Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The freshwater microalga Haematococcus pluvialis is well known as the cell factory for natural astaxanthin, which composes up to 4–7% of its total dry weight. The bioaccumulation of astaxanthin in H. pluvialis cysts seems to be a very complex process that depends on different stress conditions during its cultivation. The red cysts of H. pluvialis develop thick and rigid cell walls under stress growing conditions.
- microalga
- Haematococcus pluvialis
- biomolecular composition
- electrical treatment
1. Introduction
In recent years, the up and downstream processing problem of H. pluvialis microalgae has attracted great attention [1][2][3][4][5]. The low-cost bio-production of natural astaxanthin using this microalga is still one of the market’s most concerning issues. It remains a challenge to biosynthesize natural astaxanthin using microalgae such as H. pluvialis on a large scale. The main reason for the high cost of natural astaxanthin is that the recovery of the final product necessitates additional manufacturing steps.
The cultivation of H. pluvialis for natural astaxanthin production (known as a king of antioxidants) involves two phases, green and red. During the second one, the red cysts or aplanospores accumulate a high amount of astaxanthin, which accounts for ≈80% of the total carotenoid content. Accumulation of astaxanthin is a rather complex process that typically includes specific abiotic stress applications such as nutrient starvation [6], pH [7], illumination stresses [8][9][10], temperature [11], irradiation or pressure stresses [12] and a mixture of different stresses [13]. Particularly, the synergetic combination of different factors allowed improving of astaxanthin accumulation [13][14].
Application of electrotreatments during the growth stages allows genetic transformation, inactivation of culture contaminants, improvement of growth kinetics, accumulation of bioactives and increasing of permeability of cell walls [15][16][17]. The cells of H. pluvialis are covered by extraordinarily thick, rigid and indigestible cell walls, and the availability of internal biomolecules is very limited [18][19]. Therefore, the selective recovery of these biomolecules, and particularly astaxanthin, from H. pluvialis requires application of rather complex cell disruption and purification techniques. The topic is a very hot issue in contemporary studies of extraction from various microalgae [19]. The processing of H. pluvialis using pulsed electrotechnologies (pulsed electric fields and (or) high-voltage electric discharges) is very attractive because with proper adaptation these techniques allow effective extraction and obtaining of high purity extracts [20].
2. Main Steps Haematococcus pluvialis Biorefinery
At both laboratory and industrial scales, many important steps are included in the processing biorefinery of H. pluvialis microalgae. The general overviews of these steps are presented in Figure 1.
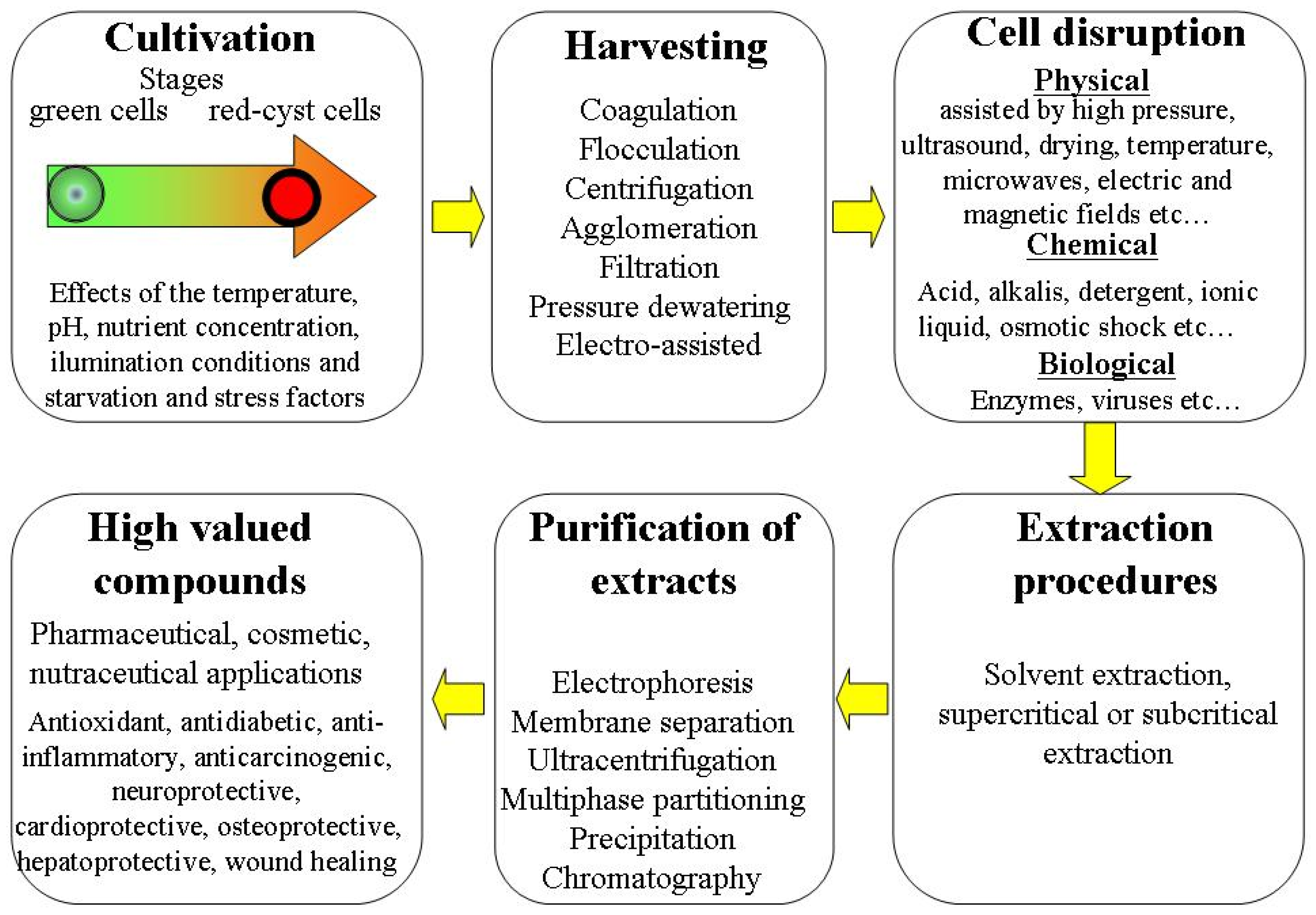
Figure 1. Overview of different steps in H. pluvialis microalgae processing biorefinery.
