Sourdough fermentation is an ancient technique to ferment cereal flour that improves bread quality, bringing nutritional and health benefits. The fermented dough has a complex microbiome composed mainly of lactic acid bacteria and yeasts. During fermentation, the production of metabolites and chemical reactions occurs, giving the product unique characteristics and high sensory quality. Mastery of fermentation allows gluten levels to adjust, delay starch digestibility, and increase the bio-accessibility of vitamins and minerals.
- sourdough bread
- microorganism
- enzymes
- probiotics
- fermentation
- microbiome
1. Introduction
Sourdough fermentation is a technique that can use several types of flour, such as wheat, rye, or other cereals, and water. The oldest process of sourdough preparation is spontaneous fermentation and acidification due to the local microbiota in a complex interaction, mainly lactic acid bacteria (LAB) and yeasts [1]. It is a traditional fermentation process used for various foods, especially baked goods, improving nutritional and health benefits [2].
Probiotics are safe for human consumption and can produce metabolites that positively influence gastrointestinal [3] and bone health diseases [4]. Beneficial effects in eczema [5], allergies [6], respiratory tract infection [7], obesity [8], and cognitive and mental health are also described [9]. Some LAB and yeasts present in sourdough fermentation are considered and presumed to be probiotics [10].
2. Sourdough Fermentation Types: Inoculum and Technology Processes
Sourdough fermentation can be classified according to the inoculum (types II, III, and I) and the technology process (types 0, II, III). According to inoculum, type I uses the microorganisms present in the dough, which results in spontaneous fermentation and utilizes back-slopping to propagate the sourdough microbiota. This process is typical in artisan bakeries and homes. Control parameters include temperature, number, and time of back-slopping in artisan bakeries. Type II inoculums are frequently utilized in industrial processes, where a starter culture is added to sourdough fermentation according to the objectives and results desirable in the final process. Type III is a hybrid and combines type II by using a starter culture with type I for propagating the sourdough with back-slopping. Type III is common in artisan bakeries and industrial ones [11].
According to the production and process, sourdough fermentation can also be classified into four major types. The simple type 0 fermentation starts with a flour–water mixture and is allowed for a limited time. In type 0 fermentation, bioactive molecules and organic acids (lactic and acetic acids) are produced, lowering the pH (pH~4). Still, it is controversial whether this fermentation is a true sourdough. The time limit is insufficient to produce other characteristic products of sourdoughs and is more known as sponge dough [11].
Type I is a traditional method for preparing sourdough and consists of spontaneous flour–water in a flour–water mixture at an ambient temperature of less than 30 °C for 24 h or less and back-slopping frequently. This fermentation increases the quality of the final baked good [12].
Type II is when a starter culture initiates the fermentation process and is used to develop desirable characteristics for the baking industry. It is a sourdough in liquid form with controlled parameters [12].
Type III can be dried or lyophilized. It is preferable in an industrial bakery because it has a higher stability quality than fresh sourdough [12]. The fermentation temperature is above 30 °C and a single time between 24–72 h [13].
The fermentation process will change depending on the added materials, such as the addition of naturally rich products in microorganisms: fruits, yogurt, or another material [14]. It is observed that it is challenging to classify sourdough fermentation due to some nuances in the process production [13]. Figure 1 summarizes the major types of production and process and their characteristics.
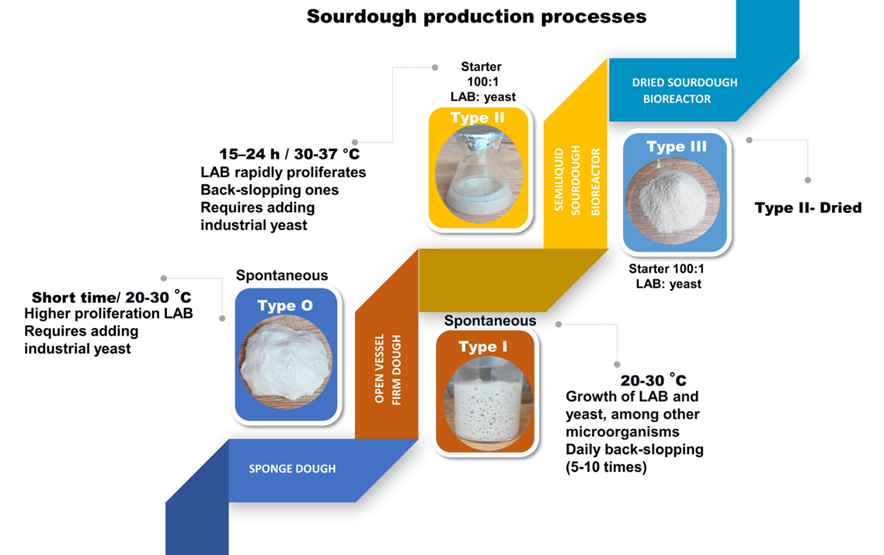
Figure 1. Sourdough production of the four types of processes. Type II and III can be scaled to an industrial level.
3. Sourdough Fermentation: Major Pathways
The sourdough fermentation process generates mainly acids, alcohols, aldehydes, esters, and ketones; it is the primary route of volatile organic compounds (VOCs) [15]. Sourdough bread has a complex profile, strongly influenced by the compounds generated during fermentation by the diverse array of microorganisms present, mainly yeasts and LAB.
Wheat flour is composed of starch (70–75%), water (14%), proteins (10–12%), non-starch polysaccharides (2–3%), lipids (2%), and soluble carbohydrates such as maltoses, sucrose, and glucose (1.55–1.85%). The starch is degraded into glucose and maltose by flour amylase and glucoamylase by some sourdough LABs. Maltose originates glucose from the enzyme maltose phosphorylase from LAB and alpha-D-glucosidase, a maltase from Saccharomyces yeasts. In the dough, the available carbon source is thus maltose, followed by sucrose, glucose, and fructose, with some trisaccharides such as maltotriose and raffinose [16].
The glucose concentration increases during fermentation because other complex carbohydrates are metabolized by LAB and yeasts. Microbial enzymes liberating glucose and galactose after cleavage can ferment the disaccharide lactose. Starting from glucose, homofermentative LAB produces lactic acid through glycolysis, while heterofermentative LAB generates, besides lactic acid, CO2, acetic acid, and/or ethanol [17]. Two major fermentation routes are found: lactic fermentation (LF) and alcoholic fermentation (AF). In lactic fermentation, the pyruvate molecules formed by glucose oxidation from the Embden–Meyerhof–Parnas or glycolytic pathway are reduced to lactic acid (homolactic fermentation).Streptococcus, Lactobacillus, and Enterococcus use this route (Figure 2A1).
