Cancer immunotherapy is a type of cancer treatment that uses the immune system to fight cancer cells. Some of these treatments stimulate the immune system, while others prime the immune system to identify better and target cancer cells. In parallel with the implementation of cancer immunotherapy, therapy-specific FDG PET/CT response criteria were explicitly designed specifically for that purpose. FDG PET/CT plays a key role in the newly developed response criteria, and several FDG PET/CT-based criteria have been proposed to address all patterns of response to therapy, including indeterminate response, pseudoprogression, and hyperprogression using several metrics, such as SUV, MTV, and TLG. This review aims to discuss the effects and side effects of cancer immunotherapy and to correlate this with the proposed criteria and relevant patterns of FDG PET/CT in lymphoma immunotherapy as applicable. Additionally, the latest updates and future prospects will be explored.
- immunotherapy
- immunotherapy in lymphoma
- FDG PET/CT
- metabolic PET parameters
- lymphoma immunotherapy response criteria
- immuno-PET
1. Introduction
2. Histrory of Cancer Immunotherapy: Classification, Previous and Current Facts
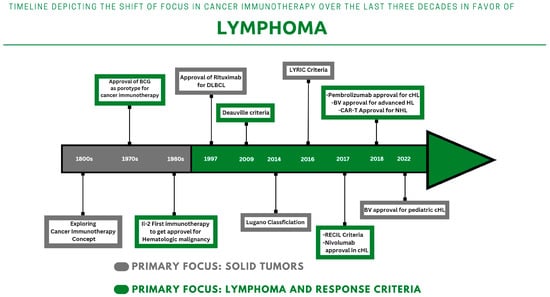
2.1. Previous Footprints
2.2. Monoclonal Antibodies: From Rituximab to Immune Checkpoint Inhibitors
| Drug Name | Class | Main Action | Treatment Protocol | Approved for |
|---|---|---|---|---|
| Rituximab | mAb 1 | CD-20 Antibody | With chemotherapy | First line for NHL 2 |
| Brentuximab Vedotin | mAb 1 | CD-30 Antibody | With chemotherapy | Advanced HL 3 |
| Nivolumab | ICI 4 | PD-1 Blockade | Standalone | cHL 5 |
| Pembrolizumab | ICI 4 | PD-1 Blockade | Standalone | Refractory cHL 5 |
| Tisagenlecleuce | CAR-T 6 | T-lymphocyte-mediated CD-19 expression | Standalone | Adult R/R DLBCL 7 |
| Lisocabtagenel maraleuecel |
CAR-T 6 | T-lymphocyte-mediated CD-19 expression | Standalone | R/R large B-cell lymphoma |
| Mosunetuzumab | BiTes 7 | Follicular Lymphoma | Standalone | R/R Follicular Lymphoma |
2.3. Adoptive Cell Therapy
2.4. Bispecific Antibodies: The Most Recent Addition to the Group
3. Current Applications of PET/CT in Lymphoma Immunotherapy
3.1. Response Patterns
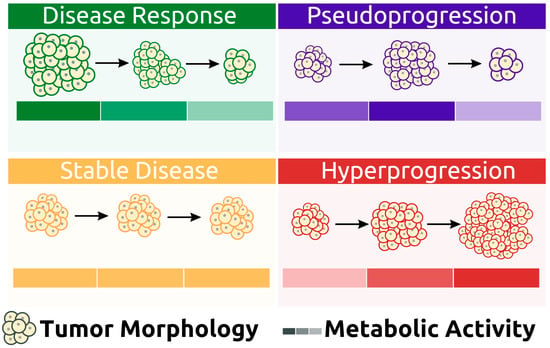
3.1.1. Pseudoprogression
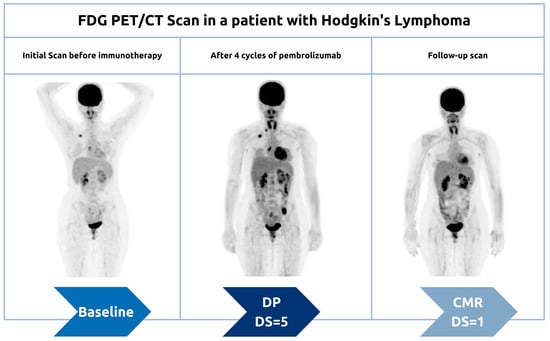
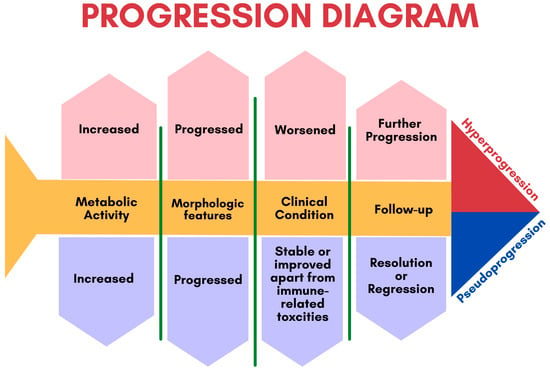
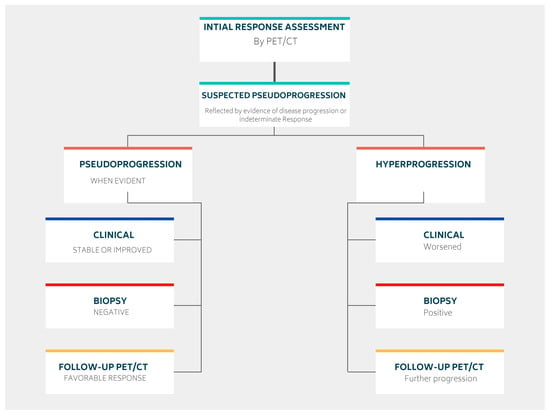
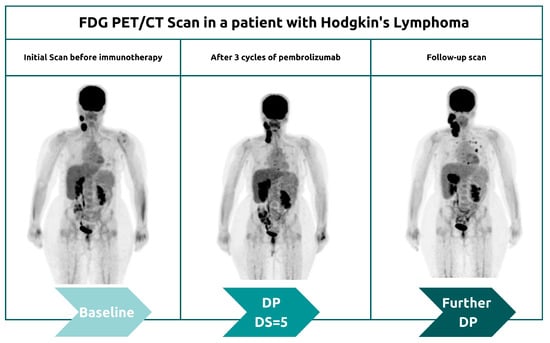
3.1.2. Hyperprogression
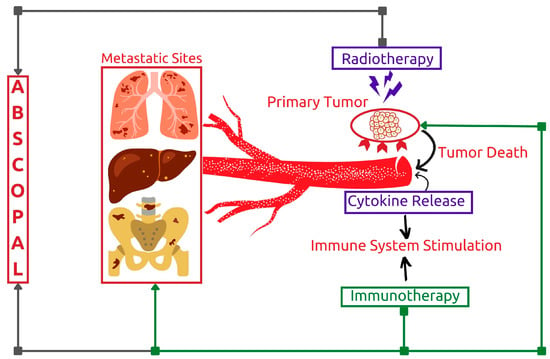
3.1.3. Potentiating Abscopal Effect
3.2. PET Response Criteria in Lymphoma
3.2.1. Lugano Classification
| Deauville 5-Point Scale (5PS) | |
| DS *1 | No uptake |
| DS2 | Uptake ≤ mediastinum |
| DS3 | Uptake > mediastinum but ≤ liver |
| DS4 | Uptake moderately higher than liver |
| DS5 | Uptake markedly higher than liver and/or new lesions + |
3.2.2. Lymphoma Response to Immunomodulatory Therapy Criteria (LYRIC)
3.2.3. Response Evaluation Criteria in Lymphoma (RECIL)
4. Influence of FDG PET/CT in Hodgkin’s Lymphoma (HL)
4.1. Immune Checkpoint Inhibitors
4.2. Brentuximab Vedotin (BV)
4.3. Chimeric Antigen Receptor Therapy (CAR-T)
5. Influence of FDG PET/CT in Non-Hodgkin’s Lymphoma (NHL)
Similarly, FDG PET/CT is of vital importance for outcome prediction and prognostication [109]. The only difference in NHL is that there are certain histologic subtypes that do not optimally express FDG avidity [109]. Indolent NHL fall under the category of variable FDG avid NHL while aggressive NHL usually have moderate to high FDG avidity (Table 3). Therefore, incorporation of FDG PET/CT is most acknowledged in response assessment of aggressive NHL.
