Cellulases from glycoside hydrolase family 48 (GH48) are critical components of natural lignocellulose-degrading systems. GH48 cellulases are broadly distributed in cellulolytic microorganisms. With the development of genomics and metatranscriptomics, diverse GH48 genes have been identified, especially in the highly efficient cellulose-degrading ruminal system. GH48 cellulases utilize an inverting mechanism to hydrolyze cellulose in a processive mode. Although GH48 cellulases are indispensable for cellulolytic bacteria, they exhibit intrinsically low cellulolytic activity. Great efforts have been made to improve their performance. Besides, GH48 cellulases greatly synergize with the complementary endoglucanases in free cellulase systems or cellulosome systems.
- GH48
- cellulase
- structure
- lignocellulose
- biomass
- biorefinery
- bioenergy
- enzyme
- protein engineering
- cellulosome
1. Key Role of Glycoside Hydrolase Family 48 Cellulases in Cellulose Degradation
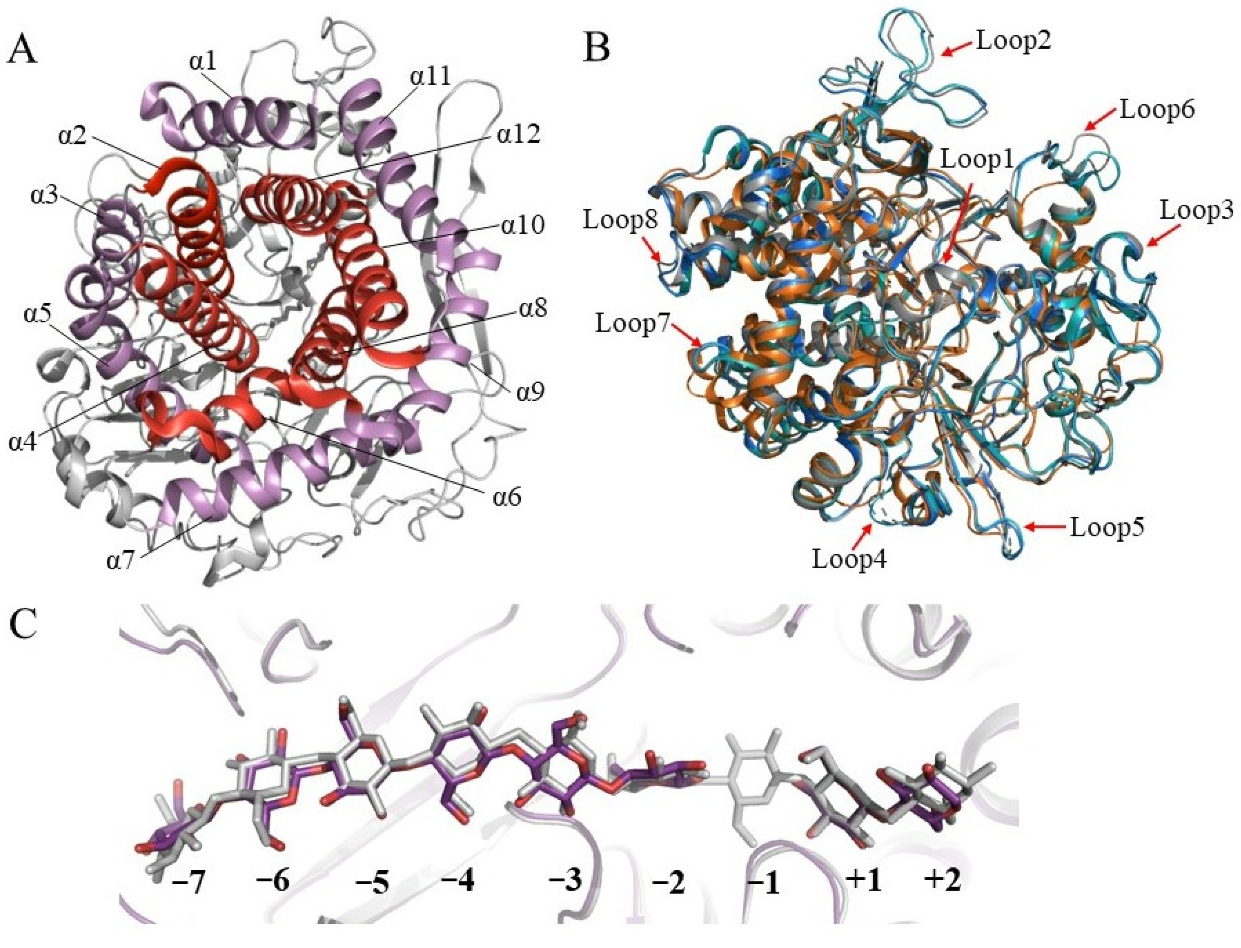
2. Structures of Glycoside Hydrolase Family 48 Cellulases
| GH48s | Activities | Origin | PDB Code (Ligand) | Form | Reference |
|---|---|---|---|---|---|
| CelS (Cel48S) | Exocellulase | C. thermocellum DSM1313 | 5YJ6 | cellulosomal | [17] |
| CelS (Cel48A) | Exocellulase | C. thermocellum F7 | 1L1Y(cellobiose), 1L2A (cellobiose, cellohexaose) | cellulosomal | [18] |
| Cel48Y | Exocellulase | C. thermocellum ATCC 27405 | free-cellulase | [19] | |
| Cel48S | Exocellulase | C. thermocellum ATCC 27405 | cellulosomal | [20] | |
| CelY | Exocellulase | Clostridium stercorarium | free-cellulase | [21] | |
| GH48 | Exocellulase | Clostridium clariflavum DSM 19732 | cellulosomal | [22] | |
| CpCel48 | Exocellulase | Clostridium phytofermentans ISDg | free-cellulase | [23] | |
| ExgS | Exocellulase | Clostridium cellulovorans ATCC 35296 | 4XWL (PEG), 4XWM (cellobiose), 4XWN (cellobiose, cellopentaose) | cellulosomal | [24] |
| Cel48F | Processive endocellulase | C. cellulolyticum H10 | 1FCE (inhibitor IG4), 1F9D (cellotetraose), 1FBW (cellohexaose), 1FAE (cellobiose), 1FBO (cellobiitol), 1F9O (inhibitor PIPS-IG3), 1G9G (glucose), 2QNO (thiocellodecaose), 1G9J (hemithiocellooligosaccharide) | cellulosomal | [14][15][16] |