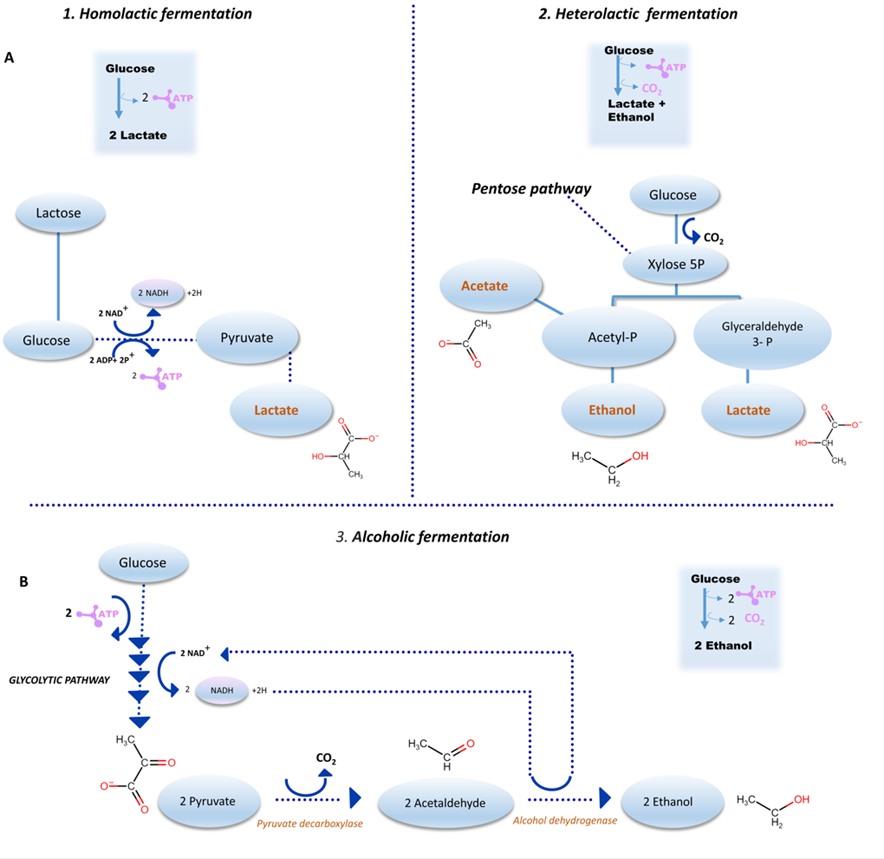
Figure 2. Major sourdough fermentation pathways. (A) Lactic fermentation. (B) Alcoholic fermentation. (A1), homolactic fermentation; (A2), heterolactic fermentation. (B3), alcoholic fermentation from yeast. The formation of ethanol and CO2 from the reduction of pyruvate characterizes alcoholic fermentation.
Another possibility is the pyruvate originating from a mixture of lactate, ethanol, and/or acetic acid through the oxidation of the coenzymes NADH + and H+ by the lactate dehydrogenase and CO2 from a glucose molecule until ribulose 5 phosphate (heterolactic fermentation). This fermentation is used by genera such as Leuconostoc, Bifidobacterium, Weissella, and some Lactobacillus. Some glycolytic enzymes are missing in these bacteria, and they use the pentose-P pathway to degrade the glucose (Figure 2A2). Classification according to the type of lactic fermentation defines three major Lactobacillus groups: group I: obligate homofermentative lactobacilli. Hexoses are exclusively fermented to lactic acid via the Embden–Meyerhof–Parnas pathway. The bacteria have the enzyme fructose-1,6-bisphosphate aldolase, but phosphoketolase is absent. Due to this, pentoses and gluconate are not fermented. This group includes the species L. acidophilus, L. delbrueckii, and L. salivarius; group II: facultative heterofermentative lactobacilli. Hexoses are fermented to lactic acid almost exclusively via the Embden–Meyerhof–Parnas pathway. The bacteria possess both aldolase and phosphoketolase enzymes and ferment not only hexoses but also pentoses. In the presence of glucose, the enzymes of the phosphogluconate pathway are inhibited. This group includes L. casei, L. paracasei, L. plantarum, and L. curvatus. Organic acids, CO2, alcohol, and H2O2 could be produced; group III: is composed of obligate heterofermentative Lactobacilli. They have the enzyme phosphoketolase but not aldolase and ferment sugars in a heterofermentative mode. Hexoses are fermented via the phosphogluconate pathway, producing lactate, ethanol (acetic acid), and CO2 in equimolar amounts. Pentoses enter this pathway and can also be fermented [18].
After the enzymatic hydrolysis of pentosans and other complex carbohydrates, pentoses such as d-xylose, ribose, and l-arabinose are liberated in rye and wheat flour. Heterofermentative strains can ferment pentoses through part of the 6-phosphogluconate pathway [19]. Pentoses and hexoses are simultaneously rather than successively fermented by sourdough Lactobacilli. Lactobacilli are essential during the fermentation of sourdoughs, and they can ferment pentose carbohydrates without producing CO2 because they have a constitutive phosphoketolase. Facultatively heterofermentative lactic acid bacteria produce the phosphoketolase enzyme in response to the presence of pentoses [20]. The contribution of LAB to the flavor of sourdough bread is associated with the production of lactic acid (fresh acidity) and acetic acid (sharp acidity). The conversion of amino acids such as phenylalanine (sweet), isoleucine (acidic), glycine, serine, and alanine (vinegar/sour) to aldehydes and ketones can form additional flavor compounds [15].
Alcoholic fermentation (AF) is characterized by the formation of ethanol and CO2 from pyruvate reduction (Figure 2B). AF is a metabolic pathway of yeasts. In this fermentation, yeast produces gas that promotes dough conditioning and increased volume and imparts desirable aromas and flavors in baked goods. A synergy occurs between the dough and the yeast, and in this research, the rate of CO2 production is determined by the activities of the glycolytic yeast enzymes. The retention of CO2 produced by yeast is a function of wheat or cereal flour. It is essential to point out that glucose formed by LAB amylases from amide is a significant source of this substrate for yeasts. Other compounds are volatile organic compounds (VOCs), which arise from this interaction between yeasts and LABs. Diverse aldehydes, alcohols, esters, ketones, lactones, sulfur compounds, furan derivatives, and hydrocarbons are found, conferring flavor and other characteristics to sourdough bread [21]. Some VOCs are due to yeast fermentation, particularly of S. cerevisiae in mixed sourdough starters, including ethanol, 2,3-butanedione, 2-methyl-1-propanol, 3-methyl-1-butanol, and phenyl ethyl alcohol [22]. Other negatively correlated compounds have been associated with the fermentation of homofermentative and facultative heterofermentative LAB, including 2,3-butanedione and acetaldehyde [23].
4. Probiotics and Postbiotics in Sourdough and the Impact on Human Health
Probiotics are live microorganisms that confer a health benefit when administered in adequate amounts. In addition, new terms have been used to name microbial metabolites from non-viable cells, including paraprobiotics, parapsychobiotics, ghost probiotics, matabiotics, tyndallized probiotics, bacterial lysates, and postbiotics. However, the last term is being mightily used for the vital concept of promoting health. Postbiotics are non-living microorganisms and is a preparation with inanimate microbial cells or their components that promote host health. Metabolites and cellular structures from microorganisms from sourdough are potential postbiotics [24].