Table 3. Status and degree of FDG avidity in each type and subtype of Lymphoma.
|
Category |
Subtype of Lymphoma |
FDG Avidity |
Degree of FDG Avidity |
|
HL 1 |
Classical |
Avid |
High |
|
Mixed cellularity |
Avid |
Moderate to high |
|
|
Lymphocyte depletion |
Avid |
Moderate to high |
|
|
Lymphocyte predominance |
Avid |
Moderate |
|
|
Aggressive NHL 2 |
Diffuse large B-cell |
Avid |
High |
|
Burkitt |
Avid |
High |
|
|
Anaplastic Large cell |
Avid |
High |
|
|
Mantle Cell |
Avid |
Moderate |
|
|
Indolent NHL 2 |
Follicular |
Variable |
Low-high |
|
Lymphoplasmacytic |
Variable |
Low-high |
|
|
Marginal zone |
Variable |
None-high |
|
|
Small lymphocytic |
Variable |
None-high |
|
|
Cutaneous Anaplastic |
Variable |
None-moderate |
1 HL: Hodgkin’s Lymphoma; 2 NHL: Non-Hodgkin’s Lymphoma.
5.1. FDG PET/CT in Diffuse Large B-Cell (DLBCL)
5.1.1. Rituximab
Since the approval of FDG PET/CT by the FDA, a number of studies have been conducted to explore the efficacy of this treatment modality. Haioun et al. were among the first to examine the prognostic and predictive value of early FDG PET/CT imaging [110]. In their study, 41% of all 90 patients received rituximab as part of the therapy protocol [110]. These patients were then followed up to determine the prognostic outcome [110]. It was concluded that patients with negative PET results had much more favorable outcomes, reflected by PFS and OS rates of 82% and 90%, respectively, as compared to 43% and 61% for those with positive PET results [110]. More recently, a group of DLBCL patients treated with R-CHOP and evaluated by FDG PET/CT at the interim stage were prospectively enrolled in a study [111]. The calculated 3-year PFS and OS rates in iPET negative patients achieved statistically significant superiority when compared to positive results [111]. Data from these studies along with others were collected to conduct a meta-analysis [112]. This meta-analysis was interested in examining the predictive role of iPET in DLBCL patients treated with R-CHOP [112]. The overall sensitivity and specificity of iPET were observed to be discouraging, justifying the need for more effort to unify response criteria [113]. Recently, a group of GOYA DLBCL patients were analyzed for data following the first line of immunochemotherapy to determine survival outcome [113]. It was found that EoT PET is an independent predictor of both PFS and OS and a promising prognostic marker for such patients [113]. The results from the previous analysis necessitate a meta-analytic study in the near future to support this evidence. Another GOYA group analysis was carried out to observe PFS rates difference between DLBCL patients receiving R-CHOP vs Obinutuzumab plus CHOP (G-CHOP) [114]. The study was unable to demonstrate any PFS benefit of G-CHOP over R-CHOP in previously untreated patients with DLBCL [114]. As of right now, metabolic PET parameters are being extensively studied in an attempt to outline their prognostic values [115][116][117]. The GOYA group of patients was analyzed using metabolic PET parameters. Tumor MTV was found to be a predictor of therapy failure in these patients. A recent study concerning baseline PET parameters in R-CHOP treated DLBCL patients was conducted [115]. Metabolic PET parameters were used including SUVmax, SUVmean, MTV and TLG [115]. The study suggests that these parameters may have a prognostic value at baseline and interim intervals [115]. In another study, tumor MTV values were found to be the most reliable parameters among all to determine survival outcome [116]. This was recently shown by another study that confirmed the predictive value of baseline and interim MTV on survival outcome [117]. It appears obvious by now that metabolic PET parameters can establish solid background for future response-adapted management approaches in NHL patients.
5.1.2. Immune Checkpoint Inhibitors (ICI)
Unlike HL, the results achieved in the previous literature using ICI in NHL are less encouraging. Despite having high safety profile, ICI failed to achieve optimal efficacy in NHL. The safety and efficacy of nivolumab in DLBCL were assessed in the previous single-arm phase II study by Ansell et al. [118]. The study acknowledged suboptimal ORR despite the highly observed safety profile [118]. On the other hand, Results from clinical trials of ICI combined with other immunochemotherapies appears more promising. Pembrolizumab was explored as a treatment for DLBCL in a study of 30 patients [119]. This study found that the combination of pembrolizumab and R-CHOP resulted in an ORR of 90%, a CR of 77%, and a 2-year PFS of 83% [119]. The findings of this trial indicate that combining the PD-L1 inhibitor atezolizumab with chemotherapy may be a promising treatment option for DLBCL. The combination of atezolizumab and R-CHOP (a type of chemotherapy) resulted in a high efficacy, with an ORR of 87.5% and durable responses in 80% of patients at 24 months [120]. Based on previous research, it appears that combining immunotherapy with chemotherapy is more likely to result in favorable outcomes in terms of response and clinical outcome.
5.1.3. Chimeric Antigen Receptor Therapy (CAR-T)
A few studies have examined the value of FDG PET/CT in CAR-T, with mixed results. Shah et al. were among the first to examine MTV in a small group of NHL patients and found that nearly half the patients had non-measurable MTV values at M1 (Table S3) [121]. These patients showed long-term remission over the following 2 years [121]. The other half presented with measurable MTV and witnessed an early relapse [121]. Cohen et al. have approached the issue differently and include both DS and ∆SUVmax for evaluation [122]. It was concluded that SUVmax prior to therapy may help determine treatment eligibility and that DS and ∆SUVmax can help identify treatment failure [122]. Recently, Galtier et al. conducted a multicentric cohort study which highlighted the high predictive values of both the 5 PS and MTV [123]. This was also previously explored by Kuhnl et al., who found that Deauville criteria may predict the risk for CAR-T failure and help direct post-CAR-T management [124]. Breen et al. have conducted more detailed analysis of SUVmax values at M1 and found that higher SUVmax values indicate higher risk for disease progression [125]. SUVmax above 10 at M1 is regarded as a significant prognostic and predictive indicator in patients with stable disease or partial response [125]. This was later confirmed by Al Zaki et al. [126].In NHL, tumor burden was validated through the use of FDG PET/CT in a retrospective study by Wang et al. [127].In fact, having high tumor burden at baseline was linked to more aggressive cytokine release syndrome [127]. Bailly et al. enrolled a group of R/R NHL patients in order to demonstrate the added value of adequate disease control prior to therapy [128]. Among all 40 patients, 33 cases were adequately managed prior to CAR-T [128]. During TT PET/CT, adequately treated patients showed more favorable outcomes in terms of event free survival when compared to others [128]. Moreover, 5 of the remainder 7 patients have witnessed early disease relapse [128]. Therefore, adequate control prior to CAR-T was linked to more favorable response in such cases. Despite the encouraging outcomes of previous studies, more research is needed with larger cohorts to get a complete picture.
5.2. FDG PET/CT in Follicular Lymphoma (FL)
Follicular lymphoma (FL) is one of the most common types of lymphoma, representing 22% of adult non-Hodgkin’s lymphomas (NHL) worldwide [129]. The disease can present with a variable clinical course, usually indolent and slow growing, while in other cases the disease may become aggressive, often characterized by histological transformation into a high-grade lymphoma (25–60%) and early death [130]. FL belongs to a group of neoplasms usually presenting with a variable FDG avidity. Therefore, permitting an overall good diagnostic accuracy using FDG PET/CT, up to 98% [131]. Although the outlook for patients with FL has improved in recent years, with a median survival that can exceed 20 years, FL is still considered incurable [132]. The main goal of treatment is usually disease control and extending patients' life expectancy [133].
5.2.1. Rituximab
In FL, the combination of rituximab and chemotherapy has been shown to improve outcomes for patients with FL. However, 20% of patients treated with this immunochemotherapy still experience disease progression within a short time frame, and 50% of them will witness death within 5 years [134]. FDG PET/CT have quickly replaced CI through the use of metabolic PET parameters. Providing more reliable indices for therapy response and outcome. In result, staging, therapy response and surveillance became more accurate. In 2011, a study by Trotman et al. was the first to provide large-scale evidence that EoT PET/CT after Immunochemotherapy treatment is a strong and independent predictor of PFS in FL [135]. This study included 160 patients from the prospective Primary Rituximab and Maintenance (PRIMA) study group [135]. Disease progression and death was significantly higher in PET-positive patients (70.7% at 42 months) compared to PET-negative patients [135]. The study also showed that the predictive value of the FDG PET/CT is independent of the state of the response by CI [135]. In result, FDG PET/CT can function as metabolic biomarker to viable disease process. Dupuis et al. have also examined prognostic role of FDG PET/CT at both interim and EoT periods [136]. This study included a total of 121 FL patients with median follow-up of 23 months. Among all patients, 116 cases have received at least 4 cycles of R-CHOP and had FDG PET/CT for response assessment [136]. iPET negative patients were found to have more favorable PFS, both at interim and EoT periods [136]. The 2-year PFS rates were 87% for EoT PET-negative patients compared to 51% for EoT PET positive patients (P .001). At interim period, 2-year PFS was 86% for iPET-negative patients compared to 61% for iPET-positive patients, respectively (P =.0046). Final PET results revealed a significant difference in two-year overall survival as well: 100% versus 88% (P =.0128) [136]. The results of the previous two studies were explore that vital aspect utilizing FDG PET/CT for response. A retrospective analysis of FOLL05 trial group was carried out by Luminari et al. [137]. The study found that patients who had negative PET scans at EoT had significantly 3-year PFS rates [137]. This suggests that PET scans can be useful in assessing response to treatment in patients with FL[137].