Each of these steps may be crucial in determination of the quantity and quality of extracted bioactive molecules. In recent years, the different methods of H. pluvialis processing optimization are intensively discussed [1][2][3]. These methods include optimization of favorable and unfavorable growing conditions on cultivation stages and screening of optimized techniques for harvesting of biomass, cell disruption, extraction and purification processes.
3. Cultivation and Growth Stages of Haematococcus pluvialis, Effects of Electrotreatment
The freshwater microalga H. pluvialis was described for the first time in 1841 [21]. It has been reported that this microscopic object can occur in green and red forms. The green forms were observed in juvenile states. In cold seasons (winter, spring, and autumn), the green forms were mainly observed [22][23]. A detailed discussion of the early studies of H. pluvialis was presented in 1899 [24]. H. pluvialis occurs in different regions of Europe, Africa, and North America [25].
3.1. Cultivation and Growth Stages
It is still acknowledged that the green microalga H. pluvialis is recognized for its capacity to produce significant quantities of natural astaxanthin via the application of different stress conditions and its high specific growth rate 1.3 d−1 [26].
H. pluvialis astaxanthin production for the market is relatively new. The induction of astaxanthin bioaccumulation in H. pluvialis cells is carried out by subjecting the cells to stress growth conditions. In the last two decades, the two-stage strategy for cultivation has been widely adopted for both laboratory and large-scale cultures. The first stage involves growing H. pluvialis under favorable conditions for green biomass production (green stage); this is followed by a second stage (red stage) in which the astaxanthin biosynthesis is induced by changing the favorable growth conditions to unfavorable ones. The natural response to the harsh environmental conditions involves the inhibition of normal growth and the accumulation of astaxanthin for the purpose of protecting the photosynthetic apparatus from photo-oxidative damage by absorbing the excess light. The role of astaxanthin is important to protect cells by scavenging free radicals and reacting with reactive oxygen species. This role is to ensure the maintenance of photosynthesis during red-stage stress [27].
Obviously, the life cycle of H. pluvialis microalgae is complicated. The two-stage culturing implies different cell forms. This life cycle can be roughly divided into motile (bi-flagellated cells) and non-motile stages (aplanospores, astaxanthin-accumulating aplanospores, red cysts cells, sporangium and zoospores released from the sporangium) [19][28][29][30]. Each transition from the motile to the non-motile cell form involves remarkable changes in the cell wall structure.
Photosynthetic activity is highest in the initial vegetative green growth phase (days 1 to 12) [7]. In this green vegetative phase, the cells do not accumulate astaxanthin and their carotenoid pattern is mainly composed of lutein (75–80%) and β-carotene (10–20%) [31]. However, the astaxanthin accumulation is triggered in the second phase by specific stress application such as nitrogen starvation, oxidative stress, salinity stress, high temperature and high irradiation. These cited factors significantly affect the astaxanthin accumulation.
The recent literature on cultivation and growth conditions of H. pluvialis is rather vast. It includes discussions of the effects of temperature [11], pH [7], nutrient concentration (nitrogen, phosphorus and carbon starvation) [6][32] and illumination conditions [8][9][10][33][34][35]. The effects of light illumination on the growth of biomass and accumulation of bioastaxanthin were analyzed, and it was demonstrated that red light is more effective for increasing the biomass, whereas blue light supports the accumulation of bioastaxanthin [10]. A comprehensive examination of the effects of different photo-, mixo- and heterotrophic cultivation conditions on H. pluvialis cell germination has been recently presented [36].
The effects of temperature (20–30.5 °C) on the cell growth and accumulation of astaxanthin has been discussed [11]. It was demonstrated that increased temperature allows enhanced astaxanthin accumulation combined with nitrogen starvation stress. It was demonstrated that the photosynthetic properties are differentially modulated in response to nitrogen starvation/high-light-illumination stress and a correct balance between these stresses is required for efficient astaxanthin production [37]. Effects of nuclear irradiation and a high concentration of carbon dioxide CO2 on enhancing the growth rate and astaxanthin yield were demonstrated [12].
The economical two-stage strategy with low light illumination in the initial phase (growth for biomass production) and a combination of high light illumination and elevated carbon dioxide levels (5 or 15%) in the second phase (astaxanthin accumulation) has been tested [8]. The applied procedures showed a significant increase (2–3 times) in the accumulation of astaxanthin. A two-step process was proposed to maximize algal growth and astaxanthin yield [13]. During the first step (biomass production), the different nitrogen sources were tested. During the second step (carotenogenesis induction), a mix of moderate stressors (mild light, nitrogen limitation, and the addition of sodium acetate) was used. The synergetic combination of different factors allowed the promotion of astaxanthin accumulation. The new sequential heterotrophy–dilution–photoinduction cultivation strategy was shown to be rather effective for production of astaxanthin from H. pluvialis [14]. The effects of continuous and interrupted (light/dark cycles) illumination conditions on the production of vegetative green cells and astaxanthin accumulation were investigated [38]. It was demonstrated that light/dark cycles are optimal to produce green cells, whereas continuous illumination is better for astaxanthin accumulation. The effects of fulvic acid on biomass growth and astaxanthin accumulation were investigated [39]. It was demonstrated that the astaxanthin content increased by 86.89% in 5 mg/L-treated cells.
Recently, the details of laboratory and commercial scale cultivation procedures in the production of natural astaxanthin derived from H. pluvialis have been discussed [40][41][42]. The effects of different cultivation conditions and strategies, technological innovations and types of cultivation systems and an economic assessment for astaxanthin production were also reviewed [43][44][45][46][47][48][49][50].
3.2. Electrotreatments during the Growth Stages
The effects of electrotreatments applied during the growth of H. pluvialis on cell viability, cell number density and accumulation of astaxanthin attract great attention. A general review of the application of different electrotechnologies for genetic transformation, inactivation of culture contaminants and improvement of growth kinetics of different species of microalgae is given in [15]. Application of electrotreatments of H. pluvialis during the different growing stages may reduce the strength and impermeability of cell walls and positively affect the accumulation of bioactives.