The sourdough microbiota changes with decreasing pH, whereas at the beginning, there is abundant Proteobacteria phylum sourdoughs, which at the end is replaced by Firmicutes [25], and among yeasts Saccharomyces cerevisiae is the most common [1].
A sourdough starter is in essence, a culture of probiotic yeast and lactic bacteria to be added to the fermentation process, besides the native microbiome of the cereals and external contaminations. Starters also can be used to propagate the sourdough as a live fermentation. Here, it is interesting to explore the technological and biological properties of the selected starter associations of bacteria/yeast to improve the quality of fermentation and bread properties.
The microbial production of bioactive peptides, organic acids, exopolysaccharides (EPS), and vitamins are considered the primary metabolites responsible for antioxidant, antimicrobial, and probiotic activities [26]. Relative to fermentative processes, many bioproducts are present in sourdough fermentation. Koistinen et al. [27] demonstrated 118 bioactive compounds in sourdough fermentation.
One important point is that most probiotics die when the bread is baked at high temperatures. However, most health benefits continue, mostly, not with probiotics at intestinal epithelial cell colonization but in sourdough dough. It is essential to note that active biomolecules could be present in the baked bread. Different conditions, varying temperatures, baking time, and fermentation parameters could influence the heat resistance of some bioproducts, and they could be present as nutrients and fibers such as β-glucan and resistant starch [28].
During the baking process, cell lysis of microorganisms delivers cellular debris that may also have beneficial properties functioning as postbiotics. Remains of microbial cell structures such as peptidoglycan from bacterial cell walls, pili, fimbriae, flagella, cell-surface-associated proteins, cell-wall-bound biosurfactants, and cell supernatants are also postbiotic components that have potential health benefits in the host [29].
Among lactic acid bacteria, one of the most versatile and adaptable is Lactiplantibacillus plantarum, with a long history of use in foods and claims of benefits as a probiotic. Lactiplantibacillus plantarum strain ITM21B has been used to prolong bread shelf-life due to the production of antimicrobial substances, such as lactic, acetic, phenyl lactic (PLA), and hydroxy-phenyl lactic (OH-PLA) acids [30].
Dietary sourdough bread increases γ-aminobutyric acid (GABA) content [31]. The γ-aminobutyric acid GABA is a non-protein amino acid synthesized from L-glutamate by the glutamate decarboxylase [32]. It is a mammalian neurotransmitter involved in critical regulatory functions, hypotensive properties, anti-depressive effects, diuretic, and antioxidant effects [33]. It is present in many medicines and supplements [34]. GABA has several physiological functions, include relaxation, sleeplessness, enhanced immunity under stress, increasing the concentration of growth hormone in plasma, preventing diabetes, inhibiting the invasion and metastasis of various types of cancer, anti-inflammatory effects, blood pressure regulation, and antioxidant effects. It is also a hormonal regulator (for review, see Diana et al., 2014 [35] and Polak et al. 2021 [36].
A study by Boakye et al. 2022 [37], described that the longer sourdough fermentation time (12 h) caused up to 69%, 69%, and 41% reductions in fructans, raffinose, and ATIs, respectively. Low FODMAPs may be valuable for people with gastrointestinal disorders.
Antioxidant and anti-inflammatory activities of peptides from cooked sourdough breads were described [38], where longer fermentation (72–96 h) increases the production of aromatic compounds with antioxidant activity [39].
LAB and yeast strains were isolated and molecularly identified from traditional Iranian sourdough. Based on total phenol production and antioxidant capacity assessments, all the identified traditional sourdough microorganisms significantly produced phenolic compounds. They showed significant antioxidant capacity improving bread’s health benefits and quality [40].
Exopolysaccharides (EPS), such as β-glucan, dextran, and inulin, are metabolites from LAB identified in sourdough fermentation. The β-glucan, for instance, is a prebiotic homopolysaccharide formed by glucose, presenting substantial health benefits, such as stabilized cholesterol levels, anti-inflammatory effects, and benefits for probiotic microorganisms [41]. The conclusions reached by Schlörmann et al. [42] provide significant insights into the general chemopreventive and prebiotic effects of LAB-generated sourdoughs and bread. In addition, exopolysaccharides are produced by LAB in sourdough, favoring great water retention. β-glucans also contribute to the viscoelastic properties of dough [41]. Some of these health benefits and others are summarized in Table 1.
Table 1. Microorganisms and the compounds they produce, and the health benefits.
|
Microorganisms |
Compound |
Benefit |
Reference |
|
Probiotics Streptococcus thermophilus, Lactobacillus plantarum, L. acidophilus, L. casei, L. delbrueckii spp. bulgaricus, Bifidobacterium breve, B. longum, and B. infantis |
Peptidase |
Alfa-gliadin degradation, reduced wheat allergenic |
[43] |
|
LAB from sourdough |
Gamma-aminobutyric acid (GABA) |
ACE-inhibitory activity |
[44] |
|
LAB from sourdough |
Multifactors |
Low-glycemic index in the white wheat bread |
[45] |
|
Lactobacillus reuteri |
Exopolysaccharide |
Antiadhesive properties, inhibition enterotoxigenic Escherichia coli |
[46] |
|
Lactobacillus brevis with S. cerevisiae var. Chevalieri; L. Fermentum; L. Fermentum with phytase |
Higher total phenolic and a lower molar ratio of lactic to acetic acid |
Reduce glycemic index |
[47] |
|
L. curvatus SAL33 and L. Brevis AM7 |
Peptide lunasin |
Cancer preventive |
[48] |
|
Bifidobacterium strains |
Phytase |
Increase iron bioavailability in bread |
[49] |
|
Weissella ciabaria MG1; L. Reuteri VIP, L. Reuteri Y2 |
Oligosaccharides |
Improved nutritional quality of sorghum bread |
[50] |
|
L. brevis |
Phytase |
Decrease phytate levels, improve mineral bio-accessibility |
[51] |
|
L. Sakei KTU05-6 |
Organic acids, bacteriocins |
Bio-preservative |
[52] |
|
Weissella confusa LBAE C39-2 |
Dextransucrase (glycoside hydrolase) |
Alfa-glucans/ oligosaccharides or glycoconjugates |
[53] |
|
L. rossiae DSM 15814 from sourdough |
Vitamin B12, folate, and riboflavin |
Nutritional improvement |
[54] |
|
Lactobacillus amylovorus DSM 19280 and Weisella cibari MG1 |
Glutamate accumulation |
NaCl reduction in bread |
[55] |
|
Traditional sourdough LAB starter culture |
Essential and non-essential amino acids, flavonoids, antioxidant peptides |
Nutritional improvement protects against oxidative stress and degenerative disease through phenolic compounds |
[56] |
|
LAB from traditional Austrian sourdoughs |
Fructose metabolized/antifungal and anti-bacillus properties |
Decrease FODMAPS/ control molds |
[57] |
|
Lactobacillus plantarum ZJUFT17 from Chinese sourdough |
In mice, decreased: the profile, insulin resistance, lipopolysaccharide, cytokines interleukin (IL)-1β, tumor necrosis factor (TNF)-α |
Managing gut microbiota, decreasing pathogenic and pro-inflammatory microbes, and stimulating anti-obesity ones |
[58] |
|
Levilactobacillus brevis TMW 1.211, Pediococcus claussenii TMW 2.340 from breweries |
O2-substituted (1,3)-β-D-glucan |
Prebiotic effect in bread, improving water binding capacity |
[59] |
|
Lactobacillus plantarum ZJUFB2 from Chinese sourdough |
Probiotic effect on gut microbiota |
Prevent insulin resistance and modulate gut microbiota. |
[60] |
|
Levilactobacillus brevis TMW 1.2112, Pediococcus claussenii TMW 2.340 |
Dietary fiber, short acid fat chain SCFA, butyrate, propionate,β-glucan |
Healthy environment in the colon, chemopreventive |
[61] |
|
Pediococcus pentosaceus F01, Levilactobacillus brevis MRS4, Lactiplantibacillus plantarum H64, and C48 |
Γ-aminobutyric acid (GABA) |
Bread from surplus bread with high nutritional value |
[62] |
|
Weissella cibaria PDER21 |
α-D-glucan |
Antioxidant properties |
[63] |
[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][41][59][42][60][61][62][63][64][65][66][65][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87]
In addition to the health effects, sourdough brings flavor, aroma, and better texture of bread due to LAB enzymatic processes.