To more accurately understand the relationship between FDG PET/CT and survival analysis, Trotman et al. have carried out recent multicentric study [138]. The study was a product of a joint analysis from three prospective studies (PRIMA, PET-FOLLICULAIRE, and FOLL05) [138]. All patient presented with a high tumor burden and were treated with first-line immunochemotherapy [138]. The study found that the EoT PET predicted both PFS and OS [138]. A negative EoT PET was associated with a significantly higher PFS and OS at four years than a positive one [138]. This suggests that the FDG PET/CT at the EoT predicts survival, so a negative study may be a good prognostic indicator for FL patients with high tumor burden. In 2018, a study assessed the prognostic value of EoT PET on a much larger scale, using data from the prospective GALLIUM study [139]. The study compared FDG PET/CT with contrast enhanced CT (CeCT) to determine which one is better for assessing therapy response [139]. Out of all 1202 patients who were enrolled in the study previously, only 595 patients had performed both modalities [139]. All patients were given immunochemotherapy as their first line of treatment and were assessed after finishing therapy [139]. It was found that PET was superior to contrast-enhanced CT for response assessment in FL patients at EoT [139]. More recently, FOLL12 prospective, randomized, open-label multicenter phase III trial was conducted [140]. The aim of this study was to compare a 2-year Rituximab maintenance therapy against a response-adapted therapy approach in FL patients [140]. Response adapted therapy protocol was found to be associated with lower PFS at 2-year interval. It is clear from previous evidence that EoT PET scans can provide accurate predictions of both PFS and OS [140].
5.2.2. Chimeric Antigen Receptor Therapy (CAR-T)
The recent approval of axicabtagene ciloleucel for r/r FL was granted after observed results from ZUMA-5 study, which demonstrated an 80% CRR and a 12-month durable response rate of 72% [141]. This offers an effective treatment option for patients who develop refractory disease [142]. A few studies have examined the role of FDG PET/CT in CAR-T for FL patients [136][137][138][139]. These were already mentioned in DLBCL section (CAR-T subheading) as previous studies have pooled aggressive NHL patients together regardless of subtype.
5.2.3. Bispecific Antibodies
More recently, the drug Mosunetuzumab has been approved for the treatment of r/r FL. A recent multicentric phase 2 study has confirmed the efficacy and safety profile of Mosunetuzumab [142]. This is the first in-class approval of a bispecific antibody targeting CD20 and CD3. The activity in FL patients is excellent, with an ORR of approximately 80% and a CR of approximately 60% [142]. However, more studies and research are needed to determine the predictive and prognostic role of FDG PET/CT. Additionally, trials are still ongoing to examine other drugs of the same class.
This entry is adapted from the peer-reviewed paper 10.3390/cancers15041063
References
- Borghaei, H.; Smith, M.R.; Campbell, K.S. Immunotherapy of Cancer. Eur. J. Pharmacol. 2009, 625, 41–54.
- Subramaniam, D.S.; Liu, S.V.; Giaccone, G. Novel Approaches in Cancer Immunotherapy. Discov. Med. 2016, 21, 267–274.
- Choi, B.K.; Kim, S.-H.; Kim, Y.H.; Kwon, B.S. Cancer Immunotherapy Using Tumor Antigen-Reactive T Cells. Immunotherapy 2018, 10, 235–245.
- Oiseth, S.J.; Aziz, M.S. Cancer Immunotherapy: A Brief Review of the History, Possibilities, and Challenges Ahead. J. Cancer Metastasis Treat. 2017, 3, 250.
- Zhang, H.; Chen, J. Current Status and Future Directions of Cancer Immunotherapy. J. Cancer 2018, 9, 1773–1781.
- Unterrainer, M.; Ruzicka, M.; Fabritius, M.P.; Mittlmeier, L.M.; Winkelmann, M.; Rübenthaler, J.; Brendel, M.; Subklewe, M.; von Bergwelt-Baildon, M.; Ricke, J.; et al. PET/CT Imaging for Tumour Response Assessment to Immunotherapy: Current Status and Future Directions. Eur. Radiol. Exp. 2020, 4, 63.
- Decker, W.K.; da Silva, R.F.; Sanabria, M.H.; Angelo, L.S.; Guimarães, F.; Burt, B.M.; Kheradmand, F.; Paust, S. Cancer Immunotherapy: Historical Perspective of a Clinical Revolution and Emerging Preclinical Animal Models. Front. Immunol. 2017, 8, 829.
- Dobosz, P.; Dzieciątkowski, T. The Intriguing History of Cancer Immunotherapy. Front. Immunol. 2019, 10, 2965.
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in Clinical Cancer Immunotherapy. Br. J. Cancer 2019, 120, 6–15.
- Fyfe, G.; Fisher, R.I.; Rosenberg, S.A.; Sznol, M.; Parkinson, D.R.; Louie, A.C. Results of Treatment of 255 Patients with Metastatic Renal Cell Carcinoma Who Received High-Dose Recombinant Interleukin-2 Therapy. J. Clin. Oncol. 1995, 13, 688–696.
- Atkins, M.B.; Lotze, M.T.; Dutcher, J.P.; Fisher, R.I.; Weiss, G.; Margolin, K.; Abrams, J.; Sznol, M.; Parkinson, D.; Hawkins, M.; et al. High-Dose Recombinant Interleukin 2 Therapy for Patients with Metastatic Melanoma: Analysis of 270 Patients Treated between 1985 and 1993. J. Clin. Oncol. 1999, 17, 2105–2116.
- de Gruijl, T.D.; Janssen, A.B.; van Beusechem, V.W. Arming Oncolytic Viruses to Leverage Antitumor Immunity. Expert Opin. Biol. Ther. 2015, 15, 959–971.
- Mondal, M.; Guo, J.; He, P.; Zhou, D. Recent Advances of Oncolytic Virus in Cancer Therapy. Hum. Vaccin. Immunother. 2020, 16, 2389–2402.
- Menotti, L.; Avitabile, E. Herpes Simplex Virus Oncolytic Immunovirotherapy: The Blossoming Branch of Multimodal Therapy. Int. J. Mol. Sci. 2020, 21, 8310.
- Grillo-López, A.J.; White, C.A.; Varns, C.; Shen, D.; Wei, A.; McClure, A.; Dallaire, B.K. Overview of the Clinical Development of Rituximab: First Monoclonal Antibody Approved for the Treatment of Lymphoma. Semin. Oncol. 1999, 26, 66–73.
- Pfreundschuh, M.; Schubert, J.; Ziepert, M.; Schmits, R.; Mohren, M.; Lengfelder, E.; Reiser, M.; Nickenig, C.; Clemens, M.; Peter, N.; et al. Six versus Eight Cycles of Bi-Weekly CHOP-14 with or without Rituximab in Elderly Patients with Aggressive CD20+ B-Cell Lymphomas: A Randomised Controlled Trial (RICOVER-60). Lancet Oncol. 2008, 9, 105–116.
- Pfreundschuh, M.; Trümper, L.; Osterborg, A.; Pettengell, R.; Trneny, M.; Imrie, K.; Ma, D.; Gill, D.; Walewski, J.; Zinzani, P.-L.; et al. CHOP-like Chemotherapy plus Rituximab versus CHOP-like Chemotherapy Alone in Young Patients with Good-Prognosis Diffuse Large-B-Cell Lymphoma: A Randomised Controlled Trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006, 7, 379–391.
- Habermann, T.M.; Weller, E.A.; Morrison, V.A.; Gascoyne, R.D.; Cassileth, P.A.; Cohn, J.B.; Dakhil, S.R.; Woda, B.; Fisher, R.I.; Peterson, B.A.; et al. Rituximab-CHOP versus CHOP Alone or with Maintenance Rituximab in Older Patients with Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2006, 24, 3121–3127.
- Coiffier, B.; Lepage, E.; Brière, J.; Herbrecht, R.; Tilly, H.; Bouabdallah, R.; Morel, P.; van den Neste, E.; Salles, G.; Gaulard, P.; et al. CHOP Chemotherapy plus Rituximab Compared with CHOP Alone in Elderly Patients with Diffuse Large-B-Cell Lymphoma. N. Engl. J. Med. 2002, 346, 235–242.
- Schulz, H.; Bohlius, J.; Skoetz, N.; Trelle, S.; Kober, T.; Reiser, M.; Dreyling, M.; Herold, M.; Schwarzer, G.; Hallek, M.; et al. Chemotherapy plus Rituximab versus Chemotherapy Alone for B-Cell Non-Hodgkin’s Lymphoma. Cochrane Database Syst. Rev. 2007, 2010, CD003805.
