Stroke constitutes the second highest cause of morbidity and mortality worldwide while also impacting the world economy, triggering substantial financial burden in national health systems. High levels of blood glucose, homocysteine, and cholesterol are causative factors for atherothrombosis. These molecules induce erythrocyte dysfunction, which can culminate in atherosclerosis, thrombosis, thrombus stabilization, and post-stroke hypoxia. Glucose, toxic lipids, and homocysteine result in erythrocyte oxidative stress. This leads to phosphatidylserine exposure, promoting phagocytosis. Phagocytosis by endothelial cells, intraplaque macrophages, and vascular smooth muscle cells contribute to the expansion of the atherosclerotic plaque.
1. Red Blood Cells Participate in Hypoxia after Atherothrombotic Stroke
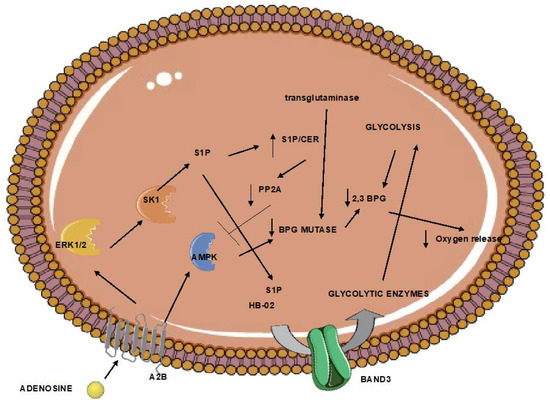
Erythrocytes regulate oxygen release. This function is mainly regulated by the formation of the glycolytic intermediate 2,3 biphosphoglycerate (2,3-BPG). This metabolite is formed by biphosphoglycerate mutase and results in the lower oxygen affinity of hemoglobin. The activity of mutase in erythrocytes increases after exposure to hypoxia. Its levels of activity are regulated by transglutaminase, which stabilizes mutase [
65]. In addition, the formation of 2,3-BPG is favored when glycolysis is upregulated. In red blood cells under hypoxia, this is mediated by adenosine receptor 2b-mediated sphingosine kinase 1 activity, and the subsequent sphingosine-1-phosphate (S1P)-induced glycolytic enzyme is release from the plasma membrane to the cytoplasm [
66]. In addition, erythrocyte adenosine receptor 2b activation during hypoxia also results in adenosine monophosphate-dependent kinase (AMPK) activation and AMPK-dependent BPG mutase [
67]. Furthermore, S1P increase also enhances AMPK activation and subsequent BPG mutase elevated activity by downregulating the activity of the pp2a phosphatase, which negatively controls AMPK [
68]. Increased levels of erythrocyte 2,3-BPG have been shown to protect from ischemia [
69], while the beneficial effects of remote ischemic conditioning are attributed to increased erythrocyte 2,3-BPG [
69].
However, obesity and aging have been associated with reduced 2,3-BPG levels [
70] and BPG mutase activity [
66], respectively (
Figure 1). Animals lacking erythrocyte adenosine receptor a2b, and consequently, BPG mutase activity, present markers of brain inflammation.
Figure 1. Erythrocytes trapped in the ischemic tissue can respond to limited oxygen through the regulation of the 2,3-bisphosphoglycerate. However, aging and obesity halter this pathway. The end result can be increased brain inflammation. (A normal arrow signifies activation. An arrow with a vertical line signifies inhibition). 2,3 BPG: 2,3 biphosphoglycerate; A2B: adenosine receptor 2B; AMPK; adenosine monophosphate-activated kinase, BPG mutase: biphosphoglycerate mutase; CER: ceramide; ERK1/2: extracellular signal-regulated kinase; HB-O2: oxygenated hemoglobin; S1P: sphingosine-1 phosphate; SK1: sphingosine kinase 1.