5. Enzymes in Sourdough
Enzymes are responsible for several biochemical events in sourdough fermentation. Although some enzymes are present in the cereal, most of them are produced by microorganisms. Figure 3 shows the sourdough fermentation process and the major enzymes involved.
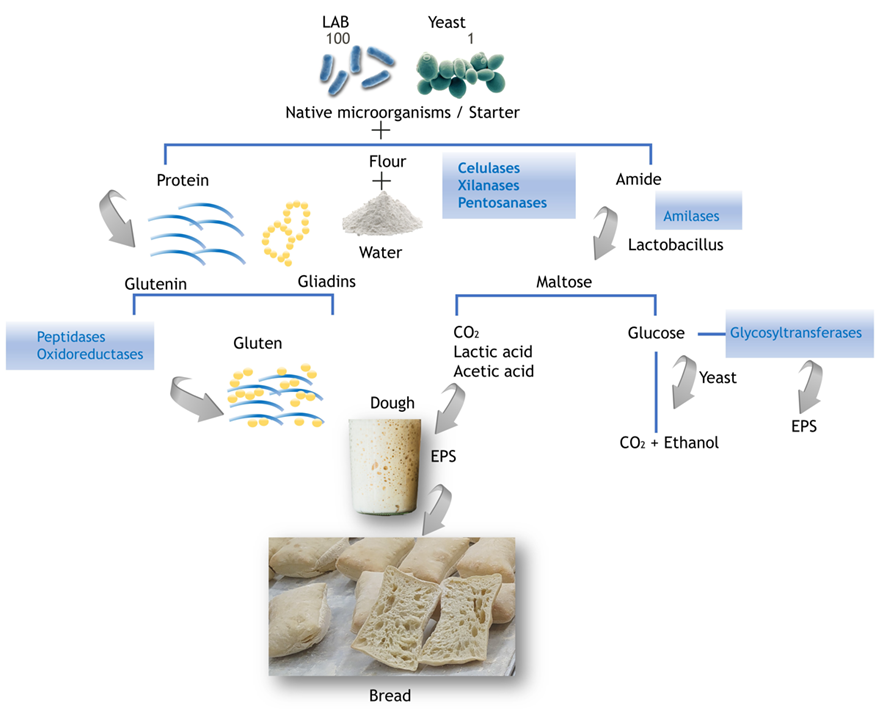
Figure 3. General scheme of bread sourdough fermentation and the major enzymes involved. EPS: Exopolysaccharides.
5.1. Transferases
Glycosyltransferases (EC 2.4) are involved in the biosynthesis of exopolysaccharides in sourdough. It is an enzymatic group produced by the LAB from sourdough microbiota, such as Lactobacillus, Leuconostoc spp., Leuconostoc citreum, and Weissella species. Glucansucrases are glycosyltransferases from LAB that split sucrose. The resulting glucose builds EPS-type α-glucan polymers such as dextran, mutan, alternan, and reuteran. Fructansucrase, found in Gram-negative and Gram-positive bacteria, is another glycosyltransferase that synthesizes levan (levansucrase) or inulin (inulosucrase). The inulosucrase is observed only in LAB [64].
EPS formed during sourdough fermentation influences the dough’s viscoelastic properties and improves the texture and shelf-life. Here, EPS can replace hydrocolloids used as bread improvers and meet consumer demands for reduced use of food additives [65].
5.2. Oxidoreductases
Glutathione reductase (EC 1.8.1.7) is the most known enzyme in this group related to sourdough bread properties. It acts on a sulfur group of donors, with NAD+ or NADP+ as the acceptor [66].
Gluten protein presents intra- and intermolecular chains of disulfide bonds between amino acids [67]. The glutathione reductase activity, identified in the microbiota of natural fermentation, interferes with these thiol bonds [68]. The glutathione reductase deficient mutant strain (Lactobacillus sanfranciscensis) produced bread with a softer texture and higher specific volume than bread made with traditional biological yeast and non-mutant Lactobacillus sanfranciscensis activity [69].
5.3. Lyases
The glutamate decarboxylase (EC 4.1.1.15) is commonly found in the sourdough environment and acts in L-glutamate’s decarboxylation to form γ-aminobutyric acid (GABA) [70].
5.4. Hydrolases
5.4.1. Amylase, Inulinase, and Their Impact on Bread Structure and Properties
The amylases produced by LAB strains reduce the aging process during bread storage [71]. Amylase-producing microorganisms in the sourdough environment are essential in converting starch into fermentable carbohydrates such as maltodextrins, maltose, sucrose, and glucose. LAB are sources of maltose phosphorylase, which generate D-glucose, β-D-glucose, 1-phosphate, and 1,6-α-glucosidase, which hydrolyzes α-(1–6)-glucooligosaccharides [72]. In addition, wheat has α-amylase, β-amylase, and glucoamylase activity, but at pH < 4.5, only glucoamylase maintains its activity. Microorganisms in the sourdough microbiota produce inulinase (β-2, 1-D-fructan-fructan-hydrolase—EC 3.2.1.7)—a glycosylase that hydrolyzes the β-2.1 bonds of fructose of the fructose polymer, inulin. Inulinase can reduce the number of oligosaccharides, disaccharides, monosaccharides, and fermentable polyols (FODMAPs) in bread [73].