The effects of pulsed electric field (PEF) treatments with square wave and exponential decay wave pulses on cell viability during the growth stages were discussed in [51]. Nanosecond PEF treatment (with very short pulses of 25–50 nm and high electric field strength 40 kV/cm) has been applied to H. pluvialis cells during the growth stage [16]. It was demonstrated that such treatment can modulate the expression of the astaxanthin biosynthesis genes. PEF treatment increased both the cell mortality (by up to 20%) and astaxanthin accumulation (by 20–30%) from the initial values. Such results were explained by the expression of carotenoid-pathway-related genes.
Electrical treatment of H. pluvialis cells was studied in the bio-electrochemical chambers [52][53]. Such treatments enhanced the nitrogen consumption and chlorophyll biosynthesis and resulted in a considerable increase in electro-treated cell number density (by 20%) after cultivation for seven days [52]. However, such treatments did not significantly affect the intracellular astaxanthin content (10% increase). The special adaptation of electrotreatment parameters (electric strength and duration) allowed a significant increase in the astaxanthin content in electrostimulated H. pluvialis (by 36.9%) [53].
Application of electrical treatment by low-temperature plasma in H. pluvialis biotechnology has been also discussed. It was demonstrated that low-temperature plasma promotes the growth of H. pluvialis [54]. The plasma treatment is widely used as a safe and environmentally friendly sterilization technology [55] and may be applied during the growth stages as a strong oxidative stress and mutagenesis tool without damaging the microalgae cells [17]. Atmospheric pressure argon dielectric barrier discharge plasma was used for mutation induction in H. pluvialis [56]. The presence of enhanced astaxanthin accumulation in the mutants was observed that was attributed to genetic modification by the mechanism of carotenogenesis. It was indicated that plasma mutation is suitable for effective H. pluvialis breeding with promising industrial applications [57]. The plasma treatment effectively improved the growth of H. pluvialis and its astaxanthin accumulation; under optimal conditions the astaxanthin content was 51.96% higher than in the starting strain. The possible mechanisms of astaxanthin accumulation induced by plasma treatment were also discussed [58][59].
4. Structure of Cell and Biomolecular Composition of Haematococcus pluvialis
4.1. Cell Structure
Figure 2 gives a schematic presentation of the cell structure of H. pluvialis at the resistant cyst stage. On the final aplanospore stage of cyst formation, the rigid and resistant multilayered cell walls are formed [60][61][62][63][64][65]. The total size of the cells at this stage is approximately 20–30 µm [28]. The cell internal compartment contains cytoplasm starch granules, sub-cellular micro-compartments of pyrenoids and astaxanthin deposited in extra-plastidial oil globules (lipid droplets). This internal space is covered by lipid membrane with thickness of ≈5 nm.
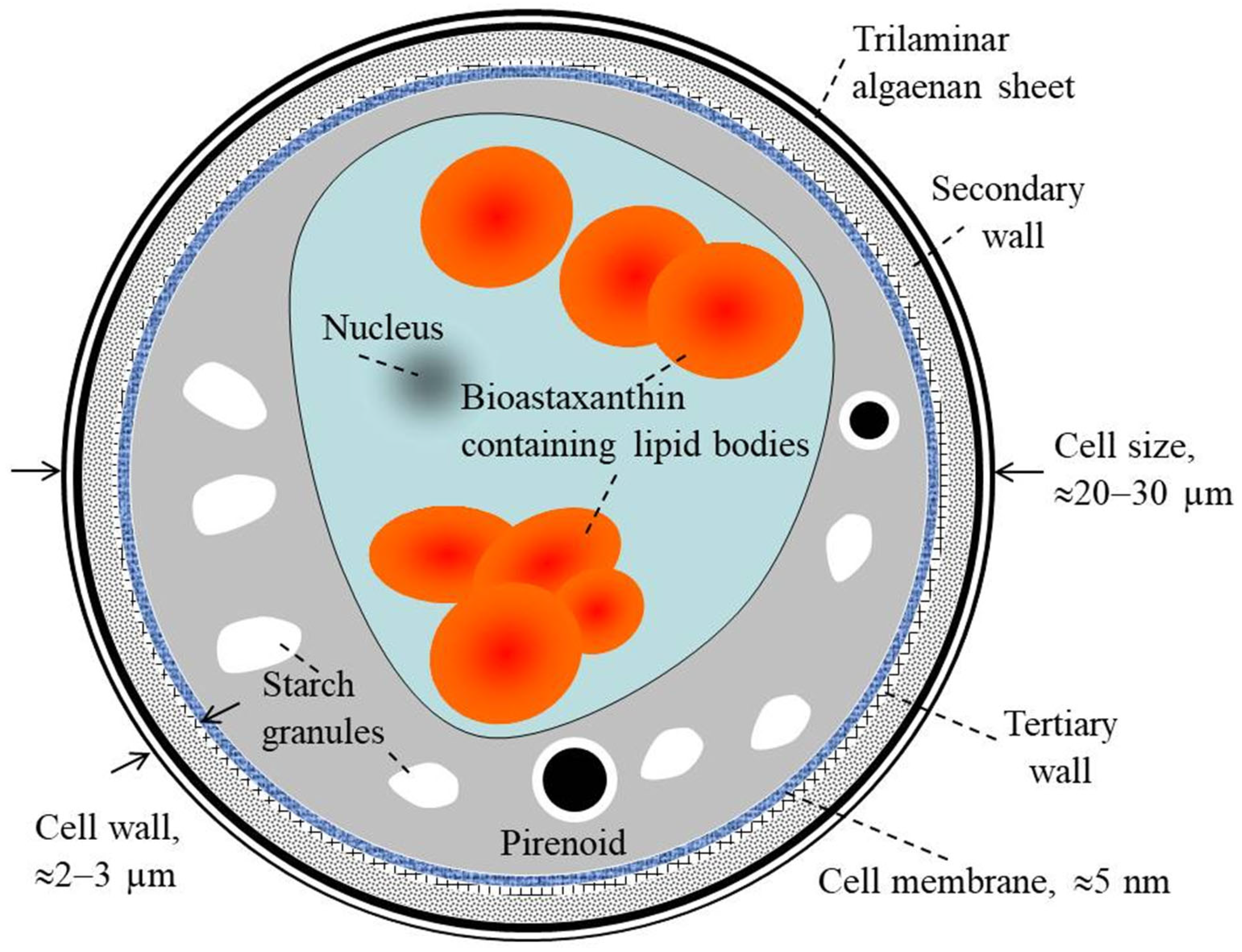
Figure 2. Description of H. pluvialis cell structure at the resistant cyst stage.