| CbCel48A | Exocellulase | C. bescii DSM 6725 | 4EL8, 4L0G (cellobiose), 4TXT (cellotriose), 4L6X |
multi- module | [13] |
| Cdan_2053 | Cellulase | Caldicellulosiruptor danielii | 6D5D (cellobiose) | multi-module | [25] |
| BlCel48B | Processive cellulase | Bacillus licheniformis DSM 13 | 7KW6 (cellobiose, cellotetraose) | free-cellulase | [26] |
| BpCel48 | Cellulase | Bacillus pmilus SAFR-032 | 5BV9 (cellobiose), 5CVY (cellobiose, cellohexaose) | free-cellulase | [27][28] |
| BpGH48 | Cellulase | Bacillus pmilus SH-B9 | 5VMA (cellobio-derived isofagomine) | free-cellulase | To be published |
| TfCel48A | Exocellulase | T. fusca YX | 4JJJ (cellobiose, cellohexaose) | free-cellulase | [29] |
| HcheGH48 | Cellulase | H. chejuensis KCTC 2396 | 4FUS (cellobiose) | free-cellulase | [30] |
| CbhB | Exocellulase | Cellulomonas fimi ATCC 484 | free-cellulase | [31] | |
| Cel48A | Exocellulase | C. ruminicola H1 | free-cellulase | [32] | |
| Cel48 | Exocellulase | Myxobacter sp. AL-1 | free-cellulase | [33] | |
| Cel48C | Exocellulase | Paenibacillus sp. BP-23 | free-cellulase | [34] |
3. Strategies and Progress of Engineering Glycoside Hydrolase Family 48 Cellulases
This entry is adapted from the peer-reviewed paper 10.3390/fermentation9030204
References
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807.
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Lukk, T.; Tuohy, M.G.; Gong, L.; Nguyen-Tri, P.; Goddard, A.D.; Bill, R.M.; Nayak, S.C.; et al. Lignocellulosic biorefineries: The current state of challenges and strategies for efficient commercialization. Renew. Sustain. Energy Rev. 2021, 148, 111258.
- Liu, Y.; Cruz-Morales, P.; Zargar, A.; Belcher, M.S.; Pang, B.; Englund, E.; Dan, Q.; Yin, K.; Keasling, J.D. Biofuels for a sustainable future. Cell 2021, 184, 1636–1647.
- Devillard, E.; Goodheart, D.B.; Karnati, S.K.R.; Bayer, E.A.; Lamed, R.; Miron, J.; Nelson, K.E.; Morrison, M. Ruminococcus albus 8 mutants defective in cellulose degradation are deficient in two processive endocellulases, Cel48A and Cel9B, both of which possess a novel modular architecture. J. Bacteriol. 2004, 186, 136–145.