5.4.2. Cellulase, Phytase, and Xylanase for Mineral Bio-Accessibility Improvement in Bread
Endocellulase acts on cellulose and β-glucan substrates, removing insoluble arabinoxylans, contributing to gluten network formation [74]. Subsequently, there is a decrease in the hardness of the bread and, consequently, an improvement in the sensory evaluations. Cellulase action may also provide an anti-staling effect, which may be related to alterations in water distribution between the starch–protein matrix [75]. The enzyme phytase or myoinositol-hexaphosphate phosphohydrolase is present in plants, bacteria, yeasts, and filamentous fungi. Xylan is the main hemicellulosic component of hardwoods and accounts for approximately 30% of the woody cell wall. Xylanases (EC 3.2.1.8) are enzymes that hydrolyze this polysaccharide. Two mechanisms can summarize the improvement in bread quality due to the addition of xylanase: (i) the removal of arabinoxylans from gluten alters the distribution of water between gluten proteins and arabinoxylans; (ii) pentosan destruction and viscosity reduction effect [75]. Xylanase and cellulase showed satisfactory results to facilitate access into the aleurone tissue for the action of phytase, improving iron bio-accessibility [76].
5.4.3. Lipase and the Baking Technology
In baking, using lipase to replace emulsifiers, such as monoglycerides, proved to be an efficient alternative to replace chemical additives [77]. Sourdough fermentation inhibits the lipase activity due to decreased pH in the system, which is an efficient alternative for neutralizing the lipase activity in wheat germ [78].
5.4.4. Peptidase and Implications for the Gluten Network
Gluten structure varies with the cultivar and cultivation conditions [79]. It is composed of gliadin (α, β, γ, ω), glutenin, and polypeptide fractions that combine with gliadin or glutenin [80].
During sourdough fermentation, the partial hydrolysis of gliadin and glutenin proteins occurs because of the acidification and activation of cereal peptidases. In addition, endogenous flour peptidases became activated at a low pH reducing the gluten disulfide bonds [17].
Several studies have focused on LAB peptidases and demonstrated that in different ways, that LAB in sourdough fermentation has a proteolytic activity for gluten hydrolysis [81]. L. rhamnosus, Pediococcus pentosaceus, and Lactobacillus curvatus used gluten as the only source of nitrogen, significantly reducing the allergenic composition of wheat and improving digestibility [82].
6. General Regulation for Microbes Used in Sourdough Bread
Searching for rules that maintain food safety is a critical concern due to the growing use of microorganisms as foods or probiotics. At this moment, there is an effort at Codex Alimentarius to harmonize rules for probiotics and the use of microorganisms in food [83].
In the United States, there are rules defined by the Food and Drug Administration (FDA) for the use of microorganisms in food production. It is necessary to qualify as a Generally Recognized as Safe for Use (GRAS) microorganism [84]. In Brazil, the biological fermenting mixtures and their microbial content are classified as a supporting and processing agent and are exempted from registration by ANVISA [85].In Europe, since 1997, with the novel food regulation, all food that started to be produced after 15 May 1997 is considered a novel food with specific rules to follow [86]. For microorganisms usually found in sourdough and used as a starter, it is challenging to define it as a novel food because sourdough predates 1997 [87].
7. Conclusions
The role of microorganisms in sourdough bread has been extensively demonstrated in the literature. Increasing information is arising about the fermentation process and other metabolic routes promoting improvements in quality and adding interesting nutritional, health, and sensory characteristics. Currently, several benefits of probiotic and postbiotics elements are well established in terms of the bread’s properties, such as rheological and organoleptic properties, as well as dough quality and human health.
This entry is adapted from the peer-reviewed paper 10.3390/fermentation9020090
References
- Arora, K.; Ameur, H.; Polo, A.; Di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty Years of Knowledge on Sourdough Fermentation: A Systematic Review. Trends Food Sci. Technol. 2021, 108, 71–83. [Google Scholar] [CrossRef]
- Bartkiene, E.; Özogul, F.; Rocha, J.M. Bread Sourdough Lactic Acid Bacteria—Technological, Antimicrobial, Toxin-Degrading, Immune System-, and Faecal Microbiota-Modelling Biological Agents for the Preparation of Food, Nutraceuticals and Feed. Foods 2022, 11, 452. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.; VandeVusse, L.; Forgie, M.; Malloy, E.; Singh, M.; Scherer, M.; Kleber, D.; Dixon, J.; Hryckowian, A.J.; Safdar, N. A Randomized Controlled Trial of an Oral Probiotic to Reduce Antepartum Group B Streptococcus Colonization and Gastrointestinal Symptoms. Am. J. Obstet. Gynecol. MFM 2023, 5, 100748. [Google Scholar] [CrossRef]
- Lee, Y.; Oh, H.; Jo, M.; Cho, H.; Park, Y. Synergistic Effect of N-3 PUFA and Probiotic Supplementation on Bone Loss Induced by Chronic Mild Stress through the Brain–Gut–Bone Axis. J. Funct. Foods 2023, 100, 105363. [Google Scholar] [CrossRef]
- Dotterud, C.K.; Storrø, O.; Johnsen, R.; Øien, T. Probiotics in Pregnant Women to Prevent Allergic Disease: A Randomized, Double-Blind Trial: Probiotics in Pregnant Women to Prevent Allergic Disease. Br. J. Dermatol. 2010, 163, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.H.A.; Hazrin-Chong, N.H.; Harith, H.H.; Wan-Mohtar, W.A.A.Q.I.; Sukor, R. Roles of Fermented Plant-, Dairy- and Meat-Based Foods in the Modulation of Allergic Responses. Food Sci. Hum. Wellness 2023, 12, 691–701. [Google Scholar] [CrossRef]
- Dekker, J.; Quilter, M.; Qian, H. Comparison of Two Probiotics in Follow-on Formula: Bifidobacterium animalis Subsp. Lactis HN019 Reduced Upper Respiratory Tract Infections in Chinese Infants. Benef. Microbes 2022, 13, 341–353. [Google Scholar] [CrossRef]