The outside part of the cell is covered with an aplanospore cell wall (with total thinness of 2–3 µm). This wall is composed of an outer primary algaenan-based wall (a very stable sporopollenin-like wall), a secondary wall (composed of mannose and cellulose with homogeneous arrangement) and a tertiary wall (composed of mannose and cellulose with heterogeneous arrangement). Therefore, the cell is protected by complex, strong and rigid cell walls.
The three layered cell structure acts as a strong barrier to different external physical and chemical stresses and provides a high resistance against extreme environmental stress conditions [18][19]. Consequently, the cell wall developed is indigestible and reduces the bio-accessibility of the internal biomolecules such as astaxanthin. Moreover, the thickness of the H. pluvialis cell wall that is reached after the stress culturing stage is considered as an important bottleneck limiting the large-scale production of natural astaxanthin.
4.2. Biomolecular Composition
The chemical constituents of extracellular extracts of H. pluvialis were recently analyzed [66]. H. pluvialis cell composition includes ash, protein, carbohydrates, dietary fiber, carotenoids and lipids.
Figure 3 presents the relative percentage proportions of ash, protein, carbohydrates total dietary fiber, carotenoids and lipids in the initial biomass and extracts obtained using supercritical CO2 extraction at optimized conditions (pressure of 350 bar, temperature of 50 °C, and CO2 flow rate of 0.47 Kg/min) for the H. pluvialis red phase [67]. The total dietary fiber and protein were observed as the main intracellular constituents in the initial biomass. Carotenoids include astaxanthin (66–70%), β-carotene (3.5–6.5%) and lutein (27–28%). The content of proteins, lipids and carotenoids in extracts obtained by supercritical CO2 was higher in comparison to that of the biomass. An inverse situation was observed for the content of total dietary fibers (58% in the biomass compared with 26% in the extract).
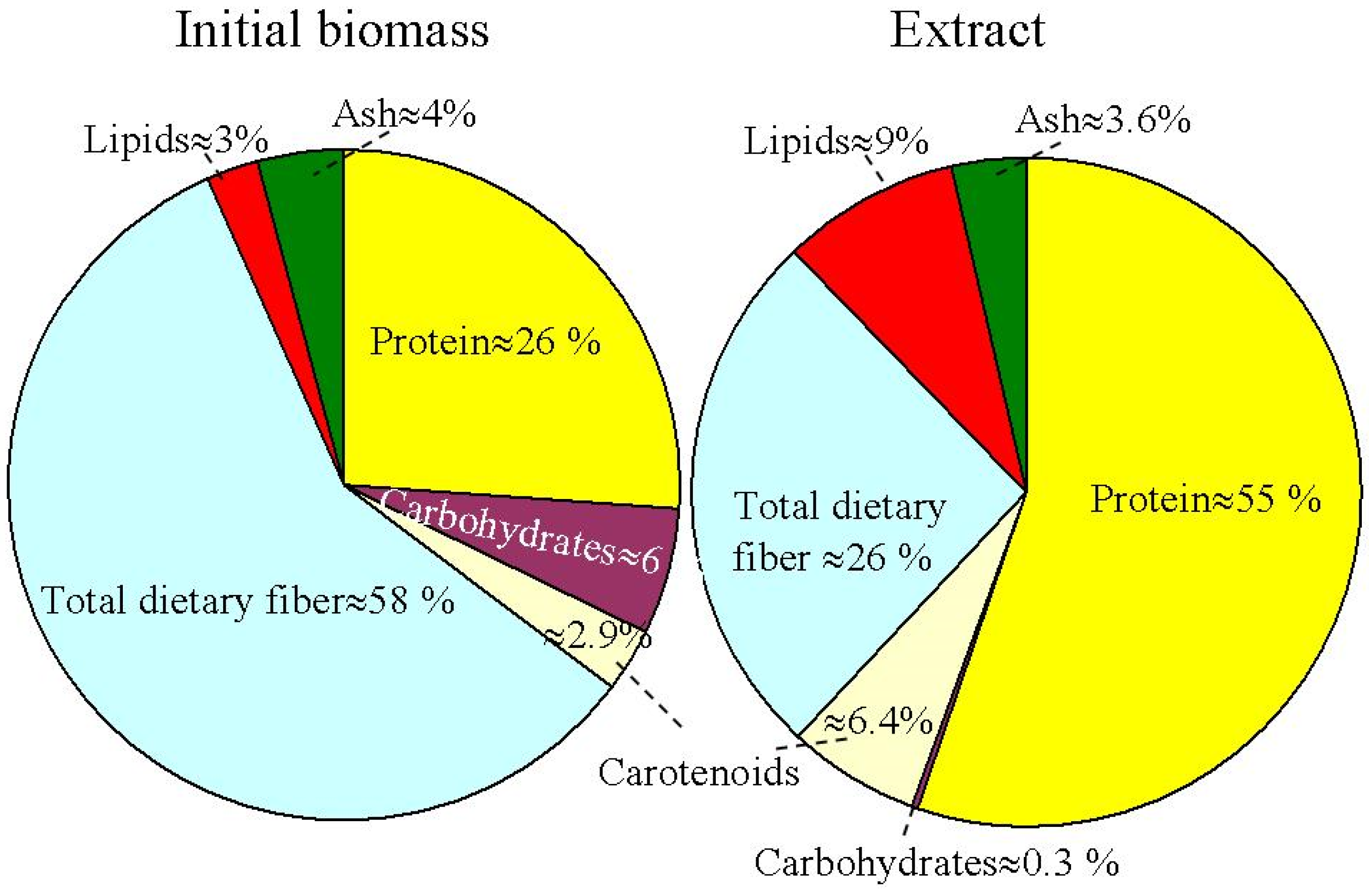
Figure 3. Initial biomass and supercritical CO2 extract relative percentage proportions for different biomolecular component compositions in the H. pluvialis red phase.