- Isaacs, A.; Lindenmann, J. Virus Interference. I. The Interferon. Proc. R. Soc. Lond. B Biol. Sci. 1957, 147, 258–267.
- Klebanoff, C.A.; Finkelstein, S.E.; Surman, D.R.; Lichtman, M.K.; Gattinoni, L.; Theoret, M.R.; Grewal, N.; Spiess, P.J.; Antony, P.A.; Palmer, D.C.; et al. IL-15 Enhances the in Vivo Antitumor Activity of Tumor-Reactive CD8+ T Cells. Proc. Natl. Acad. Sci. USA 2004, 101, 1969–1974.
- Solal-Celigny, P.; Lepage, E.; Brousse, N.; Reyes, F.; Haioun, C.; Leporrier, M.; Peuchmaur, M.; Bosly, A.; Parlier, Y.; Brice, P. Recombinant Interferon Alfa-2b Combined with a Regimen Containing Doxorubicin in Patients with Advanced Follicular Lymphoma. Groupe d’Etude Des Lymphomes de l’Adulte. N. Engl. J. Med. 1993, 329, 1608–1614.
- Cheah, C.Y.; Fowler, N.H.; Neelapu, S.S. Targeting the Programmed Death-1/Programmed Death-Ligand 1 Axis in Lymphoma. Curr. Opin. Oncol. 2015, 27, 384–391.
- Menter, T.; Bodmer-Haecki, A.; Dirnhofer, S.; Tzankov, A. Evaluation of the Diagnostic and Prognostic Value of PDL1 Expression in Hodgkin and B-Cell Lymphomas. Hum. Pathol. 2016, 54, 17–24.
- Al-Ibraheem, A.; Mottaghy, F.M.; Juweid, M.E. PET/CT in Hodgkin Lymphoma: An Update. Semin. Nucl. Med. 2022.
- Lopci, E.; Meignan, M. Current Evidence on PET Response Assessment to Immunotherapy in Lymphomas. PET Clin. 2020, 15, 23–34.
- Hutchings, M.; Radford, J.; Ansell, S.M.; Illés, Á.; Sureda, A.; Connors, J.M.; Sýkorová, A.; Shibayama, H.; Abramson, J.S.; Chua, N.S.; et al. Brentuximab Vedotin plus Doxorubicin, Vinblastine, and Dacarbazine in Patients with Advanced-stage, Classical Hodgkin Lymphoma: A Prespecified Subgroup Analysis of High-risk Patients from the ECHELON-1 Study. Hematol. Oncol. 2021, 39, 185–195.
- Mohanty, R.; Chowdhury, C.; Arega, S.; Sen, P.; Ganguly, P.; Ganguly, N. CAR T Cell Therapy: A New Era for Cancer Treatment (Review). Oncol. Rep. 2019, 42, 2183–2195.
- Abate-Daga, D.; Davila, M.L. CAR Models: Next-Generation CAR Modifications for Enhanced T-Cell Function. Mol. Ther. Oncolytics 2016, 3, 16014.
- Zhang, X.; Zhu, L.; Zhang, H.; Chen, S.; Xiao, Y. CAR-T Cell Therapy in Hematological Malignancies: Current Opportunities and Challenges. Front. Immunol. 2022, 13, 927153.
- Frigault, M.J.; Maus, M. v State of the Art in CAR T Cell Therapy for CD19+ B Cell Malignancies. J. Clin. Investig. 2020, 130, 1586–1594.
- Wang, Z.; Cao, Y.J. Adoptive Cell Therapy Targeting Neoantigens: A Frontier for Cancer Research. Front. Immunol. 2020, 11, 176.
- Ruella, M.; Kenderian, S.S. Next-Generation Chimeric Antigen Receptor T-Cell Therapy: Going off the Shelf. BioDrugs 2017, 31, 473–481.
- Yip, A.; Webster, R.M. The Market for Chimeric Antigen Receptor T Cell Therapies. Nat. Rev. Drug. Discov. 2018, 17, 161–162.
- Majzner, R.G.; Mackall, C.L. Tumor Antigen Escape from CAR T-Cell Therapy. Cancer Discov. 2018, 8, 1219–1226.
- Cahill, K.E.; Smith, S.M. Follicular Lymphoma: A Focus on Current and Emerging Therapies. Oncology 2022, 36, 97–106.
- Prigent, K.; Aide, N. 18F-Fludeoxyglucose PET/Computed Tomography for Assessing Tumor Response to Immunotherapy and Detecting Immune-Related Side Effects: A Checklist for the PET Reader. PET Clin. 2020, 15, 1–10.
- Wolchok, J.D.; Hoos, A.; O’Day, S.; Weber, J.S.; Hamid, O.; Lebbé, C.; Maio, M.; Binder, M.; Bohnsack, O.; Nichol, G.; et al. Guidelines for the Evaluation of Immune Therapy Activity in Solid Tumors: Immune-Related Response Criteria. Clin. Cancer Res. 2009, 15, 7412–7420.
- Hodi, F.S.; Hwu, W.-J.; Kefford, R.; Weber, J.S.; Daud, A.; Hamid, O.; Patnaik, A.; Ribas, A.; Robert, C.; Gangadhar, T.C.; et al. Evaluation of Immune-Related Response Criteria and RECIST v1.1 in Patients with advanced melanoma treated with Pembrolizumab. J. Clin. Oncol. 2016, 34, 1510–1517.
- Nishino, M.; Giobbie-Hurder, A.; Gargano, M.; Suda, M.; Ramaiya, N.H.; Hodi, F.S. Developing a Common Language for Tumor Response to Immunotherapy: Immune-Related Response Criteria Using Unidimensional Measurements. Clin. Cancer Res. 2013, 19, 3936–3943.
- Armand, P.; Engert, A.; Younes, A.; Fanale, M.; Santoro, A.; Zinzani, P.L.; Timmerman, J.M.; Collins, G.P.; Ramchandren, R.; Cohen, J.B.; et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J. Clin. Oncol. 2018, 36, 1428–1439.
- Borcoman, E.; Nandikolla, A.; Long, G.; Goel, S.; le Tourneau, C. Patterns of Response and Progression to Immunotherapy. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 169–178.
- Wang, Q.; Gao, J.; Wu, X. Pseudoprogression and Hyperprogression after Checkpoint Blockade. Int. Immunopharmacol. 2018, 58, 125–135.
- Chae, Y.K.; Wang, S.; Nimeiri, H.; Kalyan, A.; Giles, F.J. Pseudoprogression in Microsatellite Instability-High Colorectal Cancer during Treatment with Combination T Cell Mediated Immunotherapy: A Case Report and Literature Review. Oncotarget 2017, 8, 57889–57897.
- Cheson, B.D.; Ansell, S.; Schwartz, L.; Gordon, L.I.; Advani, R.; Jacene, H.A.; Hoos, A.; Barrington, S.F.; Armand, P. Refinement of the Lugano Classification Lymphoma Response Criteria in the Era of Immunomodulatory Therapy. Blood 2016, 128, 2489–2496.
- Tanizaki, J.; Hayashi, H.; Kimura, M.; Tanaka, K.; Takeda, M.; Shimizu, S.; Ito, A.; Nakagawa, K. Report of Two Cases of Pseudoprogression in Patients with Non–Small Cell Lung Cancer Treated with Nivolumab—Including Histological Analysis of One Case after Tumor Regression. Lung Cancer 2016, 102, 44–48.
- McGehee, E.; Patel, H.; Pearson, C.; Clements, K.; Jaso, J.M.; Chen, W.; Callan, A.; Desai, N.; Ramakrishnan Geethakumari, P. Combined Immune Checkpoint Blockade and Radiotherapy Induces Durable Remission in Relapsed Natural Killer/T-Cell Lymphoma: A Case Report and Review of the Literature. J. Med. Case Rep. 2021, 15, 221.
- Kwong, Y.-L.; Chan, T.S.Y.; Tan, D.; Kim, S.J.; Poon, L.-M.; Mow, B.; Khong, P.-L.; Loong, F.; Au-Yeung, R.; Iqbal, J.; et al. PD1 Blockade with Pembrolizumab Is Highly Effective in Relapsed or Refractory NK/T-Cell Lymphoma Failing l-Asparaginase. Blood 2017, 129, 2437–2442.
- Saâda-Bouzid, E.; Defaucheux, C.; Karabajakian, A.; Coloma, V.P.; Servois, V.; Paoletti, X.; Even, C.; Fayette, J.; Guigay, J.; Loirat, D.; et al. Hyperprogression during Anti-PD-1/PD-L1 Therapy in Patients with Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma. Ann. Oncol. 2017, 28, 1605–1611.
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.; Marabelle, A.; Soria, J.-C.; et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin. Cancer Res. 2017, 23, 1920–1928.