2. Red blood Cells Respond to Damage-Associated Molecular Patterns Released after Ischemic Stroke
Ischemia and sustained oxygen deprivation of the brain can lead to the release of damage-associated molecular patterns (DAMPs), such as ATP, extracellular histones, amyloids, and cell-free mitochondrial DNA. DAMPs can act on microglial cells and infiltrate immune cells. This event regulates both brain inflammation and tissue repair [
71]. Apart from DAMPs, pathogen-associated molecular patterns (PAMPs) are also implicated in the progression of stroke; lipopolysaccharide (LPS), a component of Gram-negative bacteria, is associated with poor prognosis in stroke [
72]. Apart from immune cells, DAMPs can also act on red blood cells (
Table 1). Erythrocytes express various protein receptors that are capable not only of scavenging but also sensing DAMPs and PAMPs. In addition, as analyzed below, several DAMPs and PAMPs trigger physicochemical alterations on red blood cells. The net effect of DAMP-induced erythrocyte dysfunction is mainly determined by the effect of erythrophagocytosis on macrophage polarization. While in the liver erythrolysis prior to erythrophagocytosis results in an anti-inflammatory phenotype of macrophages [
73], in the brain, this mechanism induces inflammation [
74]. We speculate that the relative quantities of CD47, oxidized CD47, exposed phosphatidylserine, and the level of ROS determine the ratio of erythrolysis: intact erythrophagocytosis.
Table 1. Summary of the mechanisms underlying the response of red blood cells to pathogen- and damage-associated molecular patterns.
| Damp/Pamp |
Receptor |
Effect |
| mtDNA |
TLR9 |
|
| CpG DNA |
TLR9 |
|
| ATP |
P2X7 |
|
| Extracellular histones |
TLR2? |
-
Phosphatidylserine exposure
-
Increased erythrocyte aggression and fragility
-
Calcium influx
-
Generation of reactive oxygen species
-
Activation of caspase-3
-
Hemoglobin release
-
Generation of microvesicles with externalized phosphatidylserine
|
| LPS |
- |
|
| Amyloids |
- |
-
Sphingomyelin hydrolysis
-
Phosphatidylserine exposure
-
Downregulates the activity of several glycolytic enzymes
-
Upregulates the Na+/K+ ATPase activity
-
Activation of adenylic cyclase
-
Activation of caspase-3
-
Inhibition of ATP release
-
Influences erythrocyte morphology
|
2.1. Cell-Free Mitochondrial DNA and CpG DNA
Hotz et al. [
75] were the first to report that human erythrocytes expressed the toll-like receptor 9 (TLR9) on their plasma membranes. In their study, they showed that TLR9 on erythrocyte membranes bound cell-free mitochondrial DNA (mtDNA). In fact, they reported that when the circulating levels of mtDNA were low, most TLR9s of erythrocytes had bound mtDNA. However, an increase in the circulating levels of mtDNA resulted in the saturation of the scavenging capacity of red blood cells. In addition, the loss of TLR9 on red blood cells led to increased lung injury, while the administration of red blood cells from healthy donors attenuated the CpG DNA-induced inflammation.
Subsequent reports from the same research group provided important clues for the effects of cell-free mitochondrial DNA and CpG DNA, in general, on erythrocytes. First, red blood cells from septic patients contained increased TLR9 and bound CpG DNA levels in comparison to healthy controls [
76]. Furthermore, CpG DNA was found to induce morphological changes on erythrocytes in a TLR9-dependent manner. Specifically, a redistribution of BAND3 was observed, as well as a conformational change and the loss of the “do not eat me” signaling protein CD47. It was also remarkable that CpG DNA binding to TLR9 brought about a marked increase in the levels of TLR9 on the surface of erythrocytes. Finally, it was shown that CpG DNA binding to TLR9 augmented erythrophagocytosis and inflammation in vivo.
These results provide clear evidence that red blood cells respond to CpG DNA, both as a DAMP and a PAMP.
2.2. ATP
Parker and Snow [
77] first showed that canine red blood cells expressed the P2X7 receptor, and its activation by ATP led to an increased cation flux. This effect was not seen when erythrocytes were incubated with other adenine nucleotides. It is notable that the P2X7 receptor is also expressed on the membranes of human erythrocytes [
78], and its activation by ATP increases cation flux. More importantly, the activation of the P2X7 receptor of human red blood cells leads to phosphatidylserine exposure [
79]. However, this effect was much more prominent in canine erythrocytes, perhaps due to the higher levels of P2X7 receptors [
79] expressed therein. The ATP-induced phosphatidylserine exposure of canine erythrocytes was also confirmed by Faulks et al. [
80], excluding other nucleotides as triggers for this effect. Another important finding came from the study of Sophocleous et al. [
81], who reported that the P2X7 activation-induced phosphatidylserine exposure of canine red blood cells was not altered during cellular aging. This may indicate that P2X7 is not expelled from the membrane through vesiculation.