- He, F.; Shen, X.; Cheng, R.; Marotta, F. Screening Potential Probiotics Against Obesity and Metabolism Abnormalities in the Elderly. In Gut Microbiota in Aging and Chronic Diseases; Marotta, F., Ed.; Healthy Ageing and Longevity; Springer International Publishing: Cham, Switzerland, 2023; Volume 17, pp. 375–386. ISBN 978-3-031-14022-8. [Google Scholar]
- Shi, S.; Zhang, Q.; Sang, Y.; Ge, S.; Wang, Q.; Wang, R.; He, J. Probiotic Bifidobacterium longum BB68S Improves Cognitive Functions in Healthy Older Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2022, 15, 51. [Google Scholar] [CrossRef]
- Jenkins, G.; Mason, P. The Role of Prebiotics and Probiotics in Human Health: A Systematic Review with a Focus on Gut and Immune Health. Food Nutr. J. 2022, 6, 245. [Google Scholar]
- De Vuyst, L.; Comasio, A.; Kerrebroeck, S.V. Sourdough Production: Fermentation Strategies, Microbial Ecology, and Use of Non-Flour Ingredients. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Chavan, R.S.; Chavan, S.R. Sourdough Technology-A Traditional Way for Wholesome Foods: A Review. Compr. Rev. Food Sci. Food Saf. 2011, 10, 169–182. [Google Scholar] [CrossRef]
- Van Kerrebroeck, S.; Maes, D.; De Vuyst, L. Sourdoughs as a Function of Their Species Diversity and Process Conditions, a Meta-Analysis. Trends Food Sci. Technol. 2017, 68, 152–159. [Google Scholar] [CrossRef]
- Siepmann, F.B.; Ripari, V.; Waszczynskyj, N.; Spier, M.R. Overview of Sourdough Technology: From Production to Marketing. Food Bioprocess Technol. 2018, 11, 242–270. [Google Scholar] [CrossRef]
- Limbad, M.; Gutierrez Maddox, N.; Hamid, N.; Kantono, K. Sensory and Physicochemical Characterization of Sourdough Bread Prepared with a Coconut Water Kefir Starter. Foods 2020, 9, 1165. [Google Scholar] [CrossRef] [PubMed]
- Mietton, L.; Samson, M.-F.; Marlin, T.; Godet, T.; Nolleau, V.; Guezenec, S.; Segond, D.; Nidelet, T.; Desclaux, D.; Sicard, D. Impact of Leavening Agent and Wheat Variety on Bread Organoleptic and Nutritional Quality. Microorganisms 2022, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Van Kerrebroeck, S.; Leroy, F. Microbial Ecology and Process Technology of Sourdough Fermentation. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 100, pp. 49–160. ISBN 978-0-12-812048-4. [Google Scholar]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; Gallo, G.; Settanni, L.; Berloco, M.G.; Siragusa, S.; Parente, E.; Corsetti, A.; Gobbetti, M. Genotypic and Phenotypic Diversity of Lactobacillus rossiae Strains Isolated from Sourdough. J. Appl. Microbiol. 2007, 103, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.; Gobbetti, M. Physiology and Biochemistry of Lactic Acid Bacteria. In Handbook on Sourdough Biotechnology; Springer: New York, NY, USA, 2013; pp. 183–216. ISBN 978-1-4614-5424-3. [Google Scholar]
- Warburton, A.; Silcock, P.; Eyres, G.T. Impact of Sourdough Culture on the Volatile Compounds in Wholemeal Sourdough Bread. Food Res. Int. 2022, 161, 111885. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, T.; Zhang, G.; He, G. Intraspecific Diversity and Fermentative Properties of Saccharomyces cerevisiae from Chinese Traditional Sourdough. LWT 2020, 124, 109195. [Google Scholar] [CrossRef]
- Kaseleht, K.; Paalme, T.; Mihhalevski, A.; Sarand, I. Analysis of Volatile Compounds Produced by Different Species of Lactobacilli in Rye Sourdough Using Multiple Headspace Extraction: Volatiles Produced by LAB in Sourdough. Int. J. Food Sci. Technol. 2011, 46, 1940–1946. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. Publisher Correction: The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 671. [Google Scholar] [CrossRef]
- Menezes, L.A.A.; Sardaro, M.L.S.; Duarte, R.T.D.; Mazzon, R.R.; Neviani, E.; Gatti, M.; De Dea Lindner, J. Sourdough Bacterial Dynamics Revealed by Metagenomic Analysis in Brazil. Food Microbiol. 2020, 85, 103302. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, Z.; Ramzan, R.; Abdelazez, A.; Amjad, A.; Afzaal, M.; Zhang, S.; Pan, S. Assessment of the Antimicrobial Potentiality and Functionality of Lactobacillus plantarum Strains Isolated from the Conventional Inner Mongolian Fermented Cheese Against Foodborne Pathogens. Pathogens 2019, 8, 71. [Google Scholar] [CrossRef]
- Koistinen, V.M.; Mattila, O.; Katina, K.; Poutanen, K.; Aura, A.M.; Hanhineva, K. Metabolic Profiling of Sourdough Fermented Wheat and Rye Bread. Sci. Rep. 2018, 8, 5684. [Google Scholar] [CrossRef]
- Saa, D.T.; Di Silvestro, R.; Nissen, L.; Dinelli, G.; Gianotti, A. Effect of Sourdough Fermentation and Baking Process Severity on Bioactive Fiber Compounds in Immature and Ripe Wheat Flour Bread. Leb. Wiss Technol 2018, 89, 322–328. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-Parabiotics: The New Horizons in Microbial Biotherapy and Functional Foods. Microb. Cell Factories 2020, 19, 168. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Valerio, F.; Evidente, A.; Lazzaroni, S.; Corsetti, A.; Gobbetti, M. Purification and Characterization of Novel Antifungal Compounds from the Sourdough Lactobacillus plantarum Strain 21B. Appl. Environ. Microbiol. 2000, 66, 4084–4090. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, A.; Polo, A.; Rizzello, C.G.; Acin-Albiac, M.; Montemurro, M.; Di Cagno, R.; Gobbetti, M. Feeding with Sustainably Sourdough Bread Has the Potential to Promote the Healthy Microbiota Metabolism at the Colon Level. Microbiol. Spectr. 2021, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Siragusa, S.; De Angelis, M.; Di Cagno, R.; Rizzello, C.G.; Coda, R.; Gobbetti, M. Synthesis of γ-Aminobutyric Acid by Lactic Acid Bacteria Isolated from a Variety of Italian Cheeses. Appl. Environ. Microbiol. 2007, 73, 7283–7290. [Google Scholar] [CrossRef] [PubMed]
- Sarasa, S.B.; Mahendran, R.; Muthusamy, G.; Thankappan, B.; Selta, D.R.F.; Angayarkanni, J. A Brief Review on the Non-Protein Amino Acid, Gamma-Amino Butyric Acid (GABA): Its Production and Role in Microbes. Curr. Microbiol. 2020, 77, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Khanlari, Z.; Moayedi, A.; Ebrahimi, P.; Khomeiri, M.; Sadeghi, A. Enhancement of Γ-aminobutyric Acid (GABA) Content in Fermented Milk by Using Enterococcus faecium and Weissella confuse Isolated from Sourdough. J. Food Process. Preserv. 2021, 45, e15869. [Google Scholar] [CrossRef]