- Onesti, C.E.; Frères, P.; Jerusalem, G. Atypical Patterns of Response to Immune Checkpoint Inhibitors: Interpreting Pseudoprogression and Hyperprogression in Decision Making for Patients’ Treatment. J. Thorac. Dis. 2019, 11, 35–38.
- Ferrara, R.; Mezquita, L.; Texier, M.; Lahmar, J.; Audigier-Valette, C.; Tessonnier, L.; Mazieres, J.; Zalcman, G.; Brosseau, S.; le Moulec, S.; et al. Hyperprogressive Disease in Patients with Advanced Non–Small Cell Lung Cancer Treated with PD-1/PD-L1 Inhibitors or with Single-Agent Chemotherapy. JAMA Oncol. 2018, 4, 1543.
- Mole, R.H. Whole Body Irradiation—Radiobiology or Medicine? Br. J. Radiol. 1953, 26, 234–241.
- Stone, H.B.; Peters, L.J.; Milas, L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. JNCI J. Natl. Cancer Inst. 1979, 63, 1229–1235.
- Kang, J.; Demaria, S.; Formenti, S. Current Clinical Trials Testing the Combination of Immunotherapy with Radiotherapy. J. Immunother. Cancer 2016, 4, 51.
- Golden, E.B.; Chhabra, A.; Chachoua, A.; Adams, S.; Donach, M.; Fenton-Kerimian, M.; Friedman, K.; Ponzo, F.; Babb, J.S.; Goldberg, J.; et al. Local Radiotherapy and Granulocyte-Macrophage Colony-Stimulating Factor to Generate Abscopal Responses in Patients with Metastatic Solid Tumours: A Proof-of-Principle Trial. Lancet Oncol. 2015, 16, 795–803.
- Lang, D.; Wahl, G.; Poier, N.; Graf, S.; Kiesl, D.; Lamprecht, B.; Gabriel, M. Impact of PET/CT for Assessing Response to Immunotherapy—A Clinical Perspective. J. Clin. Med. 2020, 9, 3483.
- Castello, A.; Grizzi, F.; Qehajaj, D.; Rahal, D.; Lutman, F.; Lopci, E. 18F-FDG PET/CT for Response Assessment in Hodgkin Lymphoma Undergoing Immunotherapy with Checkpoint Inhibitors. Leuk. Lymphoma 2019, 60, 367–375.
- Rossi, C.; Gilhodes, J.; Maerevoet, M.; Herbaux, C.; Morschhauser, F.; Brice, P.; Garciaz, S.; Borel, C.; Ysebaert, L.; Obéric, L.; et al. Efficacy of Chemotherapy or Chemo-Anti-PD-1 Combination after Failed Anti-PD-1 Therapy for Relapsed and Refractory Hodgkin Lymphoma: A Series from Lysa Centers. Am. J. Hematol. 2018, 93, 1042–1049.
- Dercle, L.; Seban, R.-D.; Lazarovici, J.; Schwartz, L.H.; Houot, R.; Ammari, S.; Danu, A.; Edeline, V.; Marabelle, A.; Ribrag, V.; et al. 18F-FDG PET and CT Scans Detect New Imaging Patterns of Response and Progression in Patients with Hodgkin Lymphoma Treated by Anti–Programmed Death 1 Immune Checkpoint Inhibitor. J. Nucl. Med. 2018, 59, 15–24.
- Younes, A.; Hilden, P.; Coiffier, B.; Hagenbeek, A.; Salles, G.; Wilson, W.; Seymour, J.F.; Kelly, K.; Gribben, J.; Pfreunschuh, M.; et al. International Working Group Consensus Response Evaluation Criteria in Lymphoma (RECIL 2017). Ann. Oncol. 2017, 28, 1436–1447.
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The Lugano Classification. J. Clin. Oncol. 2014, 32, 3059–3067.
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Müeller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of Imaging in the Staging and Response Assessment of Lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J. Clin. Oncol. 2014, 32, 3048–3058.
- Skusa, C.; Weber, M.-A.; Böttcher, S.; Thierfelder, K.M. Criteria-Based Imaging and Response Evaluation of Lymphoma 20 Years After Cheson: What Is New? RöFo—Fortschr. Geb. Röntgenstrahlen Bildgeb. Verfahr. 2020, 192, 657–668.
- Cheson, B.D. Rethinking Clinical Response and Outcome Assessment in a Biologic Age. Curr. Oncol. Rep. 2015, 17, 27.
- Waxman, E.S.; Lee Gerber, D. Pseudoprogression and Immunotherapy Phenomena. J. Adv. Pract. Oncol. 2020, 11, 723–731.
- Al-Ibraheem, A.; Anwer, F.; Juweid, M.E.; Shagera, Q.A.; Khalaf, A.N.; Obeidat, S.; Mansour, A.; Ma’koseh, M.; Halahleh, K.; Jaradat, I.; et al. Interim FDG-PET/CT for Therapy Monitoring and Prognostication in Hodgkin’s Lymphoma. Sci. Rep. 2022, 12, 17702.
- Zaucha, J.M.; Małkowski, B.; Chauvie, S.; Subocz, E.; Tajer, J.; Kulikowski, W.; Fijołek-Warszewska, A.; Biggi, A.; Fallanca, F.; Kobylecka, M.; et al. The Predictive Role of Interim PET after the First Chemotherapy Cycle and Sequential Evaluation of Response to ABVD in Hodgkin’s Lymphoma Patients—The Polish Lymphoma Research Group (PLRG) Observational Study. Ann. Oncol. 2017, 28, 3051–3057.
- Hutchings, M.; Kostakoglu, L.; Zaucha, J.M.; Malkowski, B.; Biggi, A.; Danielewicz, I.; Loft, A.; Specht, L.; Lamonica, D.; Czuczman, M.S.; et al. In Vivo Treatment Sensitivity Testing with Positron Emission Tomography/Computed Tomography After One Cycle of Chemotherapy for Hodgkin Lymphoma. J. Clin. Oncol. 2014, 32, 2705–2711.
- Biggi, A.; Gallamini, A.; Chauvie, S.; Hutchings, M.; Kostakoglu, L.; Gregianin, M.; Meignan, M.; Malkowski, B.; Hofman, M.S.; Barrington, S.F. International Validation Study for Interim PET in ABVD-Treated, Advanced-Stage Hodgkin Lymphoma: Interpretation Criteria and Concordance Rate Among Reviewers. J. Nucl. Med. 2013, 54, 683–690.
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 372, 311–319.
- Armand, P.; Shipp, M.A.; Ribrag, V.; Michot, J.-M.; Zinzani, P.L.; Kuruvilla, J.; Snyder, E.S.; Ricart, A.D.; Balakumaran, A.; Rose, S.; et al. Programmed Death-1 Blockade with Pembrolizumab in Patients with Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure. J. Clin. Oncol. 2016, 34, 3733–3739.
- Chen, R.; Zinzani, P.L.; Fanale, M.A.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J. Clin. Oncol. 2017, 35, 2125–2132.
- Younes, A.; Santoro, A.; Shipp, M.; Zinzani, P.L.; Timmerman, J.M.; Ansell, S.; Armand, P.; Fanale, M.; Ratanatharathorn, V.; Kuruvilla, J.; et al. Nivolumab for Classical Hodgkin’s Lymphoma after Failure of Both Autologous Stem-Cell Transplantation and Brentuximab Vedotin: A Multicentre, Multicohort, Single-Arm Phase 2 Trial. Lancet Oncol. 2016, 17, 1283–1294.
- Dercle, L.; Ammari, S.; Seban, R.-D.; Schwartz, L.H.; Houot, R.; Labaied, N.; Mokrane, F.-Z.; Lazarovici, J.; Danu, A.; Marabelle, A.; et al. Kinetics and Nadir of Responses to Immune Checkpoint Blockade by Anti-PD1 in Patients with Classical Hodgkin Lymphoma. Eur. J. Cancer 2018, 91, 136–144.
- Lepik, K.V.; Volkov, N.P.; Mikhailova, N.B.; Kondakova, E.V.; Tsvetkova, L.A.; Zalyalov, Y.R.; Lepik, E.E.; Fedorova, L.V.; Beinarovich, A.V.; Demchenkova, M.V.; et al. Long-Term Outcomes of Nivolumab Therapy in Patients with Relapsed/Refractory Classic Hodgkin’s Lymphoma after High-Dose Chemotherapy with Autologous Hematopoietic Stem Cell Transplantation in Real Clinical Practice. Klin. Onkogematol./Clin. Oncohematol. 2020, 13, 280–288.
- Fedorova, L.; Lepik, K.; Mikhailova, N.; Kondakova, E.; Kotselyabina, P.; Shmidt, D.I.; Kozlov, A.; Zalyalov, Y.; Borzenkova, E.; Baykov, V.; et al. 903P Combination of Nivolumab with Brentuximab Vedotin in Therapy of Relapsed and Refractory Hodgkin Lymphoma. Ann. Oncol. 2020, 31, S655.
