]. The gastrointestinal tract harbors numerous species with the capacity to synthesize indole and indole-containing compounds. The bacterial synthesis of indole compounds occurs via different metabolic pathways mainly involving the activity of tryptophanase, aromatic amino acid, aminotransferases, and tryptophan deaminases generating indole and a number of indole derivatives [
29]. Indoles exert significant biological effects, including immune modulation, epithelial barrier regulation, and pathogen colonization, through which they may contribute to the etiology of a variety of human diseases, including dysbiosis [
30]. More recently, an impaired microbial Trp catabolism was associated with critical COVID-19 patients [
31]. These observations suggest that the exploration of indoles to support beneficial bacterial/host symbiosis and cooperation may lead to the rational design of effective clinical interventions in human chronic inflammatory conditions in which the epithelial barrier disruption and microbial dysbiosis are causally linked [
32]. From an evolutionary perspective, many of the endogenous Trp metabolites exhibit greater activation potential for the human AhR when compared to the rodent AhR, an observation highlighting the importance of the AhR in human barrier tissues [
26].
6. Turning Indoles into Therapeutics via Targeted Delivery Technologies
Within Lactobacilli,
L. reuteri was found to convert Trp to indole-3-aldehyde (3-IAld) via the aromatic amino acid aminotransferase [
33]. Mice exposed to a tryptophan-enriched diet expand
L. reuteri in the gut that produce 3-IAld; this promotes AhR-dependent transcription of the IL-22–encoding gene by host innate lymphoid cells and thus prevents microbial infections and local inflammation [
33]. 3-IAld, like indoles, moonlights as a metabolite and signaling molecule and is increasingly being associated with the regulation of wide-ranging physiological processes [
34]. Being rapidly metabolized upon administration [
35], 3-IAld may potentially act as a rapidly metabolized AhR ligand within the range of concentrations found in murine and human gut, despite not being among the most active endogenous ligands of murine and human AhR [
36,
37].
Recently, resorting to targeted delivery technologies has highlighted the possible translation to a clinical application of 3-IAld. Targeted delivery of 3-IAld via dry powder inhalation or orally was an efficient strategy to restore immune and microbial homeostasis in [
35,
48,
49] in preclinical models of lung or gut inflammation (
Figure 1).
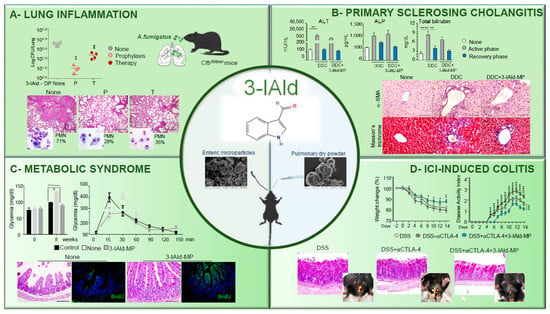
Figure 1. Therapeutic activity of 3-IAld in experimental models of human inflammatory diseases. (
A) Pulmonary insufflation of 3-IAld dry powder (DP) either before (P, prophylaxis) or after (T, therapy) prevents lung inflammatory pathology in
CftrF508del/F508del mice infected with
Aspergillus fumigatus conidia, as revealed by the fungal growth, lung histopathology, and neutrophil recruitment ([
48]). Photographs were taken using a high-resolution Olympus DP71 microscope using a 10× objective. Scale bar 400 μm. Values represent the mean ± SD of four mice per group or are representative of three experiments. Naïve, uninfected mice; None, infected mice. **
p < 0.01, one-way ANOVA—Bonferroni’s, P or T vs. None. (
B) 3-IAld microparticles (MP) reduces liver injury and fibrosis in C57BL/6 mice fed with a DDC-containing diet (active phase) and subjected to a recovery time (recovery phase) as revealed by serum levels of alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin, fibroblast activation, and collagen deposition ([
38]). Photographs were taken with a high-resolution microscope (Olympus BX51), 40× magnification (scale bars, 100 μm). **
p < 0.01, ****
p < 0.0001, DDC-treated vs untreated (none) mice, and DDC vs. DDC + 3-IAld-MP. One-way ANOVA, Bonferroni post-test. (
C) Oral administration of 3-IAld-MP decreases glycemia, promotes glucose tolerance, restores tissue architecture in the ileum, and promotes epithelial cell renewal (BrdU) in C57BL/6 mice on a high-fed diet. Control; control mice. None; high-fed diet-mice with MP alone ([
35]). Photographs were taken with a high-resolution microscope (Olympus BX51), 20× magnification (scale bars, 200 μm). ****
p < 0.001, 3-IAld-MP-treated vs MP-treated mice and None vs Control. (
D) 3-IAld-MP protects mice from immune check-point inhibitor (ICI)-induced colitis. C57BL/6 mice were treated with DSS and anti-CTLA-4 mAb with and without 3-IAld and assessed for weight change, disease activity index, colon histopathology, and gross pathology (insets) ([
41]). Photographs were taken with a high-resolution microscope (Olympus BX51), ×20 magnification (scale bars, 200 µm). wo-way analysis of variance, Bonferroni post hoc test. *
p < 0.05, **
p < 0.01, ***
p < 0.001, ****
p < 0.0001. anti-CTLA-4 + 3-IAld- versus anti-CTLA-4-treated mice.