- Diana, M.; Rafecas, M.; Quílez, J. Free Amino Acids, Acrylamide and Biogenic Amines in Gamma-Aminobutyric Acid Enriched Sourdough and Commercial Breads. J. Cereal Sci. 2014, 60, 639–644. [Google Scholar] [CrossRef]
- Polak, T.; Mejaš, R.; Jamnik, P.; Kralj Cigić, I.; Poklar Ulrih, N.; Cigić, B. Accumulation and Transformation of Biogenic Amines and Gamma-Aminobutyric Acid (GABA) in Chickpea Sourdough. Foods 2021, 10, 2840. [Google Scholar] [CrossRef] [PubMed]
- Boakye, P.G.; Kougblenou, I.; Murai, T.; Okyere, A.Y.; Anderson, J.; Bajgain, P.; Philipp, B.; LaPlante, B.; Schlecht, S.; Vogel, C.; et al. Impact of Sourdough Fermentation on FODMAPs and Amylase-Trypsin Inhibitor Levels in Wheat Dough. J. Cereal Sci. 2022, 108, 103574. [Google Scholar] [CrossRef]
- Luti, S.; Mazzoli, L.; Ramazzotti, M.; Galli, V.; Venturi, M.; Marino, G.; Lehmann, M.; Guerrini, S.; Granchi, L.; Paoli, P.; et al. Antioxidant and Anti-Inflammatory Properties of Sourdoughs Containing Selected Lactobacilli Strains Are Retained in Breads. Food Chem. 2020, 322, 126710. [Google Scholar] [CrossRef]
- Colosimo, R.; Gabriele, M.; Cifelli, M.; Longo, V.; Domenici, V.; Pucci, L. The Effect of Sourdough Fermentation on Triticum Dicoccum from Garfagnana: 1H NMR Characterization and Analysis of the Antioxidant Activity. Food Chem. 2020, 305, 125510. [Google Scholar] [CrossRef]
- Fekri, A.; Torbati, M.; Yari Khosrowshahi, A.; Bagherpour Shamloo, H.; Azadmard-Damirchi, S. Functional Effects of Phytate-Degrading, Probiotic Lactic Acid Bacteria and Yeast Strains Isolated from Iranian Traditional Sourdough on the Technological and Nutritional Properties of Whole Wheat Bread. Food Chem. 2020, 306, 125620. [Google Scholar] [CrossRef]
- Bockwoldt, J.A.; Fellermeier, J.; Steffens, E.; Vogel, R.F.; Ehrmann, M.A. β-Glucan Production by Levilactobacillus brevis and Pediococcus claussenii for In Situ Enriched Rye and Wheat Sourdough Breads. Foods 2021, 10, 547. [Google Scholar] [CrossRef]
- Schlörmann, W.; Bockwoldt, J.A.; Hübner, S.M.; Wittwer, E.; Reiners, S.; Lorkowski, S.; Dawczynski, C.; Ehrmann, M.A.; Glei, M. Use of the β-Glucan-Producing Lactic Acid Bacteria Strains Levilactobacillus brevis and Pediococcus claussenii for Sourdough Fermentation—Chemical Characterization and Chemopreventive Potential of In Situ-Enriched Wheat and Rye Sourdoughs and Breads. Nutrients 2022, 14, 1510. [Google Scholar] [CrossRef] [PubMed]
- Angelis, M.D.; Rizzello, C.G.; Fasano, A.; Clemente, M.G.; Simone, C.D.; Silano, M.; Vincenzi, M.D.; Losito, I.; Gobbetti, M. VSL#3 Probiotic Preparation Has the Capacity to Hydrolyze Gliadin Polypeptides Responsible for Celiac Sprue Probiotics and Gluten Intolerance. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2006, 1762, 80–93. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Cassone, A.; Cagno, R.D.; Gobbetti, A.M. Synthesis of Angiotensin I-Converting Enzyme (ACE)-Inhibitory Peptides and γ-Aminobutyric Acid (GABA) during Sourdough Fermentation by Selected Lactic Acid Bacteria. J. Agric. Food Chem. 2008, 56, 6936–6943.
- De Angelis, M.; Damiano, N.; Rizzello, C.G.; Cassone, A.; Di Cagno, R.; Gobbetti, M. Sourdough Fermentation as a Tool for the Manufacture of Low-Glycemic Index White Wheat Bread Enriched in Dietary Fibre. Eur. Food Res. Technol. 2009, 229, 593–601. [Google Scholar] [CrossRef]
- Wang, Y.; Gänzle, M.G.; Schwab, C. Exopolysaccharide Synthesized by Lactobacillus Reuteri Decreases the Ability of Enterotoxigenic Escherichia Coli To Bind to Porcine Erythrocytes. Appl. Environ. Microbiol. 2010, 76, 4863–4866.
- Novotni, D.; Curić, D.; Bituh, M.; Colić Barić, I.; Skevin, D.; Cukelj, N. Glycemic Index and Phenolics of Partially-Baked Frozen Bread with Sourdough. Int. J. Food Sci. Nutr. 2011, 62, 26–33.
- Rizzello, C.G.; Nionelli, L.; Coda, R.; Gobbetti, M. Synthesis of the Cancer Preventive Peptide Lunasin by Lactic Acid Bacteria during Sourdough Fermentation. Nutr. Cancer 2012, 64, 111–120.
- Sanz-Penella, J.M.; Laparra, J.M.; Sanz, Y.; Haros, M. Assessment of Iron Bioavailability in Whole Wheat Bread by Addition of Phytase-Producing Bifidobacteria. J. Agric. Food Chem. 2012, 60, 3190–3195.
- Galle, S.; Schwab, C.; Dal Bello, F.; Coffey, A.; Gänzle, M.G.; Arendt, E.K. Influence of In-Situ Synthesized Exopolysaccharides on the Quality of Gluten-Free Sorghum Sourdough Bread. Int. J. Food Microbiol. 2012, 155, 105–112.
- Sümengen, M.; Dinçer, S.; Kaya, A. Phytase Production from Lactobacillus brevis. Turk. J. Biol. 2012, 36, 533–541.
- Cizeikiene, D.; Juodeikiene, G.; Paskevicius, A.; Bartkiene, E. Antimicrobial Activity of Lactic Acid Bacteria against Pathogenic and Spoilage Microorganism Isolated from Food and Their Control in Wheat Bread. Food Control 2013, 31, 539–545.
- Amari, M.; Arango, L.F.G.; Gabriel, V.; Robert, H.; Morel, S.; Moulis, C.; Gabriel, B.; Remaud-Siméon, M.; Fontagné-Faucher, C. Characterization of a Novel Dextransucrase from Weissella confuse Isolated from Sourdough. Appl. Microbiol. Biotechnol. 2013, 97, 5413–5422.
- De Angelis, M.; Bottacini, F.; Fosso, B.; Kelleher, P.; Calasso, M.; Di Cagno, R.; Ventura, M.; Picardi, E.; van Sinderen, D.; Gobbetti, M. Lactobacillus Rossiae, a Vitamin B12 Producer, Represents a Metabolically Versatile Species within the Genus Lactobacillus. PLoS ONE 2014, 9, e107232.
- Belz, M.C.E.; Axel, C.; Arendt, E.K.; Lynch, K.M.; Brosnan, B.; Sheehan, E.M.; Coffey, A.; Zannini, E. Improvement of Taste and Shelf Life of Yeasted Low-Salt Bread Containing Functional Sourdoughs Using Lactobacillus amylovorus DSM 19280 and Weisella Cibaria MG1. Int. J. Food Microbiol. 2019, 302, 69–79.
- Omoba, O.S.; Isah, L.R. Influence of Sourdough Fermentation on Amino Acids Composition, Phenolic Profile, and Antioxidant Properties of Sorghum Biscuits. Prev. Nutr. Food Sci. 2018, 23, 220–227. [Google Scholar] [CrossRef]
- Fraberger, V.; Ammer, C.; Domig, K.J. Functional Properties and Sustainability Improvement of Sourdough Bread by Lactic Acid Bacteria. Microorganisms 2020, 8, 1895.
- Liu, T.; Li, Y.; Zhao, M.; Mo, Q.; Feng, F. Weight-Reducing Effect of Lactobacillus plantarum ZJUFT17 Isolated from Sourdough Ecosystem. Nutrients 2020, 12, 977.