- Parmar, K.; Dwarampudi, R.; Tijani, L.; Jakubski, S.; Rehman, S. Combination Therapy of Nivolumab in First Line and Relapsed/Refractory Classic Hodgkin’s Lymphoma: A Systematic Review and Meta-Analysis of Clinical Trials. Blood 2022, 140, 12031–12032.
- Bröckelmann, P.J.; Goergen, H.; Keller, U.; Meissner, J.; Ordemann, R.; Halbsguth, T.V.; Sasse, S.; Sökler, M.; Kerkhoff, A.; Mathas, S.; et al. Efficacy of Nivolumab and AVD in Early-Stage Unfavorable Classic Hodgkin Lymphoma. JAMA Oncol. 2020, 6, 872.
- Lee, H.; Flinn, I.W.; Melear, J.; Ramchandren, R.; Friedman, J.; Burke, J.M.; Linhares, Y.; Raval, M.; Chintapatla, R.; Feldman, T.A.; et al. P1089: Brentuximab Vedotin, Nivolumab, Doxorubicin, And Dacarbazine (AN+AD) for Advanced Stage Classic Hodgkin Lymphoma: Preliminary Safety and Efficacy Results from the Phase 2 Study (SGN35 027 Part B). Hemasphere 2022, 6, 979–980.
- Park, S.I.; Ansell, S.M.; Giri, S.; Svoboda, J.; Smith, S.D.; Feldman, T.; Budde, E.L.; Ness, A.J.; Choi, Y.; Bierman, P.J.; et al. Frontline PET-Directed Therapy with Brentuximab Vedotin Plus AVD Followed By Nivolumab Consolidation in Patients with Limited Stage Hodgkin Lymphoma. Blood 2022, 140, 1751–1752.
- Gibb, A.; Jones, C.; Bloor, A.; Kulkarni, S.; Illidge, T.; Linton, K.; Radford, J. Brentuximab Vedotin in Refractory CD30+ Lymphomas: A Bridge to Allogeneic Transplantation in Approximately One Quarter of Patients Treated on a Named Patient Programme at a Single UK Center. Haematologica 2013, 98, 611–614.
- Rothe, A.; Sasse, S.; Goergen, H.; Eichenauer, D.A.; Lohri, A.; Jäger, U.; Bangard, C.; Böll, B.; von Bergwelt Baildon, M.; Theurich, S.; et al. Brentuximab Vedotin for Relapsed or Refractory CD30+ Hematologic Malignancies: The German Hodgkin Study Group Experience. Blood 2012, 120, 1470–1472.
- Zinzani, P.L.; Viviani, S.; Anastasia, A.; Vitolo, U.; Luminari, S.; Zaja, F.; Corradini, P.; Spina, M.; Brusamolino, E.; Gianni, A.M.; et al. Brentuximab Vedotin in Relapsed/Refractory Hodgkin’s Lymphoma: The Italian Experience and Results of Its Use in Daily Clinical Practice Outside Clinical Trials. Haematologica 2013, 98, 1232–1236.
- Kahraman, D.; Theurich, S.; Rothe, A.; Kuhnert, G.; Sasse, S.; Scheid, C.; Dietlein, M.; Drzezga, A.; von Bergwelt-Baildon, M.; Kobe, C. 18-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography for Assessment of Response to Brentuximab Vedotin Treatment in Relapsed and Refractory Hodgkin Lymphoma. Leuk. Lymphoma 2014, 55, 811–816.
- Kedmi, M.; Khaustov, P.; Ribakovsy, E.; Benjamini, O.; Avigdor, A. Outcomes Related to FDG-PET-CT Response in Patients with Hodgkin Lymphoma Treated with Brentuximab-Vedotin at Relapse or Consolidation. Clin. Lymphoma Myeloma Leuk. 2021, 21, e929–e937.
- Connors, J.M.; Jurczak, W.; Straus, D.J.; Ansell, S.M.; Kim, W.S.; Gallamini, A.; Younes, A.; Alekseev, S.; Illés, Á.; Picardi, M.; et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 378, 331–344.
- Chen, R.W.; Ansell, S.M.; Gallamini, A.; Connors, J.M.; Savage, K.J.; Collins, G.P.; Grigg, A.; Sureda, A.M.; Ghosh, N.; Feldman, T.; et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin Lymphoma (HL): Impact of Cycle 2 PET Result on Modified Progression-Free Survival (MPFS). J. Clin. Oncol. 2018, 36, 7539.
- Ramchandren, R.; Advani, R.H.; Ansell, S.M.; Bartlett, N.L.; Chen, R.W.; Feldman, T.; Forero-Torres, A.; Friedberg, J.W.; Gopal, A.K.; Gordon, L.I.; et al. Brentuximab Vedotin (BV) plus Chemotherapy in Patients with Newly Diagnosed Advanced Stage Hodgkin Lymphoma (HL): North American Results. J. Clin. Oncol. 2018, 36, 7541.
- Eichenauer, D.A.; Plütschow, A.; Kreissl, S.; Sökler, M.; Hellmuth, J.C.; Meissner, J.; Mathas, S.; Topp, M.S.; Behringer, K.; Klapper, W.; et al. Incorporation of Brentuximab Vedotin into First-Line Treatment of Advanced Classical Hodgkin’s Lymphoma: Final Analysis of a Phase 2 Randomised Trial by the German Hodgkin Study Group. Lancet Oncol. 2017, 18, 1680–1687.
- Gavane, S.C.; Ito, K.; Moskowitz, C.H.; Moskowitz, A.J.; Schöder, H. Metabolic Tumor Volume to Predict Event Free Survival in Patients with Relapsed/Refractory HL Treated with Brentuximab Vedotin-Based Salvage Therapy. J. Clin. Oncol. 2016, 34, 11566.
- Abramson, J.S.; Arnason, J.E.; LaCasce, A.S.; Redd, R.; Barnes, J.A.; Sokol, L.; Joyce, R.; Avigan, D.; Neuberg, D.; Takvorian, R.W.; et al. Brentuximab Vedotin, Doxorubicin, Vinblastine, and Dacarbazine for Nonbulky Limited-Stage Classical Hodgkin Lymphoma. Blood 2019, 134, 606–613.
- Park, S.I.; Shea, T.C.; Olajide, O.; Reddy, N.M.; Budde, L.E.; Ghosh, N.; Deal, A.M.; Noe, J.F.; Ansell, S.M. ABVD Followed by BV Consolidation in Risk-Stratified Patients with Limited-Stage Hodgkin Lymphoma. Blood Adv. 2020, 4, 2548–2555.
- Linguanti, F.; Abenavoli, E.M.; Berti, V.; Lopci, E. Metabolic Imaging in B-Cell Lymphomas during CAR-T Cell Therapy. Cancers 2022, 14, 4700.
- Jain, T.; Bar, M.; Kansagra, A.J.; Chong, E.A.; Hashmi, S.K.; Neelapu, S.S.; Byrne, M.; Jacoby, E.; Lazaryan, A.; Jacobson, C.A.; et al. Use of Chimeric Antigen Receptor T Cell Therapy in Clinical Practice for Relapsed/Refractory Aggressive B Cell Non-Hodgkin Lymphoma: An Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Biol. Blood Marrow Transplant. 2019, 25, 2305–2321.
- Ruff, A.; Ballard, H.J.; Pantel, A.R.; Namoglu, E.C.; Hughes, M.E.; Nasta, S.D.; Chong, E.A.; Bagg, A.; Ruella, M.; Farwell, M.D.; et al. 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Following Chimeric Antigen Receptor T-Cell Therapy in Large B-Cell Lymphoma. Mol. Imaging Biol. 2021, 23, 818–826.
- Li, X.; Sun, X.; Li, J.; Liu, Z.; Mi, M.; Zhu, F.; Wu, G.; Lan, X.; Zhang, L. Interim PET/CT Based on Visual and Semiquantitative Analysis Predicts Survival in Patients with Diffuse Large B-cell Lymphoma. Cancer Med. 2019, 8, 5012–5022.
- Meignan, M.; Gallamini, A.; Meignan, M.; Gallamini, A.; Haioun, C. Report on the First International Workshop on Interim-PET Scan in Lymphoma. Leuk. Lymphoma 2009, 50, 1257–1260.
- Schmitz, C.; Hüttmann, A.; Müller, S.P.; Hanoun, M.; Boellaard, R.; Brinkmann, M.; Jöckel, K.-H.; Dührsen, U.; Rekowski, J. Dynamic Risk Assessment Based on Positron Emission Tomography Scanning in Diffuse Large B-Cell Lymphoma: Post-Hoc Analysis from the PETAL Trial. Eur. J. Cancer 2020, 124, 25–36.