- Izquierdo, J.A.; Sizova, M.V.; Lynd, L.R. Diversity of bacteria and glycosyl hydrolase family 48 genes in cellulolytic consortia enriched from thermophilic biocompost. Appl. Environ. Microbiol. 2010, 76, 3545–3553.
- Olson, D.G.; Tripathi, S.A.; Giannone, R.J.; Lo, J.; Caiazza, N.C.; Hogsett, D.A.; Hettich, R.L.; Guss, A.M.; Dubrovsky, G.; Lynd, L.R. Deletion of the Cel48S cellulase from Clostridium thermocellum. Proc. Natl. Acad. Sci. USA 2010, 107, 17727–17732.
- Young, J.; Chung, D.; Bomble, Y.J.; Himmel, M.E.; Westpheling, J. Deletion of Caldicellulosiruptor bescii CelA reveals its crucial role in the deconstruction of lignocellulosic biomass. Biotechnol. Biofuels 2014, 7, 142.
- Dai, X.; Tian, Y.; Li, J.; Luo, Y.; Liu, D.; Zheng, H.; Wang, J.; Dong, Z.; Hu, S.; Huang, L. Metatranscriptomic analyses of plant cell wall polysaccharide degradation by microorganisms in the cow rumen. Appl. Environ. Microbiol. 2015, 81, 1375–1386.
- Chu, Y.; Hao, Z.; Wang, K.; Tu, T.; Huang, H.; Wang, Y.; Bai, Y.G.; Wang, Y.; Luo, H.; Yao, B.; et al. The GH10 and GH48 dual-functional catalytic domains from a multimodular glycoside hydrolase synergize in hydrolyzing both cellulose and xylan. Biotechnol. Biofuels 2019, 12, 279.
- Lee, L.L.; Blumer-Schuette, S.E.; Izquierdo, J.A.; Zurawski, J.V.; Loder, A.J.; Conway, J.M.; Elkins, J.G.; Podar, M.; Clum, A.; Jones, P.C.; et al. Genus-wide assessment of lignocellulose utilization in the extremely thermophilic genus Caldicellulosiruptor by genomic, pangenomic, and metagenomic analyses. Appl. Environ. Microbiol. 2018, 84, e02694-17.
- Pereyra, L.P.; Hiibel, S.R.; Prieto Riquelme, M.V.; Reardon, K.F.; Pruden, A. Detection and quantification of functional genes of cellulose- degrading, fermentative, and sulfate-reducing bacteria and methanogenic archaea. Appl. Environ. Microbiol. 2010, 76, 2192–2202.
- Rettenmaier, R.; Lo, Y.K.; Schmidt, L.; Munk, B.; Lagkouvardos, I.; Neuhaus, K.; Schwarz, W.; Liebl, W.; Zverlov, V. A novel primer mixture for GH48 genes: Quantification and identification of truly cellulolytic bacteria in biogas fermenters. Microorganisms 2020, 8, 1297.
- Brunecky, R.; Alahuhta, M.; Xu, Q.; Donohoe, B.S.; Crowley, M.F.; Kataeva, I.A.; Yang, S.-J.; Resch, M.G.; Adams, M.W.W.; Lunin, V.V.; et al. Revealing nature’s cellulase diversity: The digestion mechanism of Caldicellulosiruptor bescii CelA. Science 2013, 342, 1513–1516.
- Parsiegla, G.; Juy, M.; Reverbel-Leroy, C.; Tardif, C.; Belaïch, J.P.; Driguez, H.; Haser, R. The crystal structure of the processive endocellulase CelF of Clostridium cellulolyticum in complex with a thiooligosaccharide inhibitor at 2.0 Å resolution. EMBO J. 1998, 17, 5551–5562.
- Parsiegla, G.; Reverbel-Leroy, C.; Tardif, C.; Belaich, J.P.; Driguez, H.; Haser, R. Crystal structures of the cellulase Cel48F in complex with inhibitors and substrates give insights into its processive action. Biochemistry 2000, 39, 11238–11246.
- Parsiegla, G.; Reverbel, C.; Tardif, C.; Driguez, H.; Haser, R. Structures of mutants of cellulase Cel48F of Clostridium cellulolyticum in complex with long hemithiocellooligosaccharides give rise to a new view of the substrate pathway during processive action. J. Mol. Biol. 2008, 375, 499–510.