4.2.1. Lipids, Proteins and Carbohydrates
The content of lipids, proteins and carbohydrates in the H. pluvialis cells strongly depends on the stage of cell evolution [28][41][68]. Lipid content in the H. pluvialis cells may be rather high during the green vegetative stage (≈20–25% of the total biomass). During the red-stage evolution, lipid content increases (≈32–37%) and can be manipulated by operation conditions and the stress imposed. The protein and carbohydrate contents also change with transition from the green phase to the red phase. The protein content is ≈29–45% in the green stage and it reduces up to 17–25% in the red stage. The carbohydrate content is ≈15–17% in the green stage and it increases up to 36–40% in the red stage.
4.2.2. Carotenoids
During the green stage, the other carotenoids in H. pluvialis are lutein (≈50–70% of total carotenoids), neoxanthin, β-carotene and violaxanthin (≈8–13% for each of them); there is no astaxanthin content [41][69]. During the red stage, the total carotenoid content is significantly increased (mainly due to the astaxanthin accumulation) and can reach more than 4% of the biomass dry weight. At this stage, astaxanthin is the main cell carotenoid, and the other carotenoids represent ≈5–20% of the total content.
Astaxanthin (the chemical IUPAC name is 3,3′-dihydroxy-β,β-carotene-4,4′-dione) is a liposoluble red dietary carotenoid. It has the molecular formula C40H52O4, a molecular weight of 596.86 g/mol, a boiling point of 774.04 °C, a density of 1.071 g/cm3 and a melting point of 182–183 °C. Astaxanthin contains 13 conjugated double bonds with two benzene rings and hydroxyl groups at the ends [70]. Due to the presence of hydroxyl groups, the molecule of astaxanthin is polar.
Astaxanthin is rather unstable and can be degraded during the production process due to heating (even for a very short time), long-term storage, in acidic conditions under ultraviolet irradiation and in the presence of oxygen [71]. The effect of extraction and drying methods on the antioxidant activity of astaxanthin was discussed in [72]. The general overview of different chemical and biochemical properties of astaxanthin is presented in [41].
The microalga H. pluvialis can accumulate the optical pure isomer of astaxanthin (3S, 3′S), which has high antioxidant activity (in neutralization of destructive reactive oxygen species) that is approximately 20 times stronger the synthetic astaxanthin [73]. Several studies have shown that astaxanthin accumulated within the cysts of H. pluvialis exists in an esterified form, mainly as monoesters (70%), di-esters (25%) and free form (5%) with other occurring carotenoids [46]. The astaxanthin can be esterified on one or both of its hydroxyl groups with various fatty acids such as palmitic, stearic and oleic acids [74].
For the commercialization of natural astaxanthin, the free form is only approved for the market. This means the purification of final astaxanthin product of H. pluvialis needs to pass the additional step of de-esterification (known as saponification). Two methods exist for the de-esterification of extracted astaxanthin: de-esterification through enzymolysis using a cholesterol esterase and de-esterification through saponification in darkness at low or room temperature using an alkaline solution of NaOH or KOH in methanol. However, this step of de-esterification could induce a loss of carotenoids due to their degradation. Three factors could negatively affect the final pigments: treatment time, NaOH or KOH concentration and temperature [75].
This entry is adapted from the peer-reviewed paper 10.3390/molecules28052089
References
- Zarei, Z.; Zamani, H. Biorefinery Potential of Microalga Haematococcus pluvialis to Produce Astaxanthin and Biodiesel under Nitrogen Deprivation. BioEnergy Res. 2022, 1–12.
- Khoo, K.S.; Lee, S.Y.; Ooi, C.W.; Fu, X.; Miao, X.; Ling, T.C.; Show, P.L. Recent Advances in Biorefinery of Astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2019, 288, 121606.
- Hosseini, A.; Jazini, M.; Mahdieh, M.; Karimi, K. Efficient Superantioxidant and Biofuel Production from Microalga Haematococcus pluvialis via a Biorefinery Approach. Bioresour. Technol. 2020, 306, 123100.
- Papadaki, S.; Kyriakopoulou, K.; Krokida, M. Recovery and Encapsualtion of Bioactive Extracts from Haematococcus pluvialis and Phaedodactylum Tricornutum for Food Applications. IOSR Int. Organ. Sci. Res. J. Environ. Sci. Toxicol. Food Technol. 2017, 10, 53–58.
- Nishshanka, G.K.S.H.; Liyanaarachchi, V.C.; Nimarshana, P.H.V.; Ariyadasa, T.U.; Chang, J.-S. Haematococcus pluvialis: A Potential Feedstock for Multiple-Product Biorefining. J. Clean. Prod. 2022, 344, 131103.
- Bogoçlua, M.; Keçecilerb, C.; Çokcanb, C.; Özelb, C.; Tekerekc, B.S.; Yücelb, S. The Accumulation of Astaxanthin from Haematococcus pluvialis in Different Stress Factors. J. Indian Chem. Soc. 2019, 96, 1–6.
- Dos Santos, A.C.; Lombardi, A.T. Growth, Photosynthesis and Biochemical Composition of Haematococcus pluvialis </i> at Various PH. J. Algal Biomass Util. 2017, 8, 1–15.
- Christian, D.; Zhang, J.; Sawdon, A.J.; Peng, C.-A. Enhanced Astaxanthin Accumulation in Haematococcus pluvialis Using High Carbon Dioxide Concentration and Light Illumination. Bioresour. Technol. 2018, 256, 548–551.
- Nishshanka, S.H.; Liyanaarachchi, V.C.; Premaratne, M.; Ariyadasa, T.U.; Nimarshana, V. Sustainable Cultivation of Haematococcus pluvialis for the Production of Natural Astaxanthin. In Proceedings of the Moratuwa Engineering Research Conference (MERCon), Moratuwa, Sri Lanka, 27–29 July 2021; pp. 297–302.
- Ahirwar, A.; Meignen, G.; Khan, M.J.; Sirotiya, V.; Scarsini, M.; Roux, S.; Marchand, J.; Schoefs, B.; Vinayak, V. Light Modulates Transcriptomic Dynamics Upregulating Astaxanthin Accumulation in Haematococcus: A Review. Bioresour. Technol. 2021, 340, 125707.