- Wang, X. PET/CT: Appropriate Application in Lymphoma. Chin. Clin. Oncol. 2015, 4, 4.
- Ramos, C.A.; Ballard, B.; Zhang, H.; Dakhova, O.; Gee, A.P.; Mei, Z.; Bilgi, M.; Wu, M.-F.; Liu, H.; Grilley, B.; et al. Clinical and Immunological Responses after CD30-Specific Chimeric Antigen Receptor-Redirected Lymphocytes. J. Clin. Investig. 2017, 127, 3462–3471.
- Ramos, C.A.; Grover, N.S.; Beaven, A.W.; Lulla, P.D.; Wu, M.-F.; Ivanova, A.; Wang, T.; Shea, T.C.; Rooney, C.M.; Dittus, C.; et al. Anti-CD30 CAR-T Cell Therapy in Relapsed and Refractory Hodgkin Lymphoma. J. Clin. Oncol. 2020, 38, 3794–3804.
- Voorhees, T.J.; Zhao, B.; Oldan, J.; Hucks, G.; Khandani, A.; Dittus, C.; Smith, J.; Morrison, J.K.; Cheng, C.J.; Ivanova, A.; et al. Pretherapy Metabolic Tumor Volume Is Associated with Response to CD30 CAR T Cells in Hodgkin Lymphoma. Blood Adv. 2022, 6, 1255–1263.
- “Re-Priming” RT After Incomplete Response to CAR-T in R/R NHL—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04601831?term=CAR-T%2C+FDG+PET%2FCT&cond=Lymphoma&draw=2 (accessed on 11 December 2022).
- Radiomics and Metabolomics in the Follow-up of CAR T-Cells for Refractory or Relapsed Non-Hodgkin’s Lymphoma—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05422521?term=CAR-T%2C+FDG+PET%2FCT&cond=Lymphoma&draw=2 (accessed on 11 December 2022).
- 18F-F-AraG PET Imaging to Evaluate Immunological Response to CAR T Cell Therapy in Lymphoma—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05096234?term=CAR-T%2C+FDG+PET%2FCT&cond=Lymphoma&draw=2&rank=2 (accessed on 11 December 2022).
- PD-L1 PET-Imaging During CAR T-Cell Therapy—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05404048?term=CAR-T%2C+FDG+PET%2FCT&cond=Lymphoma&draw=2&rank=1 (accessed on 11 December 2022).
- Weiler-Sagie, M.; Bushelev, O.; Epelbaum, R.; Dann, E.J.; Haim, N.; Avivi, I.; Ben-Barak, A.; Ben-Arie, Y.; Bar-Shalom, R.; Israel, O. (18)F-FDG Avidity in Lymphoma Readdressed: A Study of 766 Patients. J. Nucl. Med. 2010, 51, 25–30. https://doi.org/10.2967/jnumed.109.067892.
- Haioun, C.; Itti, E.; Rahmouni, A.; Brice, P.; Rain, J.-D.; Belhadj, K.; Gaulard, P.; Garderet, L.; Lepage, E.; Reyes, F.; et al. [18F]Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography (FDG-PET) in Aggressive Lymphoma: An Early Prognostic Tool for Predicting Patient Outcome. Blood 2005, 106, 1376–1381. https://doi.org/10.1182/blood-2005-01-0272.
- Huang, H.; Lin, J.; Guo, C.; Li, S.; Hong, H.; Li, X.; Zhang, M.; Xia, Z.; Lin, T. Predictive Value of Interim 18F-FDG PET-CT Scans on Diffuse Large B-Cell Lymphoma Treated with R-CHOP: A Prospective Study. Blood 2015, 126, 1458–1458. https://doi.org/10.1182/blood.V126.23.1458.1458.
- Sun, N.; Zhao, J.; Qiao, W.; Wang, T. Predictive Value of Interim PET/CT in DLBCL Treated with R-CHOP: Meta-Analysis. Biomed. Res. Int. 2015, 2015, 648572. https://doi.org/10.1155/2015/648572.
- Kostakoglu, L.; Martelli, M.; Sehn, L.H.; Belada, D.; Carella, A.-M.; Chua, N.; Gonzalez-Barca, E.; Hong, X.; Pinto, A.; Shi, Y.; et al. End-of-Treatment PET/CT Predicts PFS and OS in DLBCL after First-Line Treatment: Results from GOYA. Blood Adv. 2021, 5, 1283–1290. https://doi.org/10.1182/bloodadvances.2020002690.
- Sehn, L.H.; Martelli, M.; Trněný, M.; Liu, W.; Bolen, C.R.; Knapp, A.; Sahin, D.; Sellam, G.; Vitolo, U. A Randomized, Open-Label, Phase III Study of Obinutuzumab or Rituximab plus CHOP in Patients with Previously Untreated Diffuse Large B-Cell Lymphoma: Final Analysis of GOYA. J. Hematol. Oncol. 2020, 13, 71. https://doi.org/10.1186/s13045-020-00900-7.
- Zhu, L.; Meng, Y.; Guo, L.; Zhao, H.; Shi, Y.; Li, S.; Wang, A.; Zhang, X.; Shi, J.; Zhu, J.; et al. Predictive Value of Baseline 18F FDG PET/CT and Interim Treatment Response for the Prognosis of Patients with Diffuse Large B cell Lymphoma Re-ceiving R CHOP Chemotherapy. Oncol. Lett. 2020, 21, 132. https://doi.org/10.3892/ol.2020.12393.
- Cottereau, A.-S.; Lanic, H.; Mareschal, S.; Meignan, M.; Vera, P.; Tilly, H.; Jardin, F.; Becker, S. Molecular Profile and FDG-PET/CT Total Metabolic Tumor Volume Improve Risk Classification at Diagnosis for Patients with Diffuse Large B-Cell Lymphoma. Clin. Cancer Res. 2016, 22, 3801–3809. https://doi.org/10.1158/1078-0432.CCR-15-2825.
- Islam, P.; Goldstein, J.; Flowers, C.R. PET-Derived Tumor Metrics Predict DLBCL Response and Progression-Free Survival. Leuk. Lymphoma 2019, 60, 1965–1971. https://doi.org/10.1080/10428194.2018.1562181.
- Ansell, S.M.; Minnema, M.C.; Johnson, P.; Timmerman, J.M.; Armand, P.; Shipp, M.A.; Rodig, S.J.; Ligon, A.H.; Roemer, M.G.M.; Reddy, N.; et al. Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study. J. Clin. Oncol. 2019, 37, 481–489. https://doi.org/10.1200/JCO.18.00766.
- Smith, S.D.; Till, B.G.; Shadman, M.S.; Lynch, R.C.; Cowan, A.J.; Wu, Q.V.; Voutsinas, J.; Rasmussen, H.A.; Blue, K.; Ujjani, C.S.; et al. Pembrolizumab with R-CHOP in Previously Untreated Diffuse Large B-cell Lymphoma: Potential for Biomarker Driven Therapy. Br. J. Haematol. 2020, 189, 1119–1126. https://doi.org/10.1111/bjh.16494.
- Younes, A.; Burke, J.M.; Cheson, B.D.; Diefenbach, C.; Ferrari, S.; Hahn, U.H.; Hawkes, E.A.; Khan, C.; Lossos, I.S.; Musuraca, G.; et al. Safety and Efficacy of Atezolizumab in Combination with Rituximab Plus CHOP in Previously Untreated Patients with Diffuse Large B-Cell Lymphoma (DLBCL): Updated Analysis of a Phase I/II Study. Blood 2019, 134, 2874–2874. https://doi.org/10.1182/blood-2019-123368.
- Shah, N.N.; Nagle, S.J.; Torigian, D.A.; Farwell, M.D.; Hwang, W.-T.; Frey, N.; Nasta, S.D.; Landsburg, D.; Mato, A.; June, C.H.; et al. Early Positron Emission Tomography/Computed Tomography as a Predictor of Response after CTL019 Chimeric Antigen Receptor -T-Cell Therapy in B-Cell Non-Hodgkin Lymphomas. Cytotherapy 2018, 20, 1415–1418. https://doi.org/10.1016/j.jcyt.2018.10.003.
- Cohen, D.; Luttwak, E.; Beyar-Katz, O.; Hazut Krauthammer, S.; Bar-On, Y.; Amit, O.; Gold, R.; Perry, C.; Avivi, I.; Ram, R.; et al. [18F]FDG PET-CT in Patients with DLBCL Treated with CAR-T Cell Therapy: A Practical Approach of Reporting Pre- and Post-Treatment Studies. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 953–962. https://doi.org/10.1007/s00259-021-05551-5.
- Galtier, J.; Vercellino, L.; Chartier, L.; Olivier, P.; Tabouret-Viaud, C.; Mesguich, C.; di Blasi, R.; Durand, A.; Raffy, L.; Gros, F.-X.; et al. PET-Imaging Assessment for Guiding Strategy in Patients with Relapsed/Refractory Large B-Cell Lymphoma Receiving CAR T-Cells. Haematologica 2022, 108, 171–180. https://doi.org/10.3324/haematol.2021.280550.