2.3. Extracellular Histones
In 2014, Semerano et al. [
82] reported that extracellular histones induced phosphatidylserine exposure on human erythrocytes. This effect was mainly mediated by histone 4 (H4) and led to the increased activation of thrombin. Later, another research group found that extracellular histones triggered anemia and increased erythrocyte fragility and tendency to aggregate [
83]. The results of Semerano et al. were confirmed by Yeung et al. [
84], who reported histone-induced phosphatidylserine exposure on red blood cells. They found that extracellular histones induced calcium influx, generation of reactive oxygen species, and activation of caspase-3. Remarkably, these effects were attenuated with the pre-treatment of red blood cells with a neutralizing antibody for TLR2. Other investigators also found that all histones could bind to erythrocytes, albeit to different extents [
85]. All the extracellular histones induced hemoglobin release and generation of microvesicles with externalized phosphatidylserine. These results could be mediated by the opening or formation of ion pores by histones [
85].
2.4. Lipopolysaccharides (LPS)
Lipopolysaccharides can induce dramatic changes in the conformation of the erythrocyte membrane proteins. This effect is mainly attributed to the hydrophobic interactions of LPS with lipids and proteins, which can subsequently influence the protein–lipid interaction, thus disrupting the red blood cell cytoskeleton and membrane stability [
86]. These results were recapitulated in later studies, where LPS was found to increase erythrocyte osmotic fragility and decrease lipid fluidity [
87]. More recently, Brauckmann et al. [
88] reported that LPS could provoke hemolysis with direct interaction with washed red blood cells. Hence, these studies support the idea of LPS-induced physicochemical changes on erythrocytes, which could result in hemolysis.
2.5. Amyloids
First, Nicolay et al. [
89] showed that the incubation of human red blood cells with amyloid beta (1–42) induced sphingomyelin hydrolysis and phosphatidylserine exposure. This effect was amplified by the depletion of intracellular Cl
−. Other researchers reported that amyloid Aβ (25–35) downregulated the activity of several glycolytic enzymes and upregulated the Na
+/K
+ ATPase activity of rat erythrocytes in a cellular age-dependent manner [
90]. The latter results possibly hold for human red blood cells too. Aβ amyloid can uncouple the erythrocyte metabolism from oxygen saturation [
91], perhaps through the trimeric G protein activation-induced activation of adenylic cyclase and caspase 3 [
92]. This pathway also results in the inhibition of ATP release from red blood cells [
92]. Further studies identified protein kinase C and nitric oxide as important constituents of this pathway [
93,
94]. Apart from the regulation of metabolism, this pathway also influences erythrocyte morphology [
95].
3. Red Blood Cells Could Connect Non-Alcoholic Fatty Liver Disease with the Risk of Atherothrombotic Strokes
NAFLD severity is associated with an increased risk of stroke [
95]. Interestingly, the red blood cells of NAFLD patients present characteristics that are involved in the mechanisms of atherothrombosis. Our group has recently shown that erythrocytes obtained from NAFLD patients exhibited increased membrane cholesterol, sphingosine, bound chemokine MCP1 [
96], reduced levels of sphingomyelin [
97], and CD47 [
96], while also increasing the release of MCP1 [
98] and the sustained release of sphingosine-1 phosphate and lysophosphatidic acid [
98]. Previously, other researchers showed that erythrocytes of NAFLD patients and animal models also exhibited increased phosphatidylserine exposure and reactive oxygen species [
99]. Thus, we speculate that erythrocytes of NAFLD patients could partially expose patients to a greater risk for the development of atherothrombotic stroke.
This entry is adapted from the peer-reviewed paper 10.3390/neurolint15010011