- Giannelli, L.; Yamada, H.; Katsuda, T.; Yamaji, H. Effects of Temperature on the Astaxanthin Productivity and Light Harvesting Characteristics of the Green Alga Haematococcus pluvialis. J. Biosci. Bioeng. 2015, 119, 345–350.
- Cheng, J.; Li, K.; Yang, Z.; Zhou, J.; Cen, K. Enhancing the Growth Rate and Astaxanthin Yield of Haematococcus pluvialis by Nuclear Irradiation and High Concentration of Carbon Dioxide Stress. Bioresour. Technol. 2016, 204, 49–54.
- Rizzo, A.; Ross, M.E.; Norici, A.; Jesus, B. A Two-Step Process for Improved Biomass Production and Non-Destructive Astaxanthin and Carotenoids Accumulation in Haematococcus pluvialis. Appl. Sci. 2022, 12, 1261.
- Wan, M.; Zhang, Z.; Wang, J.; Huang, J.; Fan, J.; Yu, A.; Wang, W.; Li, Y. Sequential Heterotrophy--Dilution--Photoinduction Cultivation of Haematococcus pluvialis for Efficient Production of Astaxanthin. Bioresour. Technol. 2015, 198, 557–563.
- Geada, P.; Rodrigues, R.; Loureiro, L.; Pereira, R.; Fernandes, B.; Teixeira, J.A.; Vasconcelos, V.; Vicente, A.A. Electrotechnologies Applied to Microalgal Biotechnology--Applications, Techniques and Future Trends. Renew. Sustain. Energy Rev. 2018, 94, 656–668.
- Bai, F.; Gusbeth, C.; Frey, W.; Nick, P. Nanosecond Pulsed Electric Fields Modulate the Expression of the Astaxanthin Biosynthesis Genes Psy, CrtR-b and Bkt 1 in Haematococcus pluvialis. Sci. Rep. 2020, 10, 1–15.
- Nguyen, A.D.; Scarsini, M.; Poncin-Epaillard, F.; Noel, O.; Marchand, J.; Schoefs, B. Plasma Applications in Microalgal Biotechnology. In Agritech: Innovative Agriculture Using Microwaves and Plasmas. Thermal and Non-Thermal Processing; Horikoshi, S., Brodie, G., Takaki, K., Serpone, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 327–349.
- Li, Q.; Zhao, Y.; Ding, W.; Han, B.; Geng, S.; Ning, D.; Ma, T.; Yu, X. Gamma-Aminobutyric Acid Facilitates the Simultaneous Production of Biomass, Astaxanthin and Lipids in Haematococcus pluvialis under Salinity and High-Light Stress Conditions. Bioresour. Technol. 2021, 320, 124418.
- Kim, B.; Lee, S.Y.; Narasimhan, A.L.; Kim, S.; Oh, Y.-K. Cell Disruption and Astaxanthin Extraction from Haematococcus pluvialis: Recent Advances. Bioresour. Technol. 2022, 343, 126124.
- Vorobiev, E.; Lebovka, N. Processing of Foods and Biomass Feedstocks by Pulsed Electric Energy; Springer: Cham, Switzerland, 2020; ISBN 978-3-030-40917-3.
- von Flotow, J. Beobachtungen Über Haematococcus pluvialis (Observations on Haematococcus pluvialis) Verhandlungen Der Kaiserlichen Leopoldinisch-Carolinischen Deutschen Akademie Der Naturforscher 12(Abt. 2): 413–606, 3 Pls. Verhandlungen der Kais. Leopoldinisch-Carolinischen Dtsch. Akad. der Naturforscher (Proceedings Imp. Leopoldine-Carolinic Ger. Acad. Sci. 1844, 12, 413–606.
- Kützing, F.T. Über Die Verwandlung Der Infusorien in Niedere Algenformen (On the Transformation of Infusoria into Lower Forms of Algae; Verlag von W. Kohne: Nordhausen, Germany, 1844.
- Focke, G.W. Physiologische Studien; C. Schünemann: Bremen, Germany, 1847.
- Hazen, T.E. The Life History of Sphaerella lacustris (Haematococcus pluvialis). Mem. Torrey Bot. Club 1899, 6, 211–246.
- Rather, A.H.; Singh, S.; Rao, R.; Choudhary, S. Haematococcus pluvialis Astaxanthin and Its Applications. In Advances in Biotechnology and Bioscience; Kumar, S., Ed.; AkiNik Publications: New Delhi, India, 2020; Volume 4, pp. 21–35.
- Boussiba, S. Carotenogenesis in the Green Alga Haematococcus pluvialis: Cellular Physiology and Stress Response. Physiol. Plant. 2000, 108, 111–117.
- Grujić, V.J.; Todorović, B.; Kranvogl, R.; Ciringer, T.; Ambrožič-Dolinšek, J. Diversity and Content of Carotenoids and Other Pigments in the Transition from the Green to the Red Stage of Haematococcus pluvialis Microalgae Identified by HPLC-DAD and LC-QTOF-MS. Plants 2022, 11, 1026.
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531.
- Bauer, A.; Minceva, M. Techno-Economic Analysis of a New Downstream Process for the Production of Astaxanthin from the Microalgae Haematococcus pluvialis. Bioresour. Bioprocess. 2021, 8, 1–18.
- Zhang, C.; Liu, J.; Zhang, L. Cell Cycles and Proliferation Patterns in Haematococcus pluvialis. Chin. J. Oceanol. Limnol. 2017, 35, 1205–1211.
- Samorì, C.; Pezzolesi, L.; Galletti, P.; Semeraro, M.; Tagliavini, E. Extraction and Milking of Astaxanthin from Haematococcus pluvialis Cultures. Green Chem. 2019, 21, 3621–3628.
- Nunes, M.; Vieira, A.A.H.; Pinto, E.; Carneiro, R.L.; Monteiro, A.C. Carotenogenesis in Haematococcus pluvialis Cells Induced by Light and Nutrient Stresses. Pesqui. Agropecuária Bras. 2013, 48, 825–832.