Inhaled dry powder of 3-IAld demonstrated a good pharmacological and toxicological profile upon delivery for pulmonary administration and was superior to other administration modalities (oral and intranasal) in reducing the disease score [
50]. When assessed in a relevant preclinical model, the site-specific delivery of 3-IAld was an efficient strategy to control infection and restore immune homeostasis in the lungs of mice with cystic fibrosis infected with
Aspergillus fumigatus conidia. 3-IAld exerted significant control on the infection and ensuing inflammatory response administered either before (P) or after (T) the fungal challenge, as revealed by the reduced fungal growth, lung inflammatory pathology, and neutrophils recruitment (
Figure 1A).
3-IAld was also effective when microencapsulated on enteric microparticles (MP) for intestinal delivery. Although spray-drying is not a conventional preparation process for enteric formulations, scholarsfound it suitable in the manufacturing of gastro-resistant 3-IAld [
49]. As a matter of fact, demonstrated that 3-IAld is released from MP in the intestine where it activates AhR [
35]. Pharmacokinetics studies revealed that 3-IAld was detected at high levels in the intestine within 1 h after its administration at very low levels in the serum, lung, liver, and brain, suggesting a rapid local and/or systemic transformation of the molecule, likely occurring via host and/or microbial metabolic pathways, including AhR-induced Cytochrome P450 genes. However, AhR-activated genes were detected long after the sharp dropping of 3-IAld levels and could also be detected at distant organs, a finding suggesting that 3-IAld could be metabolically converted into downstream AhR agonists. Whatever the nature of the metabolite(s), scholars found that 3-IAld-MP was very active in a murine model of primary sclerosing cholangitis (PSC), a long-term liver disease characterized by a progressive course of cholestasis with liver inflammation and fibrosis. Given the contribution of the intestinal barrier dysfunction and the consequent bacterial/bacterial product translocation to the PSC pathogenesis [
51], the ability of 3-IAld to preserve epithelial barrier integrity and maintain local immune homeostasis [
35] would predict a beneficial effect in PSC. The scholars found that 3-IAld-MP reduced liver injury and fibrosis in C57BL/6 mice fed with a 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC)-containing diet (active phase) and subjected to a recovery time (recovery phase) as revealed by the reduced serum levels of alanine aminotransferase (ALT), alkaline phosphatase (ALP), and total bilirubin. 3-IAld-MP also reduced fibroblast activation and collagen deposition (
Figure 1B) in DDC-fed mice (
Figure 1B).
On the basis of the same mechanism of activity, it was not surprising to find a protective role for 3-IAld-MP in the murine model of metabolic syndrome, a disorder characterized by a cluster of diseases associated with the reduced capacity of the microbiota to metabolize Trt into AhR ligands [
54]. Accordingly, it showed that 3-IAld-MP, locally released, decreased inflammatory pathology, restored tissue architecture, and induced epithelial cell proliferation in C57BL/6 mice on a high-fed diet (
Figure 1C). Concomitantly, 3-IAld-MP prevented the metabolic complications associated with the metabolic syndrome, such as hyperglycemia (
Figure 1C). Finally, 3-IAld-MP protected mice from intestinal damage by activating the AhR/IL-22-dependent pathway in a murine model of immune checkpoint inhibitor (ICI)-induced colitis as shown by the prevention of weight loss and disease activity and by the amelioration of gross and colon intestinal pathology (
Figure 1D).
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15020506