- Kuhnl, A.; Roddie, C.; Kirkwood, A.A.; Menne, T.; Cuadrado, M.; Marzolini, M.A.V.; Osborne, W.; Sanderson, R.; O’Reilly, M.; Townsend, W.; et al. Early FDG-PET Response Predicts CAR-T Failure in Large B-Cell Lymphoma. Blood Adv. 2022, 6, 321–326. https://doi.org/10.1182/bloodadvances.2021005807.
- Breen, W.G.; Hathcock, M.A.; Young, J.R.; Kowalchuk, R.O.; Bansal, R.; Khurana, A.; Bennani, N.N.; Paludo, J.; Villasboas Bisneto, J.C.; Wang, Y.; et al. Metabolic Characteristics and Prognostic Differentiation of Aggressive Lymphoma Using One-Month Post-CAR-T FDG PET/CT. J. Hematol. Oncol. 2022, 15, 36. https://doi.org/10.1186/s13045-022-01256-w.
- al Zaki, A.; Feng, L.; Watson, G.; Ahmed, S.A.; Mistry, H.; Nastoupil, L.J.; Hawkins, M.; Nair, R.; Iyer, S.P.; Lee, H.J.; et al. Day 30 SUVmax Predicts Progression in Patients with Lymphoma Achieving PR/SD after CAR T-Cell Therapy. Blood Adv. 2022, 6, 2867–2871. https://doi.org/10.1182/bloodadvances.2021006715.
- Wang, J.; Hu, Y.; Yang, S.; Wei, G.; Zhao, X.; Wu, W.; Zhang, Y.; Zhang, Y.; Chen, D.; Wu, Z.; et al. Role of Fluorodeoxy-glucose Positron Emission Tomography/Computed Tomography in Predicting the Adverse Effects of Chimeric Antigen Receptor T Cell Therapy in Patients with Non-Hodgkin Lymphoma. Biol. Blood Marrow Transplant. 2019, 25, 1092–1098. https://doi.org/10.1016/j.bbmt.2019.02.008.
- Bailly, C.; Carlier, T.; Tessoulin, B.; Gastinne, T.; Kraeber-Bodere, F.; le Gouill, S.; Bodet-Milin, C. Prognostic Value of FDG-PET/CT Response for Patient Selection before Chimeric Antigen Receptor-T-cells Therapy in Non-Hodgkin Lymphoma. Hematol. Oncol. 2022, 40, 796–800. https://doi.org/10.1002/hon.2965.
- Armitage, J.O.; Weisenburger, D.D. New Approach to Classifying Non-Hodgkin’s Lymphomas: Clinical Features of the Major Histologic Subtypes. Non-Hodgkin’s Lymphoma Classification Project. J. Clin. Oncol. 1998, 16, 2780–2795. https://doi.org/10.1200/JCO.1998.16.8.2780.
- Dillman, R.O. Radioimmunotherapy of B-Cell Lymphoma with Radiolabelled Anti-CD20 Monoclonal Antibodies. Clin. Exp. Med. 2006, 6, 1–12. https://doi.org/10.1007/s10238-006-0087-6.
- Zinzani, P.L.; Musuraca, G.; Alinari, L.; Fanti, S.; Tani, M.; Stefoni, V.; Marchi, E.; Fina, M.; Pellegrini, C.; Castellucci, P.; et al. Predictive Role of Positron Emission Tomography in the Outcome of Patients with Follicular Lymphoma. Clin. Lymphoma Myeloma 2007, 7, 291–295. https://doi.org/10.3816/CLM.2007.n.005.
- Trotman, J.; Pettitt, A.R. Is It Time for PET-Guided Therapy in Follicular Lymphoma? Blood 2022, 139, 1631–1641. https://doi.org/10.1182/blood.2020008243.
- Barrington, S.F.; Mikhaeel, N.G. Imaging Follicular Lymphoma Using Positron Emission Tomography with [18F]Fluorodeoxyglucose: To What Purpose? J. Clin. Oncol. 2012, 30, 4285–4287. https://doi.org/10.1200/JCO.2012.45.4082.
- Casulo, C.; Byrtek, M.; Dawson, K.L.; Zhou, X.; Farber, C.M.; Flowers, C.R.; Hainsworth, J.D.; Maurer, M.J.; Cerhan, J.R.; Link, B.K.; et al. Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis from the National LymphoCare Study. J. Clin. Oncol. 2015, 33, 2516–2522. https://doi.org/10.1200/JCO.2014.59.7534.
- Trotman, J.; Fournier, M.; Lamy, T.; Seymour, J.F.; Sonet, A.; Janikova, A.; Shpilberg, O.; Gyan, E.; Tilly, H.; Estell, J.; et al. Positron Emission Tomography–Computed Tomography (PET-CT) After Induction Therapy Is Highly Predictive of Patient Outcome in Follicular Lymphoma: Analysis of PET-CT in a Subset of PRIMA Trial Participants. J. Clin. Oncol. 2011, 29, 3194–3200. https://doi.org/10.1200/JCO.2011.35.0736.
- Dupuis, J.; Berriolo-Riedinger, A.; Julian, A.; Brice, P.; Tychyj-Pinel, C.; Tilly, H.; Mounier, N.; Gallamini, A.; Feugier, P.; Soubeyran, P.; et al. Impact of [18F]Fluorodeoxyglucose Positron Emission Tomography Response Evaluation in Patients with High–Tumor Burden Follicular Lymphoma Treated with Immunochemotherapy: A Prospective Study from the Groupe d’Etudes Des Lymphomes de l’Adulte and GOELAMS. J. Clin. Oncol. 2012, 30, 4317–4322. https://doi.org/10.1200/JCO.2012.43.0934.
- Luminari, S.; Biasoli, I.; Versari, A.; Rattotti, S.; Bottelli, C.; Rusconi, C.; Merli, F.; Spina, M.; Ferreri, A.J.M.; Zinzani, P.L.; et al. The Prognostic Role of Post-Induction FDG-PET in Patients with Follicular Lymphoma: A Subset Analysis from the FOLL05 Trial of the Fondazione Italiana Linfomi (FIL). Ann. Oncol. 2014, 25, 442–447. https://doi.org/10.1093/annonc/mdt562.
- Trotman, J.; Luminari, S.; Boussetta, S.; Versari, A.; Dupuis, J.; Tychyj, C.; Marcheselli, L.; Berriolo-Riedinger, A.; Frances-chetto, A.; Julian, A.; et al. Prognostic Value of PET-CT after First-Line Therapy in Patients with Follicular Lymphoma: A Pooled Analysis of Central Scan Review in Three Multicentre Studies. Lancet Haematol. 2014, 1, e17–e27. https://doi.org/10.1016/S2352-3026(14)70008-0.
- Trotman, J.; Barrington, S.F.; Belada, D.; Meignan, M.; MacEwan, R.; Owen, C.; Ptáčník, V.; Rosta, A.; Fingerle-Rowson, G.R.; Zhu, J.; et al. Prognostic Value of End-of-Induction PET Response after First-Line Immunochemotherapy for Follicular Lymphoma (GALLIUM): Secondary Analysis of a Randomised, Phase 3 Trial. Lancet Oncol. 2018, 19, 1530–1542. https://doi.org/10.1016/S1470-2045(18)30618-1.
- Luminari, S.; Manni, M.; Galimberti, S.; Versari, A.; Tucci, A.; Boccomini, C.; Farina, L.; Olivieri, J.; Marcheselli, L.; Guerra, L.; et al. Response-Adapted Postinduction Strategy in Patients with Advanced-Stage Follicular Lymphoma: The FOLL12 Study. J. Clin. Oncol. 2022, 40, 729–739. https://doi.org/10.1200/JCO.21.01234.
- Bouchkouj, N.; Zimmerman, M.; Kasamon, Y.L.; Wang, C.; Dai, T.; Xu, Z.; Wang, X.; Theoret, M.; Purohit-Sheth, T.; George, B. FDA Approval Summary: Axicabtagene Ciloleucel for Relapsed or Refractory Follicular Lymphoma. Oncologist 2022, 27, 587–594. https://doi.org/10.1093/oncolo/oyac054.
- Budde, L.E.; Sehn, L.H.; Matasar, M.; Schuster, S.J.; Assouline, S.; Giri, P.; Kuruvilla, J.; Canales, M.; Dietrich, S.; Fay, K.; et al. Safety and Efficacy of Mosunetuzumab, a Bispecific Antibody, in Patients with Relapsed or Refractory Follicular Lymphoma: A Single-Arm, Multicentre, Phase 2 Study. Lancet Oncol. 2022, 23, 1055–1065. https://doi.org/10.1016/S1470-2045(22)00335-7.