- Xi, T.; Kim, D.G.; Roh, S.W.; Choi, J.-S.; Choi, Y.-E. Enhancement of Astaxanthin Production Using Haematococcus pluvialis with Novel LED Wavelength Shift Strategy. Appl. Microbiol. Biotechnol. 2016, 100, 6231–6238.
- Niño-Castillo, C.M.; Rodríguez-Rivera, F.C.; Díaz, L.E.; Lancheros-Díaz, A.G. Evaluation of Cell Growth Conditions for the Astaxanthin Production as of Haematococcus pluvialis Microalgae. Nova 2017, 15, 19–31.
- Pham, K.T.; Nguyen, T.C.; Luong, T.H.; Dang, P.H.; Vu, D.C.; Do, T.N.; Phi, T.C.M.; Nguyen, D.B. Influence of Inoculum Size, CO2 Concentration and LEDs on the Growth of Green Microalgae Haematococcus Pluvialis Flotow. Vietnam J. Sci. Technol. Eng. 2018, 60, 59–65.
- Bauer, A.; Minceva, M. Examination of Photo-, Mixo-, and Heterotrophic Cultivation Conditions on Haematococcus pluvialis Cyst Cell Germination. Appl. Sci. 2021, 11, 7201.
- Scibilia, L.; Girolomoni, L.; Berteotti, S.; Alboresi, A.; Ballottari, M. Photosynthetic Response to Nitrogen Starvation and High Light in Haematococcus pluvialis. Algal Res. 2015, 12, 170–181.
- Domínguez, A.; Pereira, S.; Otero, A. Does Haematococcus pluvialis Need to Sleep? Algal Res. 2019, 44, 101722.
- Zhao, Y.; Shang, M.; Xu, J.-W.; Zhao, P.; Li, T.; Yu, X. Enhanced Astaxanthin Production from a Novel Strain of Haematococcus pluvialis Using Fulvic Acid. Process Biochem. 2015, 50, 2072–2077.
- Panis, G.; Carreon, J.R. Commercial Astaxanthin Production Derived by Green Alga Haematococcus pluvialis: A Microalgae Process Model and a Techno-Economic Assessment All through Production Line. Algal Res. 2016, 18, 175–190.
- Jannel, S.; Caro, Y.; Bermudes, M.; Petit, T. Novel Insights into the Biotechnological Production of Haematococcus pluvialis-Derived Astaxanthin: Advances and Key Challenges to Allow Its Industrial Use as Novel Food Ingredient. J. Mar. Sci. Eng. 2020, 8, 789.
- Miyakawa, K. Commercial Production of Astaxanthin from the Green Alga Haematococcus pluvialis. In Carotenoids: Biosynthetic and Biofunctional Approaches; Misawa, N., Ed.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 3–10.
- Jiang, S.; Tong, S. Advances in Astaxanthin Biosynthesis in Haematococcus pluvialis. Chin. J. Biotechnol. 2019, 35, 988–997.
- Li, J.; Zhu, D.; Niu, J.; Shen, S.; Wang, G. An Economic Assessment of Astaxanthin Production by Large Scale Cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011, 29, 568–574.
- Oslan, S.N.H.; Shoparwe, N.F.; Yusoff, A.H.; Rahim, A.A.; Chang, C.S.; Tan, J.S.; Oslan, S.N.; Arumugam, K.; Ariff, A.B.; Sulaiman, A.Z.; et al. A Review on Haematococcus pluvialis Bioprocess Optimization of Green and Red Stage Culture Conditions for the Production of Natural Astaxanthin. Biomolecules 2021, 11, 256.
- Oslan, S.N.H.; Tan, J.S.; Oslan, S.N.; Matanjun, P.; Mokhtar, R.A.M.; Shapawi, R.; Huda, N. Haematococcus pluvialis as a Potential Source of Astaxanthin with Diverse Applications in Industrial Sectors: Current Research and Future Directions. Molecules 2021, 26, 6470.
- Ren, Y.; Deng, J.; Huang, J.; Wu, Z.; Yi, L.; Bi, Y.; Chen, F. Using Green Alga Haematococcus pluvialis for Astaxanthin and Lipid Co-Production: Advances and Outlook. Bioresour. Technol. 2021, 340, 125736.
- Souza, R.L.M.; Viana, W.K.R.; Moreira, M.S.M.; Soares Filho, A.A.; Lisboa, V.; de Vidigal, R.C.A.B.; Catter, K.M.; Eloy, H.R.F.; Matias, J.F.N. Technological Innovations in the Cultivation of Haematococcus pluvialis Microalgae. Sist. Gestão 2020, 15, 223–234.
- Liu, J.; van der Meer, J.P.; Zhang, L.; Zhang, Y. Cultivation of Haematococcus pluvialis for Astaxanthin Production. In Microalgal Production for Biomass and High-Value Products; Slocombe, S.P., Benemann, J.R., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 267–293.
- Zhu, X.; Meng, C.; Sun, F.; Wei, Z.; Chen, L.; Chen, W.; Tong, S.; Du, H.; Gao, J.; Ren, J. Sustainable Production of Astaxanthin in Microorganisms: The Past, Present, and Future. Crit. Rev. Food Sci. Nutr. 2022, 1–17.
- Lu, S.; Chen, G.; Wei, D. Effect of Electroporation Conditions on Cell Viability of Haematococcus pluvialis. Mod. Food Sci. Technol. 2010, 26, 554–557.
- Kim, J.Y.; Lee, C.; Jeon, M.S.; Park, J.; Choi, Y.-E. Enhancement of Microalga Haematococcus pluvialis Growth and Astaxanthin Production by Electrical Treatment. Bioresour. Technol. 2018, 268, 815–819.
- Fitriana, H.-N.; Lee, S.-Y.; Choi, S.-A.; Lee, J.-Y.; Kim, B.-L.; Lee, J.-S.; Oh, Y.-K. Electric Stimulation of Astaxanthin Biosynthesis in Haematococcus pluvialis. Appl. Sci. 2021, 11, 3348.
- Li, L.; Chen, Z.; Acheampong, A.; Huang, Q. Low-Temperature Plasma Promotes Growth of Haematococcus pluvialis and Accumulation of Astaxanthin by Regulating Histone H3 Lysine 4 Tri-Methylation. Bioresour. Technol. 2022, 343, 126095.