- Liu, Y.-J.; Liu, S.; Dong, S.; Li, R.; Feng, Y.; Cui, Q. Determination of the native features of the exoglucanase Cel48S from Clostridium thermocellum. Biotechnol. Biofuels 2018, 11, 6.
- Guimarães, B.G.; Souchon, H.; Lytle, B.L.; David Wu, J.H.; Alzari, P.M. The crystal structure and catalytic mechanism of cellobiohydrolase CelS, the major enzymatic component of the Clostridium thermocellum cellulosome. J. Mol. Biol. 2002, 320, 587–596.
- Berger, E.; Zhang, D.; Zverlov, V.V.; Schwarz, W.H. Two noncellulosomal cellulases of Clostridium thermocellum, Cel9I and Cel48Y, hydrolyse crystalline cellulose synergistically. FEMS Microbiol. Lett. 2007, 268, 194–201.
- Kruus, K.; Wang, W.K.; Ching, J.; Wu, J.H.D. Exoglucanase activities of the recombinant Clostridium thermocellum CelS, a major cellulosome component. J. Bacteriol. 1995, 177, 1641–1644.
- Bronnenmeier, K.; Kundt, K.; Riedel, K.; Schwarz, W.H.; Staudenbauer, W.L. Structure of the Clostridium stercorarium gene celY encoding the exo-1,4-β-glucanase Avicelase II. Microbiology 1997, 143, 891–898.
- Artzi, L.; Morag, E.; Shamshoum, M.; Bayer, E.A. Cellulosomal expansin: Functionality and incorporation into the complex. Biotechnol. Biofuels 2016, 9, 61.
- Zhang, X.-Z.; Zhang, Z.; Zhu, Z.; Sathitsuksanoh, N.; Yang, Y.; Zhang, Y.-H.P. The noncellulosomal family 48 cellobiohydrolase from Clostridium phytofermentans ISDg: Heterologous expression, characterization, and processivity. Appl. Microbiol. Biotechnol. 2010, 86, 525–533.
- Tsai, L.C.; Amiraslanov, I.; Chen, H.R.; Chen, Y.W.; Lee, H.L.; Liang, P.H.; Liaw, Y.C. Structures of exoglucanase from Clostridium cellulovorans: Cellotetraose binding and cleavage. Acta Crystallogr. F Struct. Biol. Commun. 2015, 71, 1264–1272.
- Conway, J.M.; Crosby, J.R.; Hren, A.P.; Southerland, R.T.; Lee, L.L.; Lunin, V.V.; Alahuhta, P.; Himmel, M.E.; Bomble, Y.J.; Adams, M.W.W.; et al. Novel multidomain, multifunctional glycoside hydrolases from highly lignocellulolytic Caldicellulosiruptor species. AIChE J. 2018, 64, 4218–4228.
- Araújo, E.A.; Dias, A.H.S.; Kadowaki, M.A.S.; Piyadov, V.; Pellegrini, V.O.A.; Urio, M.B.; Ramos, L.P.; Skaf, M.S.; Polikarpov, I. Impact of cellulose properties on enzymatic degradation by bacterial GH48 enzymes: Structural and mechanistic insights from processive Bacillus licheniformis Cel48B cellulase. Carbohydr. Polym. 2021, 264, 118059.
- Chen, M.; Bu, L.; Alahuhta, M.; Brunecky, R.; Xu, Q.; Lunin, V.V.; Brady, J.W.; Crowley, M.F.; Himmel, M.E.; Bomble, Y.J. Strategies to reduce end-product inhibition in family 48 glycoside hydrolases. Proteins Struct. Funct. Bioinform. 2016, 84, 295–304.
- Brunecky, R.; Alahuhta, M.; Sammond, D.W.; Xu, Q.; Chen, M.; Wilson, D.B.; Brady, J.W.; Himmel, M.E.; Bomble, Y.J.; Lunin, V.V. Natural diversity of glycoside hydrolase family 48 exoglucanases: Insights from structure. Biotechnol. Biofuels 2017, 10, 274.
- Kostylev, M.; Alahuhta, M.; Chen, M.; Brunecky, R.; Himmel, M.E.; Lunin, V.V.; Brady, J.; Wilson, D.B. Cel48A from Thermobifida fusca: Structure and site directed mutagenesis of key residues. Biotechnol. Bioeng. 2014, 111, 664–673.
- Sukharnikov, L.O.; Alahuhta, M.; Brunecky, R.; Upadhyay, A.; Himmel, M.E.; Lunin, V.V.; Zhulin, I.B. Sequence, structure, and evolution of cellulases in glycoside hydrolase family 48. J. Biol. Chem. 2012, 287, 41068–41077.