- Zhong, H.; Wang, J.; Abdullah; Hafeez, M.A.; Guan, R.; Feng, F. Lactobacillus plantarum ZJUFB2 Prevents High Fat Diet-Induced Insulin Resistance in Association With Modulation of the Gut Microbiota. Front. Nutr. 2021, 8, 754222.
- Verni, M.; Vekka, A.; Immonen, M.; Katina, K.; Rizzello, C.G.; Coda, R. Biosynthesis of Γ-aminobutyric Acid by Lactic Acid Bacteria in Surplus Bread and Its Use in Bread Making. J. Appl. Microbiol. 2022, 133, 76–90.
- Yilmaz, M.T.; Ispirli, H.; Taylan, O.; Alamoudi, M.; Dertli, E. Bioactive and Technological Properties of an α-D-Glucan Synthesized by Weissella cibaria PDER21. Carbohydr. Polym. 2022, 285, 119227.
- Yu, L.; Qian, Z.; Ge, J.; Du, R. Glucansucrase Produced by Lactic Acid Bacteria: Structure, Properties, and Applications. Fermentation 2022, 8, 629.
- Galle, S.; Arendt, E.K. Exopolysaccharides from Sourdough Lactic Acid Bacteria. Crit. Rev. Food Sci. Nutr. 2014, 54, 891–901.
- BRENDA Information on EC 1.8.1.7—Glutathione-Disulfide Reductase. Available online: https://www.brenda-enzymes.org/enzyme.php?ecno=1.8.1.7#reactschemes (accessed on 20 November 2022).
- Xu, D.; Tang, K.; Hu, Y.; Xu, X.; Gänzle, M.G. Effect of Glutathione Dehydrogenase of Lactobacillus sanfranciscensis on Gluten Properties and Bread Volume in Type i Wheat Sourdough Bread. J. Agric. Food Chem. 2018, 66, 9770–9776.
- Tang, K.X.; Zhao, C.J.; Gänzle, M.G. Effect of Glutathione on the Taste and Texture of Type I Sourdough Bread. J. Agric. Food Chem. 2017, 65, 4321–4328.
- Venturi, M.; Galli, V.; Pini, N.; Guerrini, S.; Granchi, L. Use of Selected Lactobacilli to Increase γ-Aminobutyric Acid (GABA) Content in Sourdough Bread Enriched with Amaranth Flour. Foods 2019, 8, 218.
- Corsetti, A.; Gobbetti, M.; De Marco, B.; Balestrieri, F.; Paoletti, F.; Russi, L.; Rossi, J. Combined Effect of Sourdough Lactic Acid Bacteria and Additives on Bread Firmness and Staling. J Agric Food Chem 2000, 48, 3044–3051.
- Gänzle, M.G. Enzymatic and Bacterial Conversions during Sourdough Fermentation. Food Microbiol. 2014, 37, 2–10.
- Struyf, N.; Vandewiele, H.; Herrera-Malaver, B.; Verspreet, J.; Verstrepen, K.J.; Courtin, C.M. Kluyveromyces marxianus Yeast Enables the Production of Low FODMAP Whole Wheat Breads. Food Microbiol. 2018, 76, 134–145.
- Melim Miguel, A.S.; Souza, T.; da Costa Figueiredo, E.V.; Paulo Lobo, B.W.; Maria, G. Enzymes in Bakery: Current and Future Trends. In Food Industry; Muzzalupo, I., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-0911-2.
- Pourmohammadi, K.; Abedi, E. Hydrolytic Enzymes and Their Directly and Indirectly Effects on Gluten and Dough Properties: An Extensive Review. Food Sci. Nutr. 2021, 9, 3988–4006.
- Baye, K.; Guyot, J.-P.; Icard-Vernière, C.; Rochette, I.; Mouquet-Rivier, C. Enzymatic Degradation of Phytate, Polyphenols and Dietary Fibers in Ethiopian Injera Flours: Effect on Iron Bioaccessibility. Food Chem. 2015, 174, 60–67.
- Gandra, K.M.; Del Bianchi, M.; Godoy, V.P.; Queiroz, F.P.C.; Steel, C.J. Aplicação de Lipase e Monoglicerídeo Em Pão de Forma Enriquecido Com Fibras. Cienc. E Tecnol. Aliment. 2008, 28, 182–192.
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel Insights on the Functional/Nutritional Features of the Sourdough Fermentation. Int. J. Food Microbiol. 2018, 302, 103–113.
- Wieser, H. Chemistry of Gluten Proteins. Food Microbiol. 2007, 24, 115–119.
- Bietz, J.; Wall, J. Identity of High Molecular Weight Gliadin and Ethanol-Soluble Glutenin Subunits of Wheat: Relation to Gluten Structure. Cereal Chem. 1980, 57, 415–421. [Google Scholar]
- Sakandar, H.A.; Usman, K.; Imran, M. Isolation and Characterization of Gluten-Degrading Enterococcus mundtii and Wickerhamomyces anomalus, Potential Probiotic Strains from Indigenously Fermented Sourdough (Khamir). LWT Food Sci. Technol. 2018, 91, 271–277. [Google Scholar] [CrossRef]
- Stefańska, I.; Piasecka-Jóźwiak, K.; Kotyrba, D.; Kolenda, M.; Stecka, K.M. Selection of Lactic Acid Bacteria Strains for the Hydrolysis of Allergenic Proteins of Wheat Flour. J. Sci. Food Agric. 2016, 96, 3897–3905.
- Codex Alimentarius. Agenda Item 11 Cx/Nfsdu 19/41/11. Joint FAO/WHO Food Standards Programme. In Proceedings of the Codex Committee on Nutrition and Foods for Special Dietary Uses Forty-First Session, Dusseldorf, Germany, 24–29 November 2019.
- FDA. Generally Recognized as Safe (GRAS). Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 10 October 2022).
- Anvisa Resolução Da Diretoria Colegiada—RDC No 241, DE 26 DE JULHO DE 2018; Imprensa Nacional: Brasília, Brazil, 2018.
- Eur Regulamento (UE) 2015/2283 do Parlamento Europeu e do Conselho. Available online: https://eur-lex.europa.eu/legal-content/PT/TXT/PDF/?uri=CELEX:32015R2283&from=EN (accessed on 6 October 2022).
- Europa Safety Assessment and Regulatory Aspects of Microorganisms in Feed and Food Applications. Available online: https://food.ec.europa.eu/system/files/2020-12/sci-com_scan-old_report_out85.pdf (accessed on 4 October 2022).
- Brandt, M.J. Industrial Production of Sourdoughs for the Baking Branch—An Overview. Int. J. Food Microbiol. 2019, 302, 3–7.
- Balachandra Nair, R.; Ramachandranna, P.C. Patenting of Microorganisms: Systems and Concerns. J. Commer. Biotechnol. 2010, 16, 337–347.
- Planalto. LEI Nº 9.279. 1996. Available online: https://www.planalto.gov.br/ccivil_03/leis/l9279.htm