- Gao, L.; Shi, X.; Wu, X. Applications and Challenges of Low Temperature Plasma in Pharmaceutical Field. J. Pharm. Anal. 2021, 11, 28–36.
- Liu, J.; Chen, J.; Chen, Z.; Qin, S.; Huang, Q. Isolation and Characterization of Astaxanthin-Hyperproducing Mutants of Haematococcus pluvialis (Chlorophyceae) Produced by Dielectric Barrier Discharge Plasma. Phycologia 2016, 55, 650–658.
- Wu, X.; Liu, Z.; Jiang, Y. Mutation Breeding of Haematococcus pluvialis by Atmospheric and Room Temperature Plasma and Isolation of High-Producing Mutants. J. Food Saf. Qual. 2016, 7, 3781–3787.
- Chen, Z.; Li, L.; Zhang, H.; Huang, Q. Stimulation of Biomass and Astaxanthin Accumulation in Haematococcus pluvialis Using Low-Temperature Plasma (LTP). Bioresour. Technol. Rep. 2020, 9, 100385.
- Chen, Z.; Chen, J.; Liu, J.; Li, L.; Qin, S.; Huang, Q. Transcriptomic and Metabolic Analysis of an Astaxanthin-Hyperproducing Haematococcus pluvialis Mutant Obtained by Low-Temperature Plasma (LTP) Mutagenesis under High Light Irradiation. Algal Res. 2020, 45, 101746.
- Bowen, W.R. Ultrastructural Aspects of the Cell Boundary of Haematococcus pluvialis. Trans. Am. Microsc. Soc. 1967, 86, 36–43.
- Hagen, C.; Siegmund, S.; Braune, W. Ultrastructural and Chemical Changes in the Cell Wall of Haematococcus pluvialis (Volvocales, Chlorophyta) during Aplanospore Formation. Eur. J. Phycol. 2002, 37, 217–226.
- Damiani, M.C.; Leonardi, P.I.; Pieroni, O.I.; Cáceres, E.J. Ultrastructure of the Cyst Wall of Haematococcus pluvialis (Chlorophyceae): Wall Development and Behaviour during Cyst Germination. Phycologia 2006, 45, 616–623.
- D’Hondt, E.; Martin-Juarez, J.; Bolado, S.; Kasperoviciene, J.; Koreiviene, J.; Sulcius, S.; Elst, K.; Bastiaens, L. Cell Disruption Technologies. In Microalgae-Based Biofuels and Bioproducts. From Feedstock Cultivation to End-products; Muñoz, R., Gonzalez-Fernandez, C., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 133–154.
- Praveenkumar, R.; Lee, J.; Vijayan, D.; Lee, S.Y.; Lee, K.; Sim, S.J.; Hong, M.E.; Kim, Y.-E.; Oh, Y.-K. Morphological Change and Cell Disruption of Haematococcus pluvialis Cyst during High-Pressure Homogenization for Astaxanthin Recovery. Appl. Sci. 2020, 10, 513.
- Xu, R.; Zhang, L.; Yu, W.; Liu, J. A Strategy for Interfering with the Formation of Thick Cell Walls in Haematococcus pluvialis by down-Regulating the Mannan Synthesis Pathway. Bioresour. Technol. 2022, 362, 127783.
- Zhu, F.; Shi, B.; Li, Y.; Yang, H.; Sun, S.; Yao, S.; Yin, X.; Zhang, J. Chemical Constituents of Extracellular Extract of Haematococcus pluvialis. Chem. Nat. Compd. 2019, 55, 578–579.
- Marino, T.; Iovine, A.; Casella, P.; Martino, M.; Chianese, S.; Larocca, V.; Musmarra, D.; Molino, A. From Haematococcus pluvialis Microalgae a Powerful Antioxidant for Cosmetic Applications. Chem. Eng. Trans. 2020, 79, 271–276.
- Shahid, A.; Khan, F.; Ahmad, N.; Farooq, M.; Mehmood, M.A. Microalgal Carbohydrates and Proteins: Synthesis, Extraction, Applications, and Challenges. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M.A., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 433–468.
- Jung, S.M.; Jeon, J.; Park, T.H.; Yoon, J.H.; Lee, K.S.; Shin, H.W. Enhancing Production-Extraction and Antioxidant Activity of Astaxanthin from Haematococcus pluvialis. J. Environ. Biol. 2019, 40, 924–931.
- Li, X.; Wang, X.; Duan, C.; Yi, S.; Gao, Z.; Xiao, C.; Agathos, S.N.; Wang, G.; Li, J. Biotechnological Production of Astaxanthin from the Microalga Haematococcus pluvialis. Biotechnol. Adv. 2020, 43, 107602.
- Bjørklund, G.; Gasmi, A.; Lenchyk, L.; Shanaida, M.; Zafar, S.; Mujawdiya, P.K.; Lysiuk, R.; Antonyak, H.; Noor, S.; Akram, M.; et al. The Role of Astaxanthin as a Nutraceutical in Health and Age-Related Conditions. Molecules 2022, 27, 7167.
- Zhao, X.; Zhang, X.; Fu, L.; Zhu, H.; Zhang, B. Effect of Extraction and Drying Methods on Antioxidant Activity of Astaxanthin from Haematococcus pluvialis. Food Bioprod. Process. 2016, 99, 197–203.
- Lemoine, Y.; Schoefs, B. Secondary Ketocarotenoid Astaxanthin Biosynthesis in Algae: A Multifunctional Response to Stress. Photosynth. Res. 2010, 106, 155–177.
- Cheng, X.; Shah, M. Bioextraction of Astaxanthin Adopting Varied Techniques and Downstream Processing Methodologies. In Global Perspectives on Astaxanthin: From Industrial Production to Food, Health, and Pharmaceutical Applications; Ravishankar, G.A., Rao, A.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 313–339.
- Galarza, J.I.; Vega, B.O.A.; Villón, J.; Henríquez, V. Deesterification of Astaxanthin and Intermediate Esters from Haematococcus pluvialis Subjected to Stress. Biotechnol. Rep. 2019, 23, e00351.
This entry is offline, you can click here to edit this entry!