- Shen, H.; Gilkes, N.R.; Kilburn, D.G.; Miller Jr, R.C.; Warren, R.A. Cellobiohydrolase B, a second exo-cellobiohydrolase from the cellulolytic bacterium Cellulomonas fimi. Biochem. J. 1995, 311, 67–74.
- Cai, S.; Li, J.; Hu, F.Z.; Zhang, K.; Luo, Y.; Janto, B.; Boissy, R.; Ehrlich, G.; Dong, X. Cellulosilyticum ruminicola, a newly described rumen bacterium that possesses redundant fibrolytic-protein-encoding genes and degrades lignocellulose with multiple carbohydrate- borne fibrolytic enzymes. Appl. Environ. Microbiol. 2010, 76, 3818–3824.
- Ramírez-Ramírez, N.; Romero-García, E.R.; Calderón, V.C.; Avitia, C.I.; Téllez-Valencia, A.; Pedraza-Reyes, M. Expression, characterization and synergistic interactions of Myxobacter Sp. AL-1 Cel9 and Cel48 glycosyl hydrolases. Int. J. Mol. Sci. 2008, 9, 247–257.
- Sánchez, M.M.; Pastor, F.I.J.; Diaz, P. Exo-mode of action of cellobiohydrolase Cel48C from Paenibacillus sp. BP-23. A unique type of cellulase among Bacillales. Eur. J. Biochem. 2003, 270, 2913–2919.
- Kurasin, M.; Väljamäe, P. Processivity of cellobiohydrolases is limited by the substrate. J. Biol. Chem. 2011, 286, 169–177.
- Pellegrini, V.O.A.; Bernardes, A.; Rezende, C.A.; Polikarpov, I. Cellulose fiber size defines efficiency of enzymatic hydrolysis and impacts degree of synergy between endo- and exoglucanases. Cellulose 2018, 25, 1865–1881.
- Morag, E.; Halevy, I.; Bayer, E.A.; Lamed, R. Isolation and properties of a major cellobiohydrolase from the cellulosome of Clostridium thermocellum. J. Bacteriol. 1991, 173, 4155–4162.
- Irwin, D.C.; Zhang, S.; Wilson, D.B. Cloning, expression and characterization of a family 48 exocellulase, Cel48A, from Thermobifida fusca. Eur. J. Biochem. 2000, 267, 4988–4997.
- Penneru, S.K.; Saharay, M.; Krishnan, M. CelS-catalyzed processive cellulose degradation and cellobiose extraction for the production of bioethanol. J. Chem. Inf. Model. 2022, 62, 6628–6638.
- Bu, L.; Crowley, M.F.; Himmel, M.E.; Beckham, G.T. Computational investigation of the pH dependence of loop flexibility and catalytic function in glycoside hydrolases. J. Biol. Chem. 2013, 288, 12175–12186.
- Bu, L.; Nimlos, M.R.; Shirts, M.R.; Ståhlberg, J.; Himmel, M.E.; Crowley, M.F.; Beckham, G.T. Product binding varies dramatically between processive and nonprocessive cellulase enzymes. J. Biol. Chem. 2012, 287, 24807–24813.
- Zhang, H.; Zhang, J.-L.; Sun, L.; Niu, X.-D.; Wang, S.; Shan, Y.-M. Molecular dynamics simulation of the processive endocellulase Cel48F from Clostridium cellulolyticum: A novel “water-control mechanism” in enzymatic hydrolysis of cellulose. J. Mol. Recognit. 2014, 27, 438–447.
- Qian, M.; Guan, S.; Shan, Y.; Zhang, H.; Wang, S. Structural and molecular basis of cellulase Cel48F by computational modeling: Insight into catalytic and product release mechanism. J. Struct. Biol. 2016, 194, 347–356.
- Lee, H.-J.; Kang, T.-G.; Kim, Y.-W.; Lee, H.-S.; Kim, S.-K. Functional expression and extracellular secretion of Clostridium thermocellum Cel48S cellulase in Escherichia coli via the signal recognition particle-dependent translocation pathway. Enzyme Microb. Technol. 2021, 151, 109918.
- Smith, M.A.; Rentmeister, A.; Snow, C.D.; Wu, T.; Farrow, M.F.; Mingardon, F.; Arnold, F.H. A diverse set of family 48 bacterial glycoside hydrolase cellulases created by structure-guided recombination. FEBS J. 2012, 279, 4453–4465.
